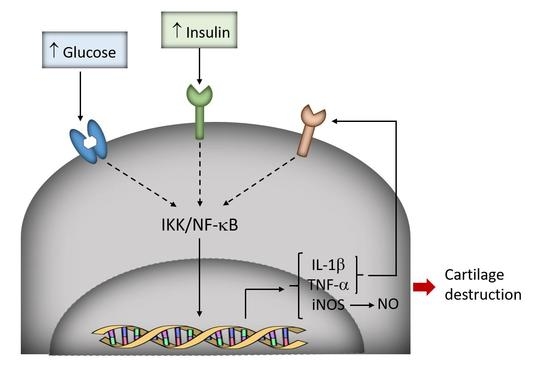
Hyperglycemia and Hyperinsulinemia-Like Conditions Independently Induce Inflammatory Responses in Human Chondrocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chondrocyte Isolation and Treatments
2.2. Nitric Oxide Production
2.3. Western Blot Analysis
2.4. Total RNA Extraction and Quantitative Real-Time RT-PCR (qRT-PCR)
2.5. Statistical Analysis
3. Results
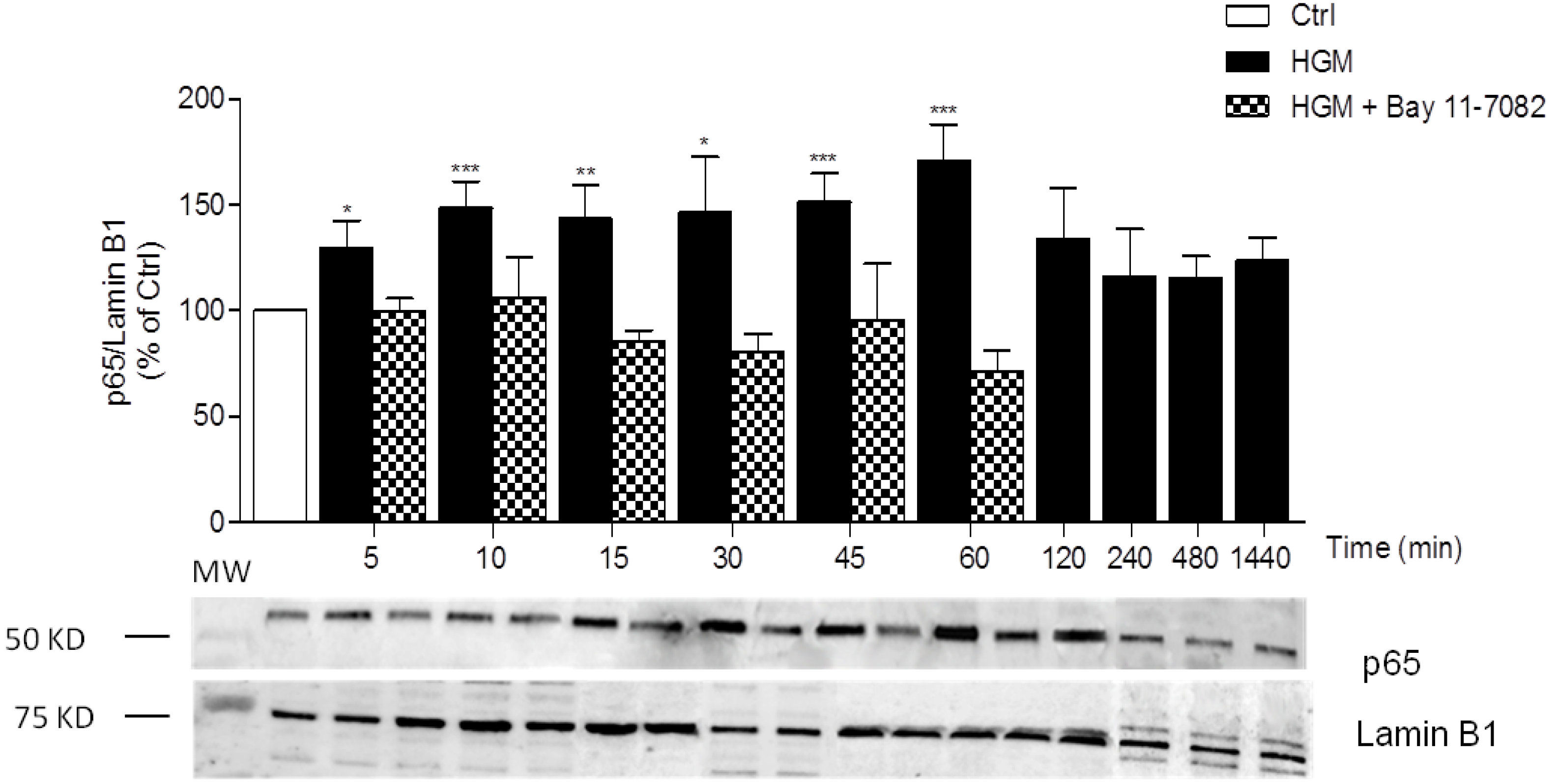
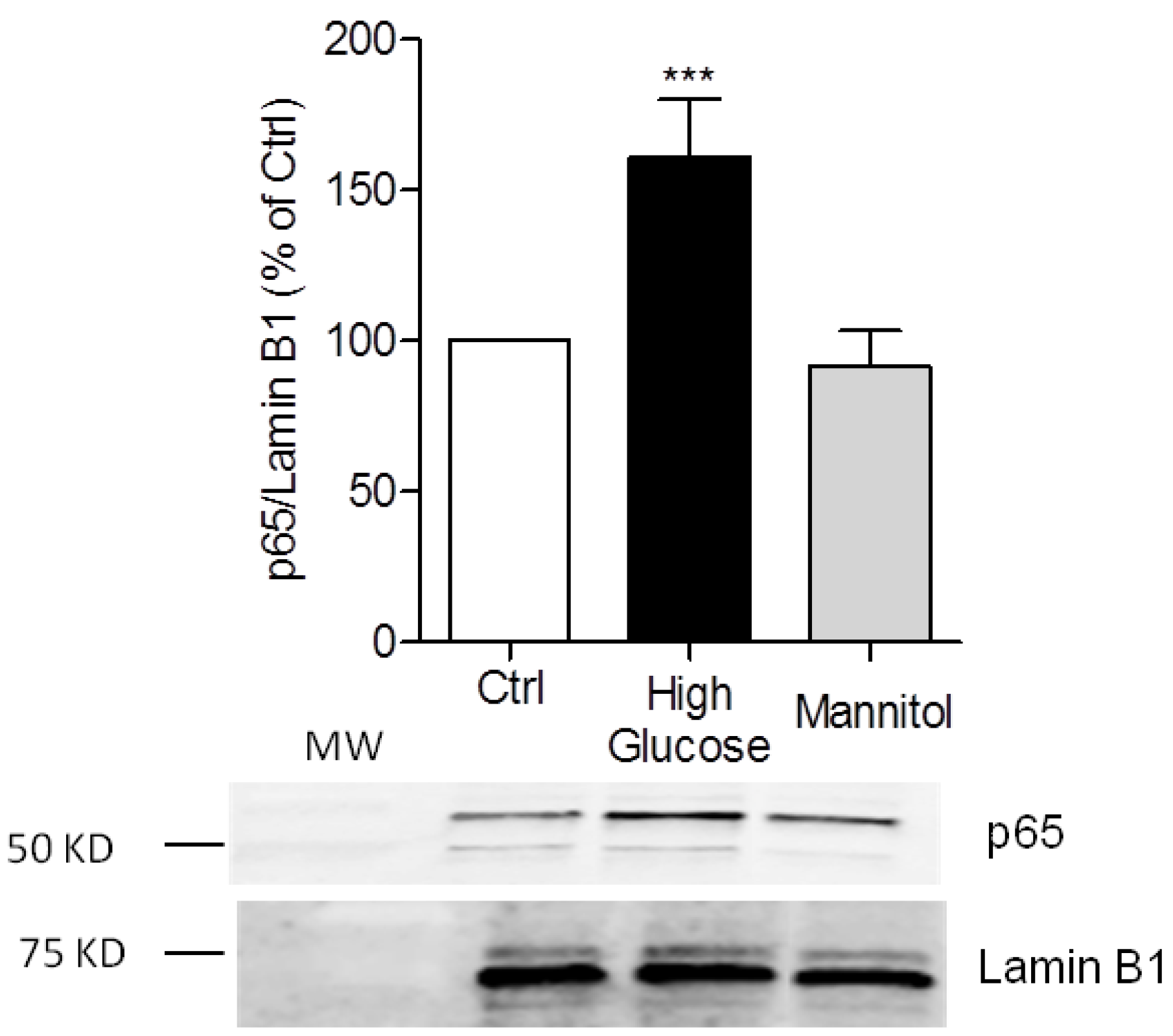
3.1. High-Glucose Induces NF-κB p65 Translocation to the Nucleus
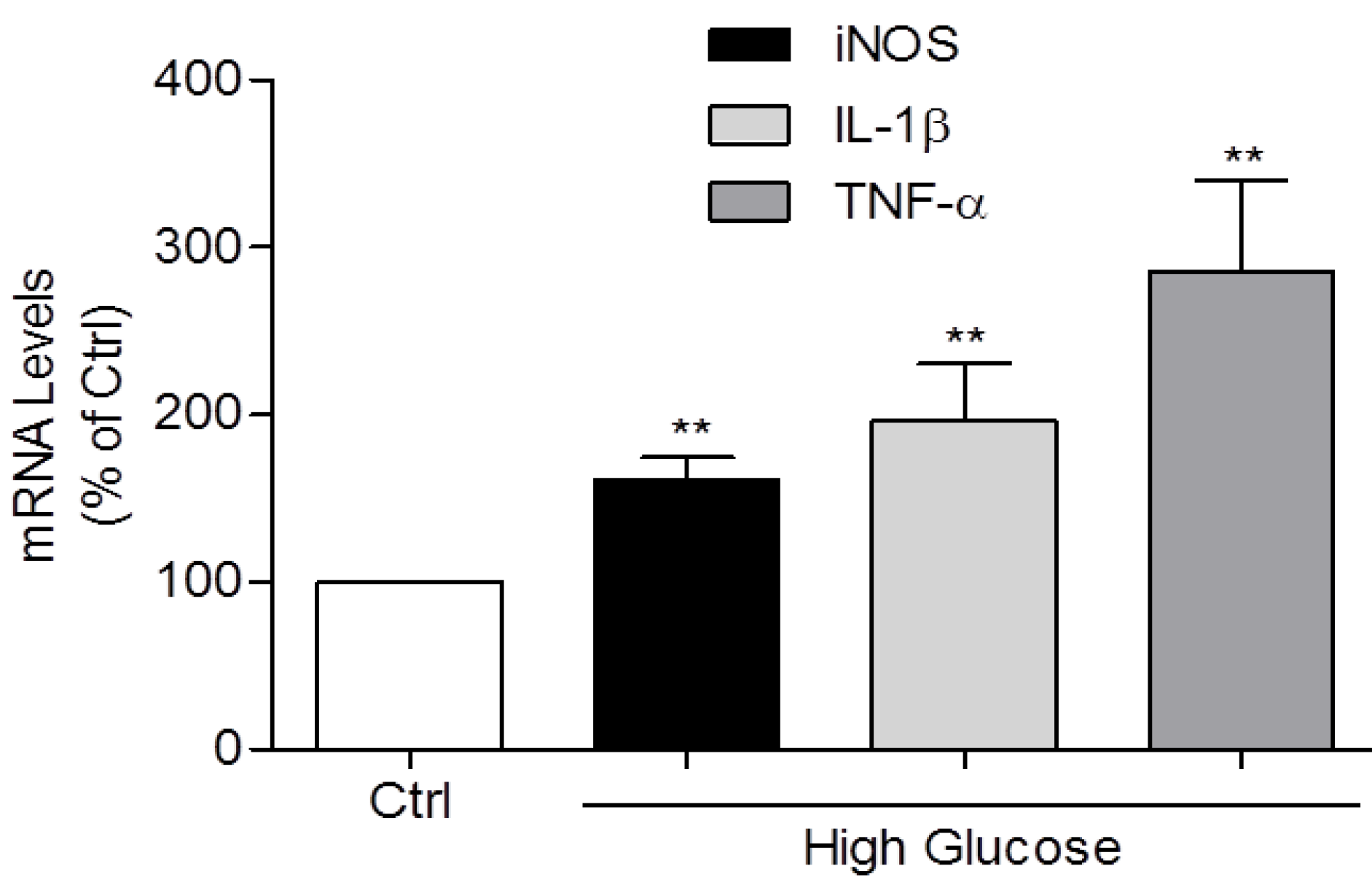
3.2. Induction of IL-1β, TNF-α, and iNOS Expression and NO Production by High Glucose
3.3. High Insulin Induced NF-κB p65 Translocation to the Nucleus
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. Signaling transduction: Target in osteoarthritis. Curr. Opin. Rheumatol. 2004, 16, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Kleyer, A.; Perricone, C.; Sahinbegovic, E.; Iagnocco, A.; Zwerina, J.; Lorenzini, R.; Aschenbrenner, F.; Berenbaum, F.; D’Agostino, M.A.; et al. Diabetes Is an Independent Predictor for Severe Osteoarthritis: Results from a longitudinal cohort study. Diabetes Care 2012, 36, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Plaza, M.; Castro-Santana, L.E.; Font, Y.M.; Mayor, A.M.; Vila, L.M. Association of hand or knee osteoarthritis with diabetes mellitus in a population of Hispanics from Puerto Rico. J. Clin. Rheumatol. 2013, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Lopez de Figueroa, P.; Blanco, F.J.; Mendes, A.F.; Carames, B. Insulin decreases autophagy and leads to cartilage degradation. Osteoarthr. Cartil. 2015, 24, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Lopez de Figueroa, P.; Nogueira-Recalde, U.; Centeno, A.; Mendes, A.F.; Blanco, F.J.; Carames, B. Diabetes-accelerated experimental osteoarthritis is prevented by autophagy activation. Osteoarthr. Cartil. 2016, 24, 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- Lotz, M.K.; Carames, B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat. Rev. Rheumatol. 2011, 7, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.M.; Johnson, T.R.; Ilan, J.; Moskowitz, R.W. Glucose regulation of the IGF response system in chondrocytes: Induction of an IGF-I-resistant state. Am. J. Physiol. 1999, 276, R1164–R1171. [Google Scholar] [PubMed]
- McNulty, A.L.; Stabler, T.V.; Vail, T.P.; McDaniel, G.E.; Kraus, V.B. Dehydroascorbate transport in human chondrocytes is regulated by hypoxia and is a physiologically relevant source of ascorbic acid in the joint. Arthritis Rheumatol. 2005, 52, 2676–2685. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.C.; Goncalves, J.; Judas, F.; Mobasheri, A.; Lopes, C.; Mendes, A.F. Impaired glucose transporter-1 degradation and increased glucose transport and oxidative stress in response to high glucose in chondrocytes from osteoarthritic versus normal human cartilage. Arthritis Res. Ther. 2009, 11, R80. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.C.; Rufino, A.T.; Judas, F.; Tenreiro, C.; Lopes, M.C.; Mendes, A.F. Expression and function of the insulin receptor in normal and osteoarthritic human chondrocytes: Modulation of anabolic gene expression, glucose transport and GLUT-1 content by insulin. Osteoarthr. Cartil. 2011, 19, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Chan, D.C.; Lan, K.C.; Wang, C.C.; Chen, C.M.; Chao, S.C.; Tsai, K.S.; Yang, R.S.; Liu, S.H. PPARgamma is involved in the hyperglycemia-induced inflammatory responses and collagen degradation in human chondrocytes and diabetic mouse cartilages. J. Orthop. Res. 2015, 33, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Laiguillon, M.C.; Courties, A.; Houard, X.; Auclair, M.; Sautet, A.; Capeau, J.; Feve, B.; Berenbaum, F; Sellam, J. Characterization of diabetic osteoarthritic cartilage and role of high glucose environment on chondrocyte activation: Toward pathophysiological delineation of diabetes mellitus-related osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.C.; Judas, F.; Lopes, M.C.; Mendes, A.F. Nitric oxide synthase isoforms and NF-κB activity in normal and osteoarthritic human chondrocytes: Regulation by inducible nitric oxide. Nitric Oxide 2008, 19, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Stadler, J.; Stefanovic-Racic, M.; Billiar, T.R.; Curran, R.D.; McIntyre, L.A.; Georgescu, H.I.; Simmons, R.L.; Evans, C.H. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J. Immunol. 1991, 147, 3915–3920. [Google Scholar] [PubMed]
- Liu-Bryan, R.; Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Strickson, S.; Campbell, D.G.; Emmerich, C.H.; Knebel, A.; Plater, L.; Ritorto, M.S.; Shpiro, N.; Cohen, P. The anti-inflammatory drug BAY 11-7082 suppresses the MyD88-dependent signalling network by targeting the ubiquitin system. Biochem. J. 2013, 451, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Nolan, T.; Hands, R.E.; Bustin, S.A. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 2006, 1, 1559–1582. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-κB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.F.; Carvalho, A.P.; Caramona, M.M.; Lopes, M.C. Role of nitric oxide in the activation of NF-κB, AP-1 and NOS II expression in articular chondrocytes. Inflamm. Res. 2002, 51, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Peters, M.A.; Wehmeyer, C.; Strietholt, S.; Koers-Wunrau, C.; Bertrand, J.; Heitzmann, M.; Hillmann, A.; Sherwood, J.; Seyfert, C.; et al. Regulation of matrixmetalloproteinase-3 and matrixmetalloproteinase-13 by SUMO-2/3 through the transcription factor NF-κB. Ann. Rheum. Dis. 2013, 72, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Rufino, A.T.; Ribeiro, M.; Sousa, C.; Judas, F.; Salgueiro, L.; Cavaleiro, C.; Mendes, A.F. Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. Eur. J. Pharmacol. 2015, 750, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.F.; Caramona, M.M.; Carvalho, A.P.; Lopes, M.C. Differential roles of hydrogen peroxide and superoxide in mediating IL-1-induced NF-κB activation and iNOS expression in bovine articular chondrocytes. J. Cell. Biochem. 2003, 88, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Kellner, K.; Schulz, M.B.; Gopferich, A.; Blunk, T. Insulin in tissue engineering of cartilage: A potential model system for growth factor application. J. Drug Target. 2001, 9, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Guo, S. Insulin signaling, resistance, and the metabolic syndrome: Insights from mouse models into disease mechanisms. J. Endocrinol. 2014, 220, T1–T23. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Geng, Y.J.; Bolli, R.; Rokosh, G.; Ferdinandy, P; Patterson, C.; De Caterina, R. Co-Activation of Nuclear Factor-κB and Myocardin/Serum Response Factor Conveys the Hypertrophy Signal of High Insulin Levels in Cardiac Myoblasts. J. Biol. Chem. 2014, 298, 19585–19598. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rufino, A.T.; Ribeiro, M.; Pinto Ferreira, J.; Judas, F.; Mendes, A.F. Hyperglycemia and Hyperinsulinemia-Like Conditions Independently Induce Inflammatory Responses in Human Chondrocytes. J. Funct. Morphol. Kinesiol. 2017, 2, 15. https://doi.org/10.3390/jfmk2020015
Rufino AT, Ribeiro M, Pinto Ferreira J, Judas F, Mendes AF. Hyperglycemia and Hyperinsulinemia-Like Conditions Independently Induce Inflammatory Responses in Human Chondrocytes. Journal of Functional Morphology and Kinesiology. 2017; 2(2):15. https://doi.org/10.3390/jfmk2020015
Chicago/Turabian StyleRufino, Ana Teresa, Madalena Ribeiro, João Pinto Ferreira, Fernando Judas, and Alexandrina Ferreira Mendes. 2017. "Hyperglycemia and Hyperinsulinemia-Like Conditions Independently Induce Inflammatory Responses in Human Chondrocytes" Journal of Functional Morphology and Kinesiology 2, no. 2: 15. https://doi.org/10.3390/jfmk2020015