Target Type Modulates the Effect of Task Demand on Reflexive Focal Attention
Abstract
:1. Introduction
2. Experiment 1: Shape Detection
2.1. Materials and Methods
2.1.1. Participants
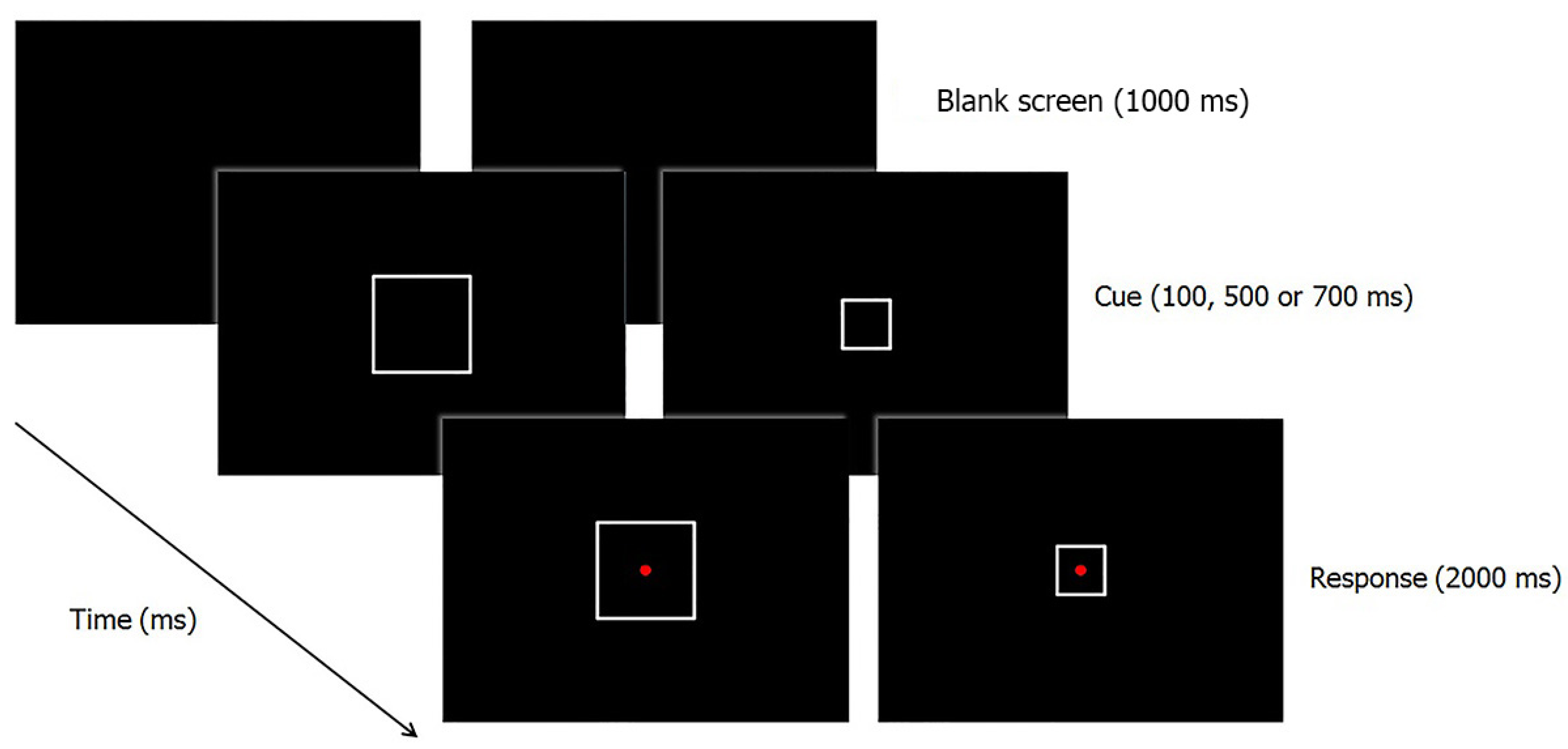
2.1.2. Apparatus, Stimuli and Procedure
2.1.3. Statistical Analyses
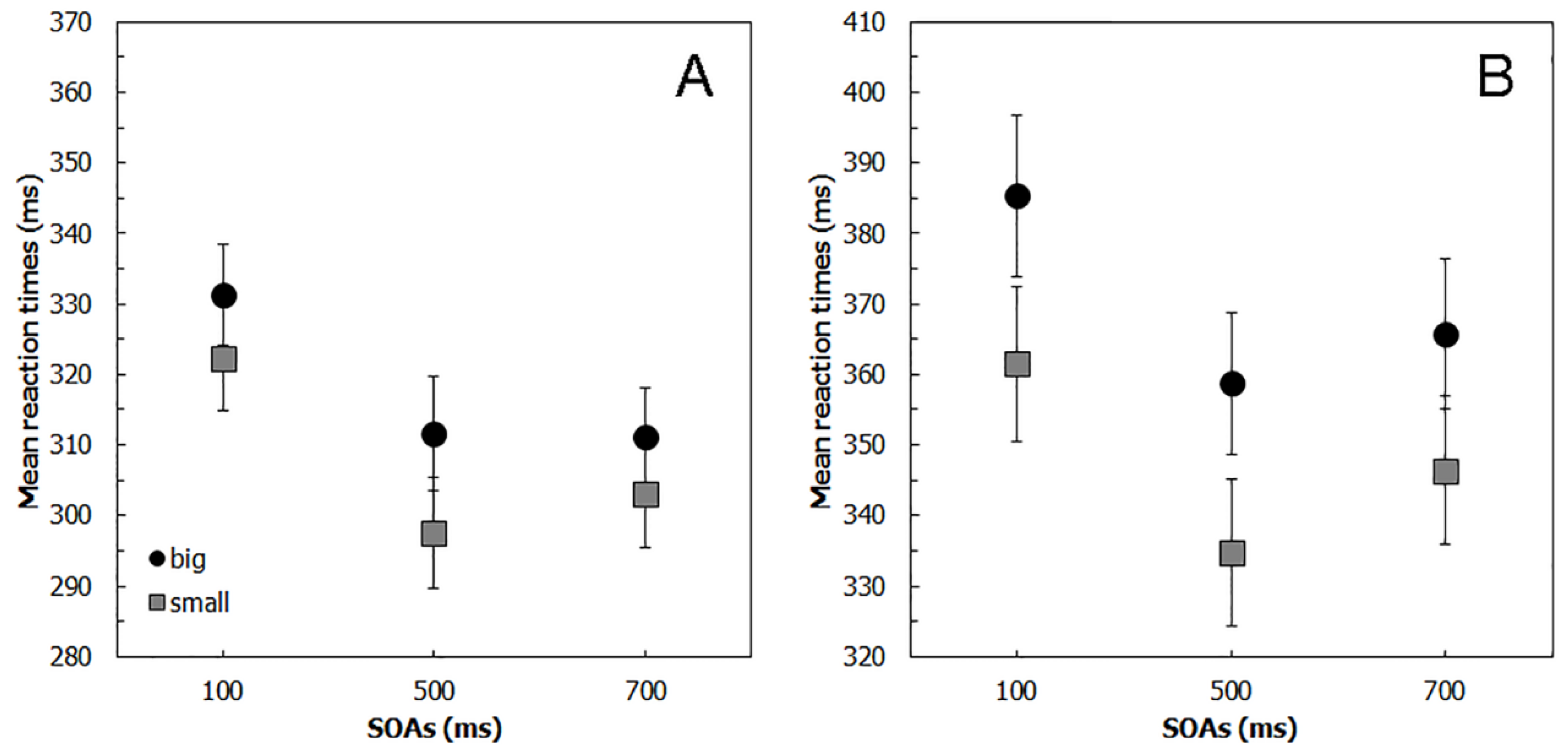
2.2. Results
3. Experiment 2: Shape Discrimination
3.1. Materials and Methods
3.1.1. Participants
3.1.2. Apparatus, Stimuli and Procedure
3.2. Results
4. Experiment 3: Letter Detection
4.1. Materials and Methods
4.1.1. Participants
4.1.2. Apparatus, Stimuli and Procedure
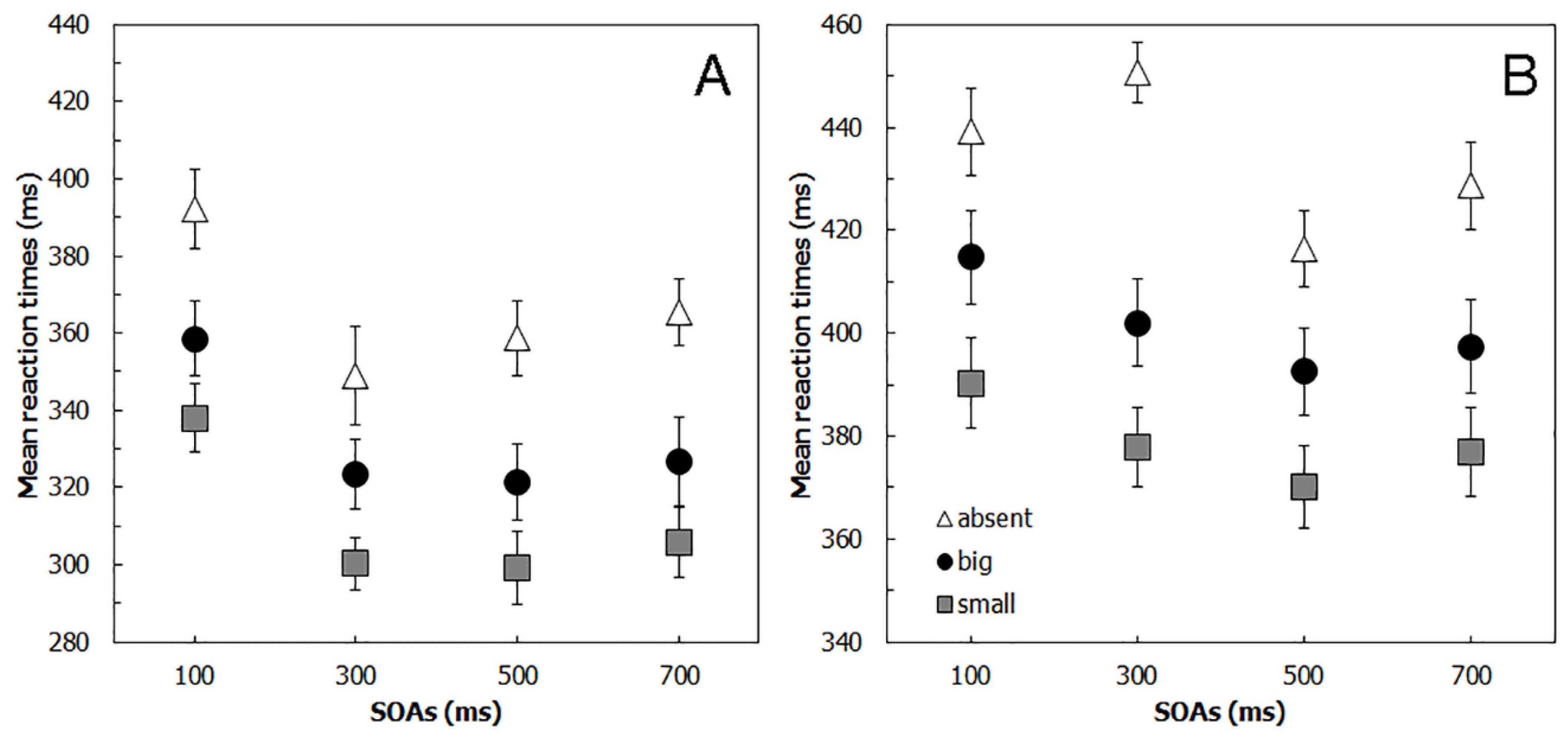
4.2. Results
5. Experiment 4: Letter Discrimination
5.1. Materials and Methods
5.1.1. Participants
5.1.2. Apparatus, Stimuli and Procedure
5.2. Results
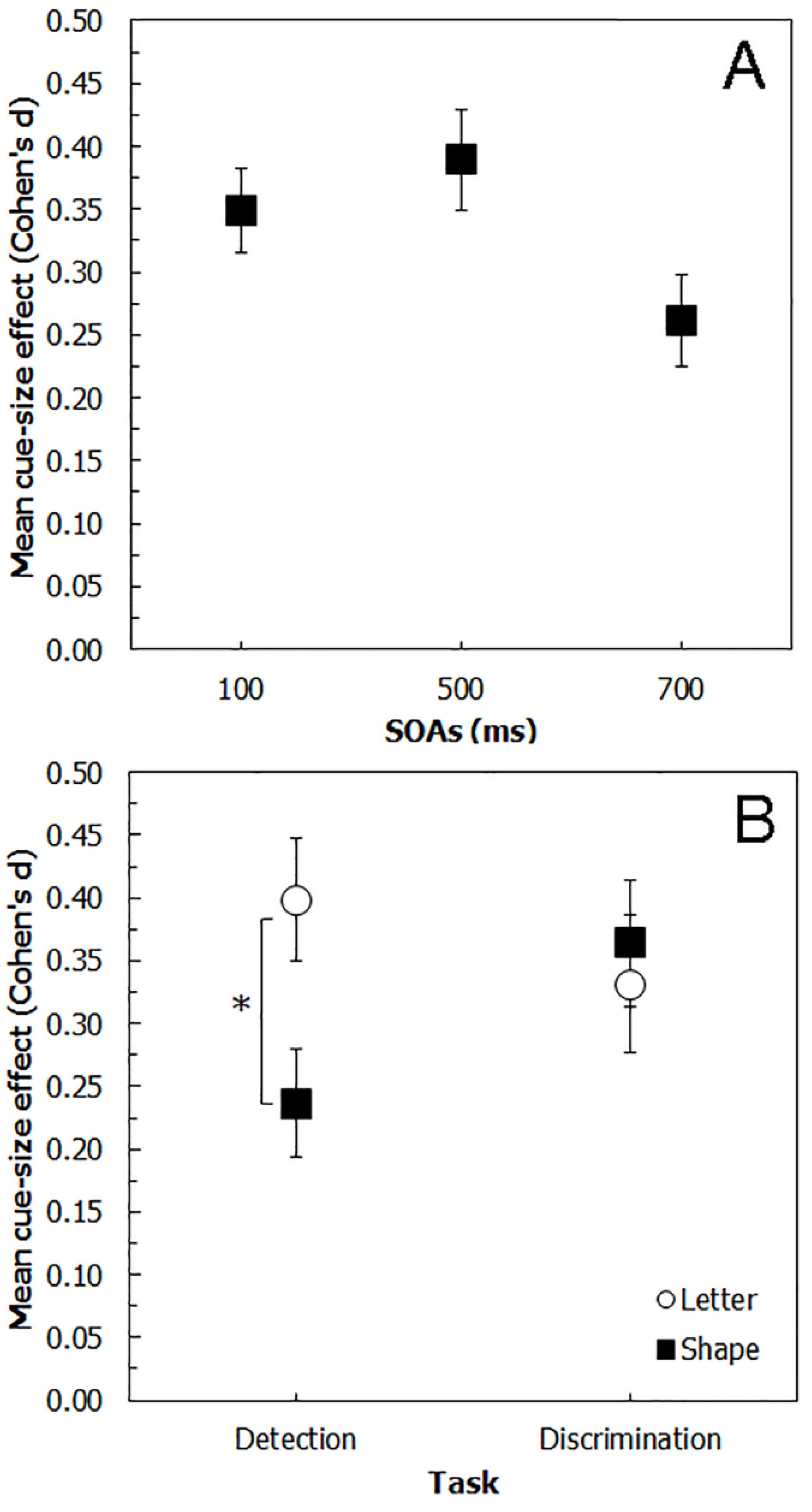
6. Comparing Experiments 1, 2, 3 and 4
7. Discussion
Acknowledgments
Author contributions
Conflicts of Interest
References
- Chelazzi, L.; Corbetta, M. Cortical mechanisms of visuospatial attention in the primate brain. New Cogn. Neurosci. 2000, 2, 667–686. [Google Scholar]
- He, S.; Cavanagh, P.; Intriligator, J. Attentional resolution. Trends Cogn. Sci. 1997, 1, 115–121. [Google Scholar] [CrossRef]
- Turatto, M.; Benso, F.; Facoetti, A.; Galfano, G.; Mascetti, G.G.; Umiltà, C. Automatic and voluntary focusing of attention. Atten. Percept. Psychophys. 2000, 62, 935–952. [Google Scholar] [CrossRef]
- Albonico, A.; Malaspina, M.; Bricolo, E.; Martelli, M.; Daini, R. Temporal dissociation between the focal and orientation components of spatial attention in central and peripheral vision. Acta Psychol. 2016, 171, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, C.W.; James, J.D.S. Visual attention within and around the field of focal attention: A zoom lens model. Percept. Psychophys. 1986, 40, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, C.W.; Yeh, Y.-Y. Allocation of attention in the visual field. J. Exp. Psychol. Hum. Percept. Perform. 1985, 11, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Castiello, U.; Umiltà, C. Size of the attentional focus and efficiency of processing. Acta Psychol. 1990, 73, 195–209. [Google Scholar] [CrossRef]
- Maringelli, F.; Umiltà, C. The control of the attentional focus. Eur. J. Cogn. Psychol. 1998, 10, 225–246. [Google Scholar]
- Brefczynski, J.A.; DeYoe, E.A. A physiological correlate of the ’spotlight’ of visual attention. Nat. Neurosci. 1999, 2, 370–374. [Google Scholar] [PubMed]
- Müller, N.G.; Bartelt, O.A.; Donner, T.H.; Villringer, A.; Brandt, S.A. A physiological correlate of the ‘zoom lens’ of visual attention. J. Neurosci. 2003, 23, 3561–3565. [Google Scholar]
- Benso, F.; Turatto, M.; Mascetti, G.G.; Umiltà, C. The time course of attentional focusing. Eur. J. Cogn. Psychol. 1998, 10, 373–388. [Google Scholar]
- Jefferies, L.N.; Ghorashi, S.; Kawahara, J.-I.; Di Lollo, V. Ignorance is bliss: The role of observer expectation in dynamic spatial tuning of the attentional focus. Atten. Percept. Psychophys. 2007, 69, 1162–1174. [Google Scholar] [CrossRef]
- Herrmann, K.; Montaser-Kouhsari, L.; Carrasco, M.; Heeger, D.J. When size matters: Attention affects performance by contrast or response gain. Nat. Neurosci. 2010, 13, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, L.N.; Gmeindl, L.; Yantis, S. Attending to illusory differences in object size. Atten. Percept. Psychophys. 2014, 76, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Van der Lubbe, R.H.; Vogel, R.O.; Postma, A. Different effects of exogenous cues in a visual detection and discrimination task: Delayed attention withdrawal and/or speeded motor inhibition? J. Cogn. Neurosci. 2005, 17, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Correa, Á.; Lupiáñez, J.; Milliken, B.; Tudela, P. Endogenous temporal orienting of attention in detection and discrimination tasks. Atten. Percept. Psychophys. 2004, 66, 264–278. [Google Scholar] [CrossRef]
- Lupiáñez, J.; Milán, E.G.; Tornay, F.J.; Madrid, E.; Tudela, P. Does IOR occur in discrimination tasks? Yes, it does, but later. Atten. Percept. Psychophys. 1997, 59, 1241–1254. [Google Scholar]
- Bergen, J.R.; Julesz, B. Rapid discrimination of visual patterns. IEEE Trans. Syst. Man Cybern. 1983, 857–863. [Google Scholar] [CrossRef]
- Nagy, A.L.; Sanchez, R.R. Critical color differences determined with a visual search task. J. Opt. Soc. Am. A 1990, 7, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Pashler, H. Target-distractor discriminability in visual search. Atten. Percept. Psychophys. 1987, 41, 285–292. [Google Scholar] [CrossRef]
- Friesen, C.K.; Kingstone, A. Abrupt onsets and gaze direction cues trigger independent reflexive attentional effects. Cognition 2003, 87, B1–B10. [Google Scholar] [CrossRef]
- Friesen, C.K.; Kingstone, A. The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychon. Bull. Rev. 1998, 5, 490–495. [Google Scholar] [CrossRef]
- Egeth, H. Attention and preattention. Psychol. Learn. Motiv. 1977, 11, 277–320. [Google Scholar]
- LaBerge, D. Spatial extent of attention to letters and words. J. Exp. Psychol. Hum. Percept. Perform. 1983, 9, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Bashinski, H.S.; Bacharach, V.R. Enhancement of perceptual sensitivity as the result of selectively attending to spatial locations. Atten. Percept. Psychophys. 1980, 28, 241–248. [Google Scholar] [CrossRef]
- Bonnel, A.-M.; Miller, J. Attentional effects on concurrent psychophysical discriminations: Investigations of a sample-size model. Atten. Percept. Psychophys. 1994, 55, 162–179. [Google Scholar] [CrossRef]
- Jonides, J. Voluntary versus automatic control over the mind’s eye’s movement. Atten. Perform. IX 1981, 9, 187–203. [Google Scholar]
- Laberge, D.; Brown, V. Variations in size of the visual field in which targets are presented: An attentional range effect. Atten. Percept. Psychophys. 1986, 40, 188–200. [Google Scholar] [CrossRef]
- Posner, M.I. Orienting of attention. Q. J. Exp. Psychol. 1980, 32, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Facoetti, A.; Paganoni, P.; Turatto, M.; Marzola, V.; Mascetti, G.G. Visual-spatial attention in developmental dyslexia. Cortex 2000, 36, 109–123. [Google Scholar] [CrossRef]
- Van der Heijden, A.H. Selective Attention in Vision; Routledge: Abingdon-on-Thames, UK, 2003. [Google Scholar]
- Theeuwes, J.; van der Burg, E.; Belopolsky, A. Detecting the presence of a singleton involves focal attention. Psychon. Bull. Rev. 2008, 15, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Enns, J.T.; Rensink, R.A. Influence of scene-based properties on visual search. Science 1990, 247, 721. [Google Scholar] [CrossRef] [PubMed]
- Enns, J.T.; Rensink, R.A. Preattentive recovery of three-dimensional orientation from line drawings. Psychol. Rev. 1991, 98, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Rensink, R.A.; Cavanagh, P. The influence of cast shadows on visual search. Perception 2004, 33, 1339–1358. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Cavanagh, P. Facial organization blocks access to low-level features: An object inferiority effect. J. Exp. Psychol. Hum. Percept. Perform. 1995, 21, 901–913. [Google Scholar] [CrossRef]
- Wang, Q.; Cavanagh, P.; Green, M. Familiarity and pop-out in visual search. Atten. Percept. Psychophys. 1994, 56, 495–500. [Google Scholar] [CrossRef]
- McClelland, J.L.; Rumelhart, D.E. An interactive activation model of context effects in letter perception: I. An account of basic findings. Psychol. Rev. 1981, 88, 375–407. [Google Scholar] [CrossRef]
- Teichner, W.H.; Krebs, M.J. Laws of visual choice reaction time. Psychol. Rev. 1974, 81, 75–98. [Google Scholar] [CrossRef] [PubMed]
- James, K.H.; Gauthier, I. Letter processing automatically recruits a sensory–motor brain network. Neuropsychologia 2006, 44, 2937–2949. [Google Scholar] [CrossRef] [PubMed]
- Kaye, D.B.; Brown, S.W.; Post, T.A.; Plude, D.J. The development of letter processing efficiency. Mem. Cogn. 1981, 9, 378–388. [Google Scholar] [CrossRef]
- Johnston, J.C.; McClelland, J.L. Perception of letters in words: Seek not and ye shall find. Science 1974, 184, 1192–1194. [Google Scholar] [CrossRef] [PubMed]
- Nandy, A.S.; Tjan, B.S. The nature of letter crowding as revealed by first-and second-order classification images. J. Vis. 2007, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Juola, J.F.; Ward, N.J.; McNamara, T. Visual search and reading of rapid serial presentations of letter strings, words, and text. J. Exp. Psychol. Gen. 1982, 111, 208–227. [Google Scholar] [CrossRef]
- Usai, M.C.; Umiltà, C.; Nicoletti, R. Limits in controlling the focus of attention. Eur. J. Cogn. Psychol. 1995, 7, 411–439. [Google Scholar] [CrossRef]
- Franceschini, S.; Gori, S.; Ruffino, M.; Pedrolli, K.; Facoetti, A. A causal link between visual spatial attention and reading acquisition. Curr. Biol. 2012, 22, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Gabrieli, J.D.; Norton, E.S. Reading abilities: Importance of visual-spatial attention. Curr. Biol. 2012, 22, R298–R299. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.M.; Ferreira, F. Effects of foveal processing difficulty on the perceptual span in reading: Implications for attention and eye movement control. J. Exp. Psychol. Learn. Mem. Cogn. 1990, 16, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Facoetti, A.; Lorusso, M.L.; Paganoni, P.; Cattaneo, C.; Galli, R.; Mascetti, G.G. The time course of attentional focusing in dyslexic and normally reading children. Brain Cogn. 2003, 53, 181–184. [Google Scholar] [CrossRef]
- Greenwood, P.M.; Parasuraman, R.; Haxby, J.V. Changes in visuospatial attention over the adult lifespan. Neuropsychologia 1993, 31, 471–485. [Google Scholar] [CrossRef]
- Jefferies, L.N.; Roggeveen, A.B.; Enns, J.T.; Bennett, P.J.; Sekuler, A.B.; Di Lollo, V. On the time course of attentional focusing in older adults. Psychol. Res. 2015, 79, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Eta-squared and partial eta-squared in fixed factor anova designs. Educ. Psychol. Meas. 1973, 33, 107–112. [Google Scholar] [CrossRef]
- Talgar, C.P.; Pelli, D.G.; Carrasco, M. Covert attention enhances letter identification without affecting channel tuning. J. Vis. 2004, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Casco, C.; Tressoldi, P.E.; Dellantonio, A. Visual selective attention and reading efficiency are related in children. Cortex 1998, 34, 531–546. [Google Scholar] [CrossRef]
- Ackerman, P.T.; Dykman, R.A.; Gardner, M.Y. Counting rate, naming rate, phonological sensitivity, and memory span: Major factors in dyslexia. J. Learn. Disabil. 1990, 23, 325–327. [Google Scholar] [CrossRef] [PubMed]
- August, G.J.; Garfinkel, B.D. Comorbidity of ADHD and reading disability among clinic-referred children. J. Abnorm. Child. Psychol. 1990, 18, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, M. Visual attention: The past 25 years. Vis. Res. 2011, 51, 1484–1525. [Google Scholar] [CrossRef] [PubMed]
- LaBerge, D. Attentional Processing: The Brain’s Art of Mindfulness; Harvard University Press: Cambridge, MA, USA, 1995; Volume 2. [Google Scholar]
- Wolfe, J. Visual attention. Seeing 2000, 2, 335–386. [Google Scholar]
- Wolfe, J.M. Visual search in continuous, naturalistic stimuli. Vis. Res. 1994, 34, 1187–1195. [Google Scholar] [CrossRef]
- Correa, Á.; Lupiáñez, J.; Madrid, E.; Tudela, P. Temporal attention enhances early visual processing: A review and new evidence from event-related potentials. Brain Res. 2006, 1076, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Correa, Á.; Sanabria, D.; Spence, C.; Tudela, P.; Lupiáñez, J. Selective temporal attention enhances the temporal resolution of visual perception: Evidence from a temporal order judgment task. Brain Res. 2006, 1070, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Moher, J.; Ashinoff, B.K.; Egeth, H.E. Detection is unaffected by the deployment of focal attention. Front. Psychol. 2013, 4, 284. [Google Scholar] [CrossRef] [PubMed]
- He, Z.J.; Nakayama, K. Surfaces versus features in visual search. Nature 1992, 359, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.L.; Shaw, P. Optimal allocation of cognitive resources to spatial locations. J. Exp. Psychol. Hum. Percept. Perform. 1977, 3, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Cohn, T.E. Absolute threshold: Analysis in terms of uncertainty. J. Opt. Soc. Am. A 1981, 71, 783–785. [Google Scholar] [CrossRef]
- Kinchla, R.A. Attention. Annu. Rev. Psychol. 1992, 43, 711–742. [Google Scholar] [CrossRef] [PubMed]
- Castiello, U.; Umilta, C. Splitting focal attention. J. Exp. Psychol. Hum. Percept. Perform. 1992, 18, 837–848. [Google Scholar] [CrossRef] [PubMed]
| SOAs (ms) | ||||
|---|---|---|---|---|
| Experiment | 100 | 300 | 500 | 700 |
| Experiment 1 (random block) | 0.226 ± 0.076 | --- | 0.308 ± 0.095 | 0.176 ± 0.092 |
| Experiment 1 (fixed block) | −0.055 ± 0.097 | --- | −0.086 ± 0.113 | −0.016 ± 0.110 |
| Experiment 2 | 0.393 ± 0.058 | --- | 0.427 ± 0.057 | 0.272 ± 0.064 |
| Experiment 3 | 0.386 ± 0.071 | 0.405 ± 0.099 | 0.482 ± 0.107 | 0.328 ± 0.068 |
| Experiment 4 | 0.388 ± 0.059 | 0.307 ± 0.042 | 0.340 ± 0.050 | 0.266 ± 0.064 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albonico, A.; Malaspina, M.; Daini, R. Target Type Modulates the Effect of Task Demand on Reflexive Focal Attention. Vision 2017, 1, 13. https://doi.org/10.3390/vision1020013
Albonico A, Malaspina M, Daini R. Target Type Modulates the Effect of Task Demand on Reflexive Focal Attention. Vision. 2017; 1(2):13. https://doi.org/10.3390/vision1020013
Chicago/Turabian StyleAlbonico, Andrea, Manuela Malaspina, and Roberta Daini. 2017. "Target Type Modulates the Effect of Task Demand on Reflexive Focal Attention" Vision 1, no. 2: 13. https://doi.org/10.3390/vision1020013






