Comparing Macular Thickness Measurements in Patients with Diabetic Macular Edema with the Optos Spectral OCT/SLO and Heidelberg Spectralis HRA + OCT
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Data Collection
4.2. Data Analysis
Author Contributions
Conflicts of Interest
Abbreviations
| OCT | Optical coherence tomography |
| HRA | Heidelberg Retina Angiograph |
| SD-OCT | Spectral-domain optical coherence tomography |
| TD-OCT | Time-domain optical coherence tomography |
| SLO | Scanning laser ophthalmoscopy |
| DME | Diabetic macular edema |
| BRVO | Branch retinal vein occlusion |
| BCVA | Best corrected visual acuity |
| ETDRS | Early treatment diabetic retinopathy study |
| RPE | Retinal pigment epithelium |
References
- Fong, D.S.; Aiello, L.P.; Ferris, F.L., III; Klein, R. Diabetic retinopathy. Diabetes Care 2004, 27, 2540–2553. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.; Moss, S.E.; Cruickshanks, K.J. The Wisconsin epidemiologic study of diabetic retinopathy, XV: The long-term incidence of macular oedema. Ophthalmology 1995, 102, 7–16. [Google Scholar] [CrossRef]
- Antcliff, R.J.; Marshall, J. The pathogenesis of edema in diabetic maculopathy. Semin. Ophthalmol. 1999, 14, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, B.L.; Malukiewicz, G.; Stafiej, J.; Lesiewska-Junk, H.; Raczynska, D. The diagnostic function of OCT in diabetic maculopathy. Mediators Inflamm. 2013, 2013, 434560. [Google Scholar] [CrossRef] [PubMed]
- Diabetic Retinopathy Clinical Research Network. The relationship between OCT-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology 2007, 114, 525–536. [Google Scholar]
- Hatef, E.; Colantuoni, E.; Wang, J.; Ibrahim, M.; Shulman, M.; Adhi, F.; Sepah, Y.J.; Channa, R.; Khwaja, A.; Nguyen, Q.D.; et al. The relationship between macular sensitivity and retinal thickness in eyes with diabetic macular edema. Am. J. Ophthalmol. 2011, 152, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Midena, E.; Pilotto, E.; Radin, P.P.; Chiesa, L.; Cavarzeran, F. Diabetic macular edema: Correlation between microperimetry and optical coherence tomography findings. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3044–3051. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Yamamoto, S.; Mizunoya, S.; Hoshina, A.; Arai, M.; Takatsuna, Y. Correlation of retinal sensitivity measured with fundus-related microperimetry to visual acuity and retinal thickness in eyes with diabetic macular edema. Eye 2006, 20, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Funatsu, H.; Mimura, T.; Harino, S.; Shimada, K. Functional-morphologic correlates in patients with branch retinal vein occlusion and macular edema. Retina 2011, 31, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Sayegh, R.G.; Kiss, C.G.; Simader, C.; Kroisamer, J.; Montuoro, A.; Mittermüller, T.J.; Azhary, M.; Bolz, M.; Kreil, D.P.; Schmidt-Erfurth, U. A systematic correlation of morphology and function using spectral domain optical coherence tomography and microperimetry in patients with geographic atrophy. Br. J. Ophthalmol. 2014, 98, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.V.; Coupland, S.G.; Stitt, D.M.; Hamilton, J.; Brownstein, J.J.; Damji, K.F. Efficacy of SLO-microperimetry and humphrey for evaluating macular sensitivity changes in advanced glaucoma. Can. J. Ophthalmol. 2013, 48, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Bottega, E.; Casciano, M.; Pilotto, E.; Convento, E.; Midena, E. Microperimetry and fundus autofluorescence in diabetic macular oedema: Subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina 2010, 30, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Senturk, F.; Ozdemir, H.; Karacorlu, M.; Karacorlu, S.A.; Uysal, O. Retinal sensitivity improvement after intravitreal triamcinolone acetonide injection for macular edema secondary to branch retinal vein occlusion. Indian J. Ophthalmol. 2013, 61, 3–7. [Google Scholar]
- Wong, W.T.; Kam, W.; Cunningham, D.; Harrington, M.; Hammel, K.; Meyerle, C.B.; Cukras, C.; Chew, E.Y.; Sadda, S.R.; Ferris, F.L. Treatment of geographic atrophy by the topical administration of OT-551: Results of phase II clinical trial. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6131–6139. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Schnurrbusch, U.E.; Ceklic, L.; Brinkmann, C.K.; Iliev, M.E.; Frey, M.; Rothenbuehler, S.P.; Enzmann, V.; Wolf, S. Macular Thickness measurements in healthy eyes using six different optical coherence tomography instruments. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3432–3437. [Google Scholar] [CrossRef] [PubMed]
- Ozkok, A.; Akkan, J.C.; Tamcelik, N.; Erdogan, M.; Comlekoglu, D.U.; Yildirim, R. Comparison of retinal nerve fiber layer and macular thickness measurements with Stratus OCT and OPKO/OTI OCT devices in healthy subjects. Int. J. Ophthalmol. 2015, 8, 98–103. [Google Scholar] [PubMed]
- Pierro, L.; Giatsidis, S.M.; Mantovani, E.; Gagliardi, M. Macular thickness interoperator and intraoperator reproducibility in healthy eyes using 7 optical coherence tomography instruments. Am. J. Ophthalmol. 2010, 150, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Heussen, F.M.; Ouyang, Y.; McDonnell, E.C.; Narala, R.; Ruiz-Garcia, H.; Walsh, A.C.; Sadda, S.R. Comparison of manually corrected retinal thickness measurements from multiple spectral-domain optical coherence tomography instruments. Br. J. Ophthalmol. 2012, 96, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Pierro, L.; Gagliardi, M.; Iuliano, L.; Ambrosi, A.; Bandello, F. Retinal nerve fiber layer thickness reproducibility using seven different OCT instruments. Invest. Ophthalmol. Vis. Sci. 2012, 53, 5912–5920. [Google Scholar] [CrossRef] [PubMed]
- Forte, R.; Cennamo, G.L.; Finelli, M.L.; de Crecchio, G. Comparison of time domain Stratus OCT and spectral domain SLO/OCT for assessment of macular thickness and volume. Eye 2009, 23, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Kakinoki, M.; Miyake, T.; Sawada, O.; Sawada, T.; Kawamura, H.; Ohji, M. Comparison of macular thickness in diabetic macular edema using spectral-domain optical coherence tomography and time-domain optical coherence tomography. J. Ophthalmol. 2012, 2012, 959721. [Google Scholar] [CrossRef] [PubMed]
- Lammer, J.; Scholda, C.; Prünte, C.; Benesch, T.; Schmidt-Erfurth, U.; Bolz, M. Retinal thickness and volume measurements in diabetic macular edema: A comparison of four optical coherence tomography systems. Retina 2011, 31, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Hatef, E.; Khwaja, A.; Rentiya, Z.; Ibrahim, M.; Shulman, M.; Turkcuoglu, P.; Sepah, Y.; Wang, J.; Channa, R.; Bittencourt, M.; et al. Comparison of time domain and spectral domain optical coherence tomography in measurement of macular thickness in macular edema secondary to diabetic retinopathy and retinal vein occlusion. J. Ophthalmol. 2012, 2012, 354783. [Google Scholar] [CrossRef] [PubMed]
- Suzuma, K.; Yamada, Y.; Liu, M.; Tsuiki, E.; Fujikawa, A.; Kitaoka, T. Comparing central retinal thickness in diabetic macular edema measured by two different spectral-domain optical coherence tomography devices. Jpn. J. Ophthalmol. 2011, 55, 620–624. [Google Scholar] [CrossRef] [PubMed]
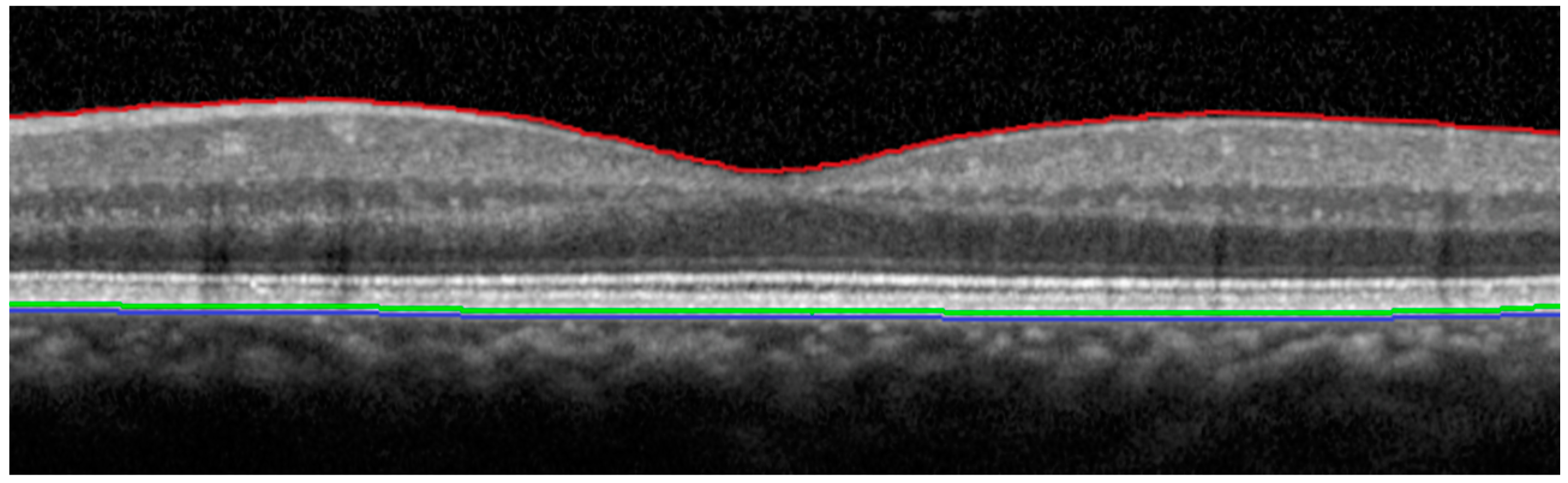
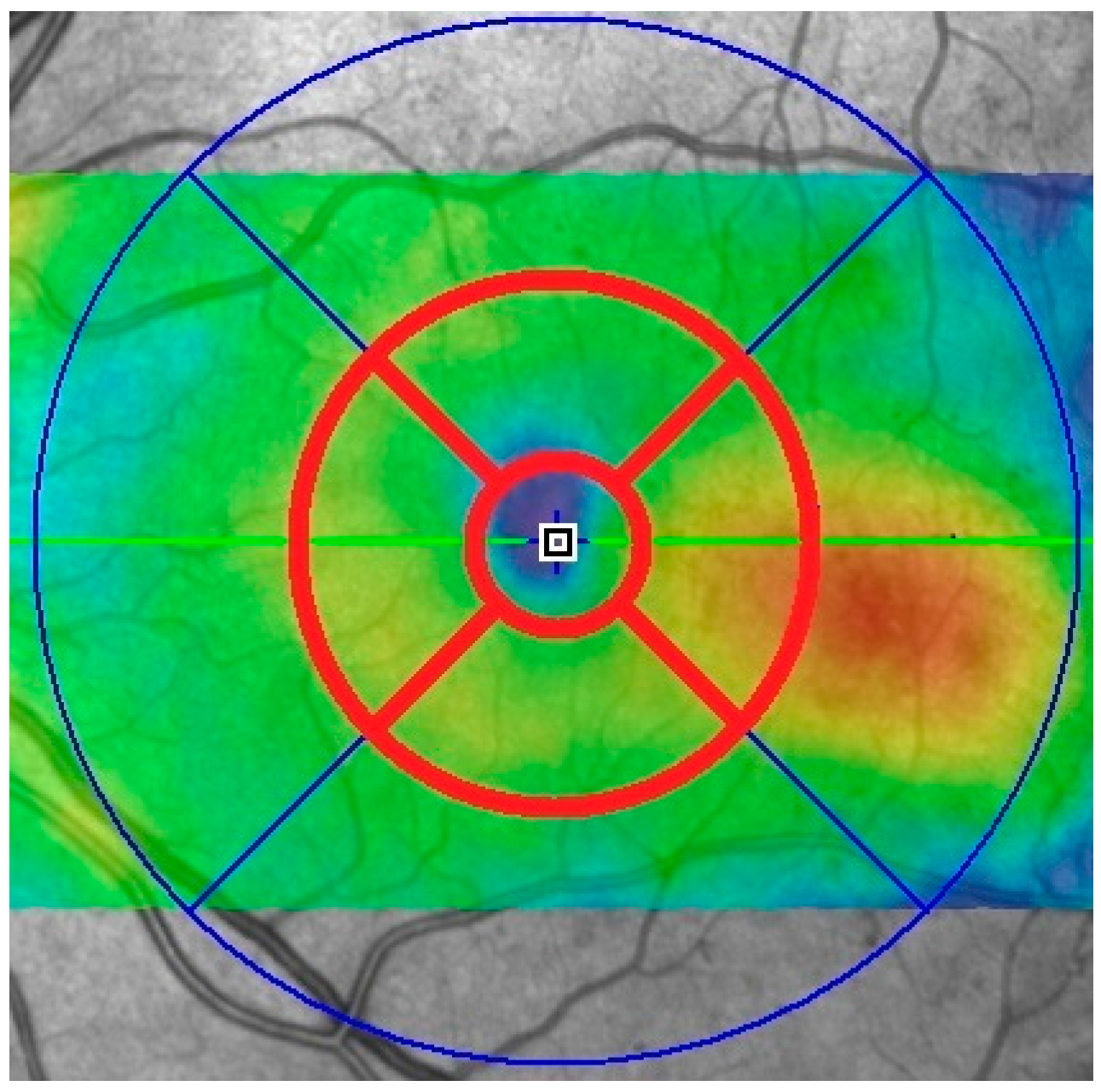
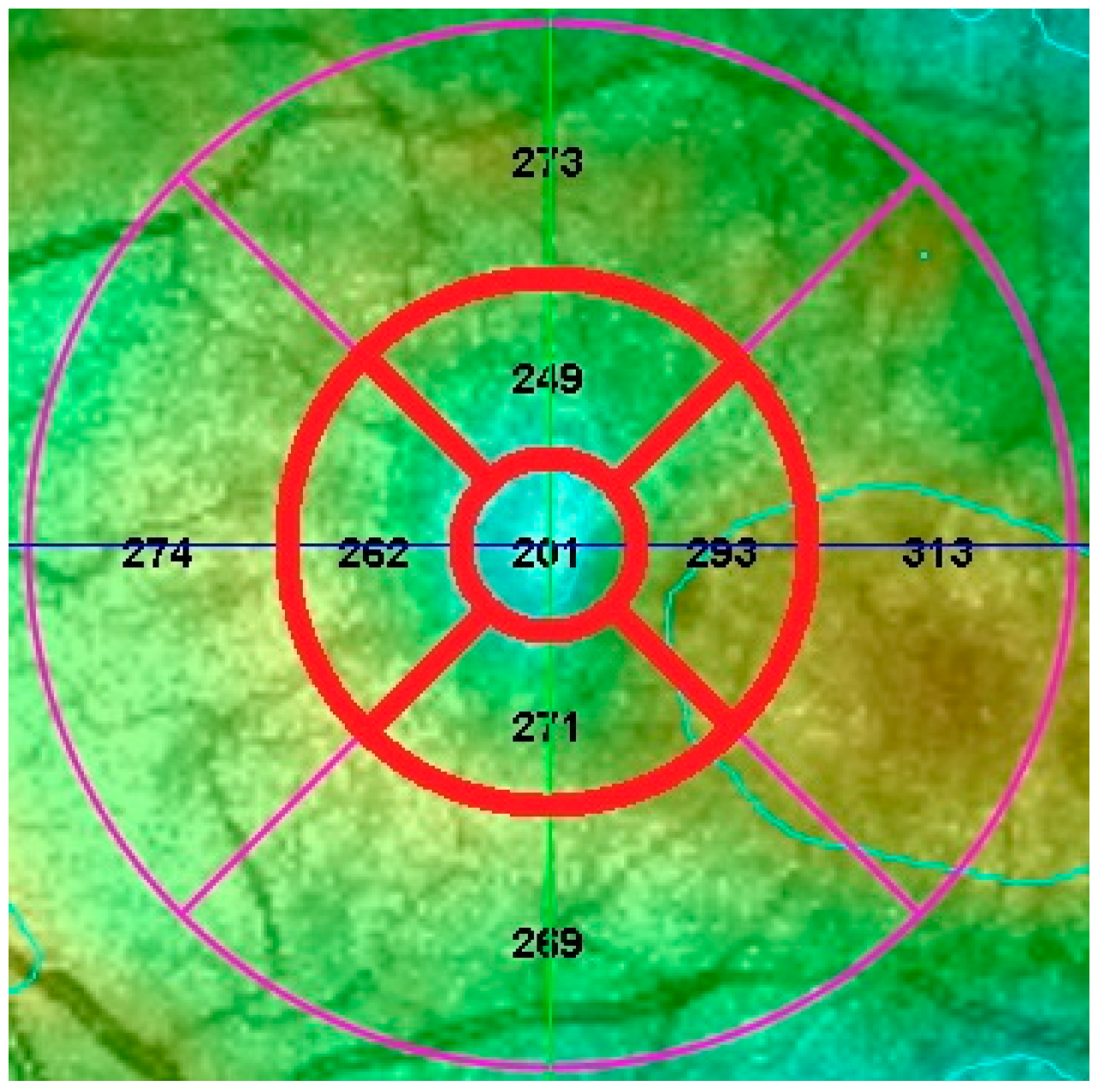
| Devices/Statistical Comparison | Central Zone | Superior Zone | Temporal Zone | Inferior Zone | Nasal Zone |
|---|---|---|---|---|---|
| Heidelberg Spectralis HRA + OCT | 310 | 343 | 344 | 332 | 340 |
| Optos Spectral OCT/SLO | 237 | 298 | 297 | 289 | 290 |
| Absolute difference | 73 | 45 | 47 | 43 | 50 |
| p-value | 1.96 × 10−14 | 5.46 × 10−1 | 3.51 × 10−8 | 1.63 × 10−13 | 2.85 × 10−12 |
| Pearson Correlation | 0.752 | 0.85 | 0.928 | 0.839 | 0.823 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sachdev, A.; Edington, M.; Morjaria, R.; Chong, N.V. Comparing Macular Thickness Measurements in Patients with Diabetic Macular Edema with the Optos Spectral OCT/SLO and Heidelberg Spectralis HRA + OCT. Vision 2017, 1, 2. https://doi.org/10.3390/vision1010002
Sachdev A, Edington M, Morjaria R, Chong NV. Comparing Macular Thickness Measurements in Patients with Diabetic Macular Edema with the Optos Spectral OCT/SLO and Heidelberg Spectralis HRA + OCT. Vision. 2017; 1(1):2. https://doi.org/10.3390/vision1010002
Chicago/Turabian StyleSachdev, Amun, Magdalena Edington, Rupal Morjaria, and Ngaihang Victor Chong. 2017. "Comparing Macular Thickness Measurements in Patients with Diabetic Macular Edema with the Optos Spectral OCT/SLO and Heidelberg Spectralis HRA + OCT" Vision 1, no. 1: 2. https://doi.org/10.3390/vision1010002







