The Effect of Resistance Training on Telomere Length in Women Recovering from Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Approach to the Problem
2.2. Subjects
2.3. Procedures
2.3.1. Resistance Training Intervention and Measurement of Muscular Strength
2.3.2. Blood Markers and Telomere Length
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Adherence and Adverse Events
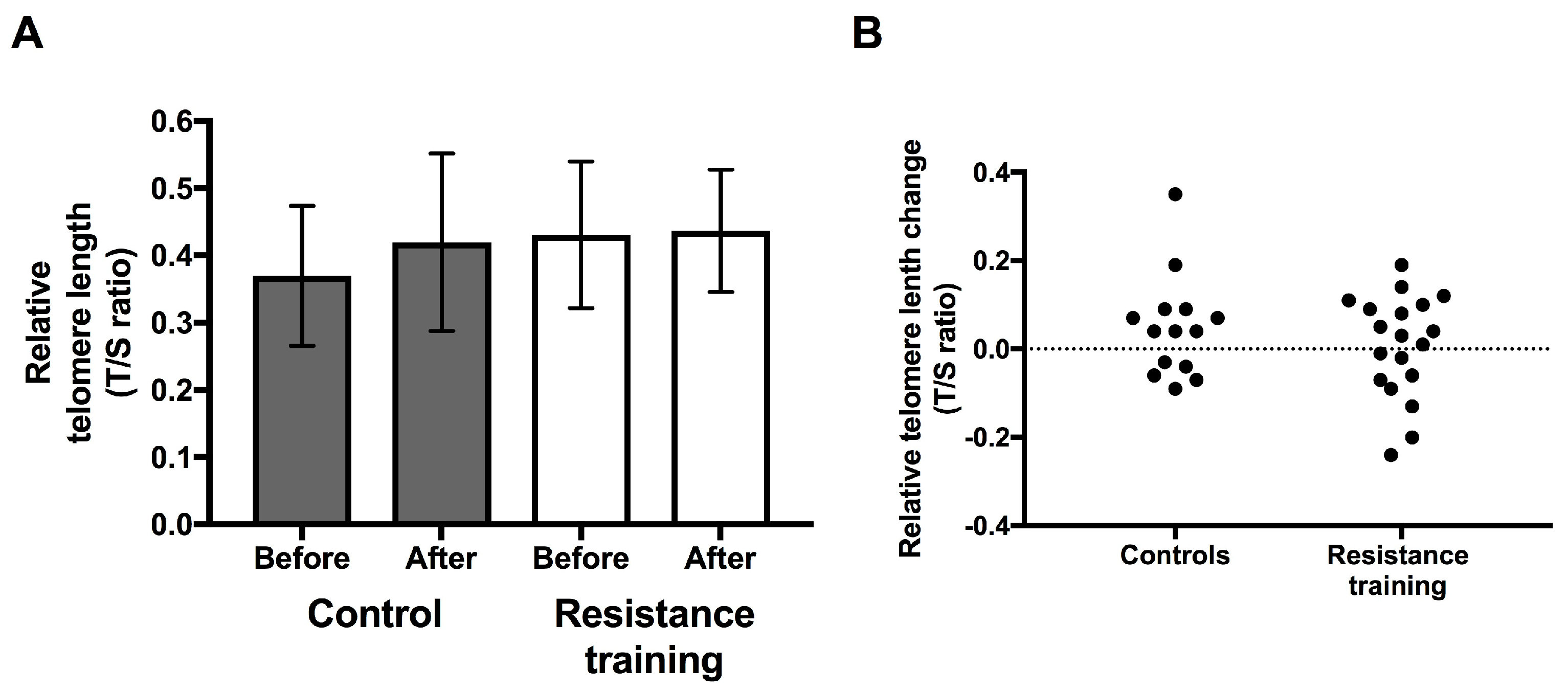
3.3. Telomere Length
3.4. Response to the Resistance Training Intervention
3.5. Relationships between Telomere Length and Muscular Strength
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- DeSantis, C.; Ma, J.; Bryan, L.; Jemal, A. Breast cancer statistics, 2013. CA Cancer J. Clin. 2014, 64, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Wentzensen, I.M.; Mirabello, L.; Pfeiffer, R.M.; Savage, S.A. The association of telomere length and cancer: A meta-analysis. Cancer Epidemiol. Prev. Biomark. 2011, 20, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Terry, M.B.; Gurvich, I.; Liao, Y.; Senie, R.T.; Santella, R.M. Short telomere length and breast cancer risk: A study in sister sets. Cancer Res. 2007, 67, 5538–5544. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.P.; Fujii, H.; Goronzy, J.J.; Weyand, C.M. Telomeres and immunological diseases of aging. Gerontology 2010, 56, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Epel, E. How “reversible” is telomeric aging? Cancer Prev. Res. 2012, 5, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Ornish, D.; Lin, J.; Chan, J.M.; Epel, E.; Kemp, C.; Weidner, G.; Marlin, R.; Frenda, S.J.; Magbanua, M.J.; Daubenmier, J.; et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013, 14, 1112–1120. [Google Scholar] [CrossRef]
- Puterman, E.; Lin, J.; Krauss, J.; Blackburn, E.H.; Epel, E.S. Determinants of telomere attrition over 1 year in healthy older women: Stress and health behaviors matter. Mol. Psychiatry 2015, 20, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Garland, S.N.; Johnson, B.; Palmer, C.; Speck, R.M.; Donelson, M.; Xie, S.X.; DeMichele, A.; Mao, J.J. Physical activity and telomere length in early stage breast cancer survivors. Breast Cancer Res. 2014, 16, 413. [Google Scholar] [CrossRef] [PubMed]
- Denham, J.; O’Brien, B.J.; Charchar, F.J. Telomere length maintenance and cardio-metabolic disease prevention through exercise training. Sports Med. 2016, 46, 1213–1237. [Google Scholar] [CrossRef] [PubMed]
- Ennour-Idrissi, K.; Tetu, B.; Maunsell, E.; Poirier, B.; Montoni, A.; Rochette, P.J.; Diorio, C. Association of telomere length with breast cancer prognostic factors. PLoS ONE 2016, 11, e0161903. [Google Scholar] [CrossRef] [PubMed]
- Kadi, F.; Ponsot, E.; Piehl-Aulin, K.; Mackey, A.; Kjaer, M.; Oskarsson, E.; Holm, L. The effects of regular strength training on telomere length in human skeletal muscle. Med. Sci. Sports Exerc. 2008, 40, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Tosevska, A.; Franzke, B.; Hofmann, M.; Vierheilig, I.; Schober-Halper, B.; Oesen, S.; Neubauer, O.; Wessner, B.; Wagner, K.H. Circulating cell-free DNA, telomere length and bilirubin in the vienna active ageing study: Exploratory analysis of a randomized, controlled trial. Sci. Rep. 2016, 6, 38084. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Furtado, C.L.; Ramos, F.K.P.; Kogure, G.S.; Santana-Lemos, B.A.; Ferriani, R.A.; Calado, R.T.; dos Reis, R.M. A nonrandomized trial of progressive resistance training intervention in women with polycystic ovary syndrome and its implications in telomere content. Reprod. Sci. 2016, 23, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Hagstrom, A.D.; Marshall, P.W.; Lonsdale, C.; Papalia, S.; Cheema, B.S.; Toben, C.; Baune, B.T.; Fiatarone Singh, M.A.; Green, S. The effect of resistance training on markers of immune function and inflammation in previously sedentary women recovering from breast cancer: A randomized controlled trial. Breast Cancer Res. Treat. 2016, 155, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Ohira, T.; Schmitz, K.H.; Ahmed, R.L.; Yee, D. Effects of weight training on quality of life in recent breast cancer survivors: The weight training for breast cancer survivors (WTBS) study. Cancer 2006, 106, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Cheema, B.; Gaul, C. Full-body exercise training improves fitness and quality of life in survivors of breast cancer. J. Strength Cond. Res. 2006, 20, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Hagstrom, A.D.; Marshall, P.W.; Lonsdale, C.; Cheema, B.S.; Fiatarone Singh, M.A.; Green, S. Resistance training improves fatigue and quality of life in previously sedentary breast cancer survivors: A randomised controlled trial. Eur. J. Cancer Care 2016, 25, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Hagstrom, A.D.; Shorter, K.A.; Marshall, P.W.M. Changes in unilateral upper limb muscular strength and emg activity following a 16 week strength training intervention survivors of breast cancer. J. Strength Cond. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Denham, J.; O’Brien, B.J.; Prestes, P.R.; Brown, N.J.; Charchar, F.J. Increased expression of telomere-regulating genes in endurance athletes with long leukocyte telomeres. J. Appl. Physiol. 2016, 120, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Denham, J. Lack of association between pbmc telomere length and endurance exercise. J. Appl. Biomed. 2017, 15, 9–13. [Google Scholar] [CrossRef]
- Denham, J.; Marques, F.Z.; Charchar, F.J. Leukocyte telomere length variation due to DNA extraction method. BMC Res. Notes 2014, 7, 877. [Google Scholar] [CrossRef] [PubMed]
- Duggan, C.; Risques, R.; Alfano, C.; Prunkard, D.; Imayama, I.; Holte, S.; Baumgartner, K.; Baumgartner, R.; Bernstein, L.; Ballard-Barbash, R.; et al. Change in peripheral blood leukocyte telomere length and mortality in breast cancer survivors. J. Natl. Cancer Inst. 2014, 106, dju035. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Epel, E.; Blackburn, E. Telomeres and lifestyle factors: Roles in cellular aging. Mutat. Res. 2012, 730, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Buxton, J.L.; Das, S.; Rodriguez, A.; Kaakinen, M.; Couto Alves, A.; Sebert, S.; Millwood, I.Y.; Laitinen, J.; O’Reilly, P.F.; Jarvelin, M.R.; et al. Multiple measures of adiposity are associated with mean leukocyte telomere length in the northern finland birth cohort 1966. PLoS ONE 2014, 9, e99133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordfjall, K.; Eliasson, M.; Stegmayr, B.; Melander, O.; Nilsson, P.; Roos, G. Telomere length is associated with obesity parameters but with a gender difference. Obesity 2008, 16, 2682–2689. [Google Scholar] [CrossRef] [PubMed]
| Variable | RT Group (n = 19) | Control Group (n = 14) | p-Value |
|---|---|---|---|
| Age (years) | 50.8 ± 8.3 | 52.1 ± 8.5 | 0.67 |
| Body mass index (BMI) (kg/m2) | 27.7 ± 4.3 | 30.3 ± 6.3 | 0.23 |
| Weight (kg) | 72.7 ± 9.3 | 80.1 ± 16.7 | 0.15 |
| Time since treatment (months) | 8.5 ± 8.8 | 8.4 ± 10.5 | 0.99 |
| Chemotherapy (n) | 16 | 12 | 0.90 |
| Radiotherapy (n) | 18 | 13 | 0.82 |
| Hormone therapy (n) | 16 | 13 | 0.45 |
| Variable (%) | r-Value | p-Value |
|---|---|---|
| Upper body strength change in the surgical arm | 0.035 | 0.85 |
| Upper body strength change in the other arm | 0.05 | 0.78 |
| Lower body strength change | −0.02 | 0.93 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagstrom, A.D.; Denham, J. The Effect of Resistance Training on Telomere Length in Women Recovering from Breast Cancer. J. Funct. Morphol. Kinesiol. 2018, 3, 9. https://doi.org/10.3390/jfmk3010009
Hagstrom AD, Denham J. The Effect of Resistance Training on Telomere Length in Women Recovering from Breast Cancer. Journal of Functional Morphology and Kinesiology. 2018; 3(1):9. https://doi.org/10.3390/jfmk3010009
Chicago/Turabian StyleHagstrom, Amanda D., and Joshua Denham. 2018. "The Effect of Resistance Training on Telomere Length in Women Recovering from Breast Cancer" Journal of Functional Morphology and Kinesiology 3, no. 1: 9. https://doi.org/10.3390/jfmk3010009





