A Novel Model of Meibomian Gland Dysfunction Induced with Complete Freund’s Adjuvant in Rabbits
Abstract
:1. Introduction
2. Results
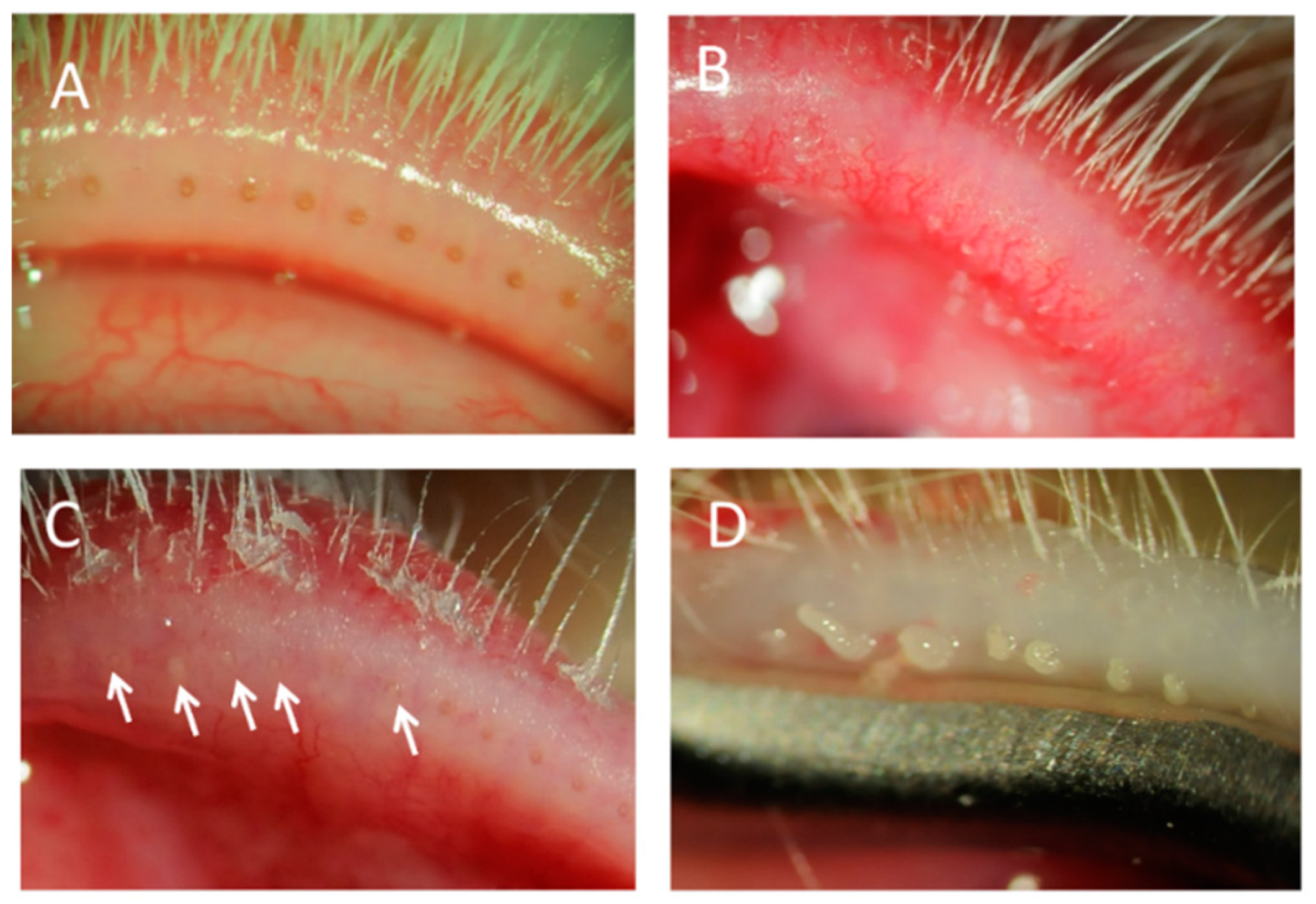
2.1. MGD with Meibomitis Assessed through Slit Lamp Examination
2.2. Histopathologic Analysis of MGD with Meibomitis
2.3. Treatment with Tob/Dex
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Induction of the MGD with Meibomitis Model
4.3. Slit Lamp Examination
4.4. Histologic Analysis
4.5. Treatment of MGD with Meibomitis
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Knop, E.; Knop, N.; Millar, T.; Obata, H.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1938–1978. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.D.; Shimazaki, J.; Benitez-del-Castillo, J.M.; Craig, J.P.; McCulley, J.P.; Den, S.; Foulks, G.N. The international workshop on meibomian gland dysfunction: Report of the definition and classification subcommittee. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- Mathers, W.D. Ocular evaporation in meibomian gland dysfunction and dry Eye. Ophthalmology 1993, 100, 347–351. [Google Scholar] [CrossRef]
- Baudouin, C.; Messmer, E.M.; Aragona, P.; Geerling, G.; Akova, Y.A.; Benítez-del-Castillo, J.; Boboridis, K.G.; Merayo-Lloves, J.; Rolando, M.; Labetoulle, M. Revisiting the vicious circle of dry eye disease: A focus on the pathophysiology of meibomian gland dysfunction. Br. J. Ophthalmol. 2016, 100, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.K.; Foulks, G.N.; Bron, A.J.; Glasgow, B.J.; Dogru, M.; Tsubota, K.; Lemp, M.A.; Sullivan, D.A. The International workshop on meibomian gland dysfunction: Executive summary. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Sledge, S.M.; Khimji, H.; Borchman, D.; Oliver, A.L.; Michael, H.; Dennis, E.K.; Gerlach, D.; Bhola, R.; Stephen, E. Evaporation and hydrocarbon chain conformation of surface lipid films. Ocul. Surf. 2016, 14, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Yan, X. Emerging treatment options for meibomian gland dysfunction. Clin. Ophthalmol. 2013, 7, 1797–1803. [Google Scholar] [PubMed]
- Thode, A.R.; Latkany, R.A. Current and emerging therapeutic strategies for the treatment of meibomian gland dysfunction (MGD). Drugs 2015, 75, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Okura, M.; Kawashima, M.; Katagiri, M.; Shirasawa, T.; Tsubota, K. New eye cleansing product improves makeup-related ocular problems. J. Ophthalmol. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kam, W.R.; Ding, J.; Sullivan, D.A. One man’s poison is another man’s meat: Using azithromycin-induced phospholipidosis to promote ocular surface health. Toxicology 2014, 320, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Foulks, G.N.; Borchman, D.; Yappert, M.; Kim, S.-H.; McKay, J.W. Topical azithromycin therapy for meibomian gland dysfunction: Clinical response and lipid alterations. Cornea 2010, 29, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Miyake, H.; Oda, T.; Katsuta, O.; Seno, M.; Nakamura, M. Meibomian gland dysfunction model in hairless mice fed a special diet with limited lipid content. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3268–3275. [Google Scholar] [CrossRef] [PubMed]
- Geerling, G.; Tauber, J.; Baudouin, C.; Goto, E.; Matsumoto, Y.; O’Brien, T.; Rolando, M.; Tsubota, K.; Nichols, K.K. The international workshop on meibomian gland dysfunction: Report of the subcommittee on management and treatment of meibomian gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2050–2064. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Teramukai, S.; Kinoshita, S. Meibomian glands and ocular surface inflammation. Ocul. Surf. 2015, 13, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Chung, B.; Kim, K.S.; Seo, K.Y.; Choi, B.J.; Kim, T. Effects of topical loteprednol etabonate on tear cytokines and clinical outcomes in moderate and severe meibomian gland dysfunction: Randomized clinical trial. Am. J. Ophthalmol. 2014, 158, 1172–1183.e1. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Rajagopalan, S.; Rodrigues, M. Meibomian gland changes in the rhino (hrrhhrrh) mouse. Investig. Ophthalmol. Vis. Sci. 1988, 29, 1190–1194. [Google Scholar]
- Yagyu, H.; Kitamine, T.; Osuga, J.I.; Tozawa, R.I.; Chen, Z.; Kaji, Y.; Oka, T.; Perrey, S.; Tamura, Y.; Ohashi, K.; et al. Absence of ACAT-1 attenuates atherosclerosis but causes dry eye and cutaneous xanthomatosis mice with congenital hyperlipidemia. J. Biol. Chem. 2000, 275, 21324–21330. [Google Scholar] [CrossRef] [PubMed]
- Naito, A.; Yoshida, H.; Nishioka, E.; Satoh, M.; Azuma, S.; Yamamoto, T.; Nishikawa, S.; Inoue, J. TRAF6-deficient mice display hypohidrotic ectodermal dysplasia. Proc. Natl. Acad. Sci. USA 2002, 99, 8766–8771. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.-Y.; Smith, J.A.; Schlessinger, D.; Chan, C.-C. X-linked anhidrotic ectodermal dysplasia disruption yields a mouse model for ocular surface disease and resultant blindness. Am. J. Pathol. 2005, 167, 89–95. [Google Scholar] [CrossRef]
- Wang, Y.C.; Li, S.; Chen, X.; Ma, B.; He, H.; Liu, T.; Yu, J.; Zhang, L.; Chen, Y.; Liu, Z.; Li, W. Meibomian gland absence related dry eye in ectodysplasin a mutant mice. Am. J. Pathol. 2016, 186, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Knop, E.; Knop, N.; Sullivan, D.A.; List, E.O.; Kopchick, J.J.; Kam, W.R.; Ding, J. Growth hormone influence on the morphology and size of the mouse meibomian gland. J. Ophthalmol. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, O.M.A.; Dogru, M.; Matsumoto, Y.; Igarashi, A.; Kojima, T.; Wakamatsu, T.H.; Inaba, T.; Shimizu, T.; Shimazaki, J.; Tsubota, K. Oxidative stress induced age dependent meibomian gland dysfunction in Cu, Zn-superoxide dismutase-1 (SOD1) knockout mice. PLoS ONE 2014, 9, e99328. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Rife, L.; Nii, D.; Luttrull, J.K.; Wilson, L.; Smith, R.E. In vivo biomicroscopy and photography of meibomian glands in a rabbit model of meibomian gland dysfunction. Investig. Ophthalmol. Vis. Sci. 1982, 22, 660–667. [Google Scholar]
- Ohnishi, Y.; Kohno, T. Polychlorinated biphenyls poisoning in monkey eye. Investig. Ophthalmol. Vis. Sci. 1979, 18, 981–984. [Google Scholar]
- Lambert, R.W. Pathogenesis of blepharoconjunctivitis complicating laboratory model. Investig. Ophthalmol. Vis. Sci. 1988, 29, 1559–1564. [Google Scholar]
- Watters, G.A.; Turnbull, P.R.; Swift, S.; Petty, A.; Craig, J.P. Ocular surface microbiome in meibomian gland dysfunction in Auckland, New Zealand. Clin. Exp. Ophthalmol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Albietz, J.M.; Lenton, L.M. Effect of antibacterial honey on the ocular flora in tear deficiency and meibomian gland disease. Cornea 2006, 25, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.E.; Moore, J.E.; Jiru, X.; Moore, J.E.; Goodall, E.A.; Dooley, J.S.G.; Hayes, V.E.A.; Dartt, D.A.; Downes, C.S.; Moore, T.C.B. Ocular pathogen or commensal: A PCR-based study of surface bacterial flora in normal and dry eyes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5616–5623. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, J.M.; McCulley, J.P. Bacterial lipases and chronic blepharitis. Investig. Ophthalmol. Vis. Sci. 1986, 27, 486–491. [Google Scholar]
- Groden, L.R.; Murphy, B.; Rodnite, J.; Genvert, G.I. Lid flora in blepharitis. Cornea 1991, 10, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Obata, H. Anatomy and histopathology of human meibomian gland. Cornea 2002, 21, S70–S74. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Yappert, M.C.; Foulks, G.N. Changes in human meibum lipid with meibomian gland dysfunction using principal component analysis. Exp. Eye Res. 2010, 91, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Butovich, I.A.; Lu, H.; McMahon, A.; Ketelson, H.; Senchyna, M.; Meadows, D.; Campbell, E.; Molai, M.; Linsenbardt, E. Biophysical and morphological evaluation of human normal and dry eye meibum using hot stage polarized light microscopy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Ong, B.L.; Hodson, S.A.; Wigham, T.; Miller, F.; Larke, J.R. Evidence for keratin proteins in normal and abnormal human meibomian fluids. Curr. Eye Res. 1991, 10, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Bell, J.; Wells, E.; Neravetla, S.; Greenstone, V. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3805–3817. [Google Scholar] [CrossRef] [PubMed]
- Butovich, I.A.; Lu, H.; McMahon, A.; Eule, J.C. Toward an animal model of the human tear film: Biochemical comparison of the mouse, canine, rabbit, and human meibomian lipidomes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6881–6896. [Google Scholar] [CrossRef] [PubMed]
- Maurice, D. The effect of the low blink rate in rabbits on topical drug penetration. J. Ocul. Pharmacol. Ther. 1995, 11, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.E.; Markoulli, M.; Zhao, Z.; Willcox, M.D. Tear film break-up time in rabbits. Clin. Exp. Optom. 2013, 96, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.C.; Gery, I.; Kohn, L.D.; Nussenblatt, R.B.; Mozes, E.; Singer, D.S. Periocular inflammation in mice with experimental systemic lupus erythematosus. A new experimental blepharitis and its modulation. J. Immunol. 1995, 154, 4830–4835. [Google Scholar] [PubMed]



| Treatment | Days after randomization | Telangiectasia Score (Mean ± SE) | Plugged Orifice Score (Mean ± SE) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Temporal | Central | Nasal | Total | Temporal | Central | Nasal | Total | ||
| Saline | 0 | 0.3 ± 0.2 | 0.7 ± 0.2 | 0.3 ± 0.2 | 1.3 ± 0.4 | 0.1 ± 0.1 | 0.6 ± 0.2 | 0.1 ± 0.1 | 0.9 ± 0.3 |
| 7 | 1.3 ± 0.3 | 1.3 ±0.2 | 0.3 ± 0.2 | 2.9 ± 0.4 | 0.9 ± 0.1 | 1.3 ± 0.3 | 0.4 ± 0.3 | 2.6 ± 0.4 | |
| 11 | 1.1 ± 0.3 | 1.4 ±0.3 | 0.4 ± 0.2 | 3.0 ± 0.6 | 1.1 ± 0.1 | 1.9 ± 0.1 | 0.7 ± 0.4 | 3.7 ± 0.5 | |
| Tobramycin/Dexamethasone | 0 | 0.0 ± 0.0 | 1.0 ±0.2 | 0.1 ± 0.1 | 1.1 ± 0.3 | 0.1 ± 0.1 | 0.7 ± 0.2 | 0.0 ± 0.0 | 0.9 ± 0.3 |
| 7 | 0.3 ± 0.2 * | 0.3 ±0.2 ** | 0.1 ± 0.1 | 0.7 ± 0.5 ** | 0.6 ± 0.3 | 0.7 ± 0.2 | 0.1 ± 0.1 | 1.4 ± 0.4 | |
| 11 | 0.4 ± 0.2 | 0.6 ± 0.2 * | 0.1 ± 0.1 | 1.1 ± 0.3 * | 0.6 ± 0.2 * | 0.9 ± 0.3 ** | 0.3 ± 0.2 | 1.7 ± 0.4 ** | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyake, H.; Oda, T.; Katsuta, O.; Seno, M.; Nakamura, M. A Novel Model of Meibomian Gland Dysfunction Induced with Complete Freund’s Adjuvant in Rabbits. Vision 2017, 1, 10. https://doi.org/10.3390/vision1010010
Miyake H, Oda T, Katsuta O, Seno M, Nakamura M. A Novel Model of Meibomian Gland Dysfunction Induced with Complete Freund’s Adjuvant in Rabbits. Vision. 2017; 1(1):10. https://doi.org/10.3390/vision1010010
Chicago/Turabian StyleMiyake, Hideki, Tomoko Oda, Osamu Katsuta, Masaharu Seno, and Masatsugu Nakamura. 2017. "A Novel Model of Meibomian Gland Dysfunction Induced with Complete Freund’s Adjuvant in Rabbits" Vision 1, no. 1: 10. https://doi.org/10.3390/vision1010010






