The Effect of Stimulus Size and Eccentricity on Attention Shift Latencies
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1 Participants
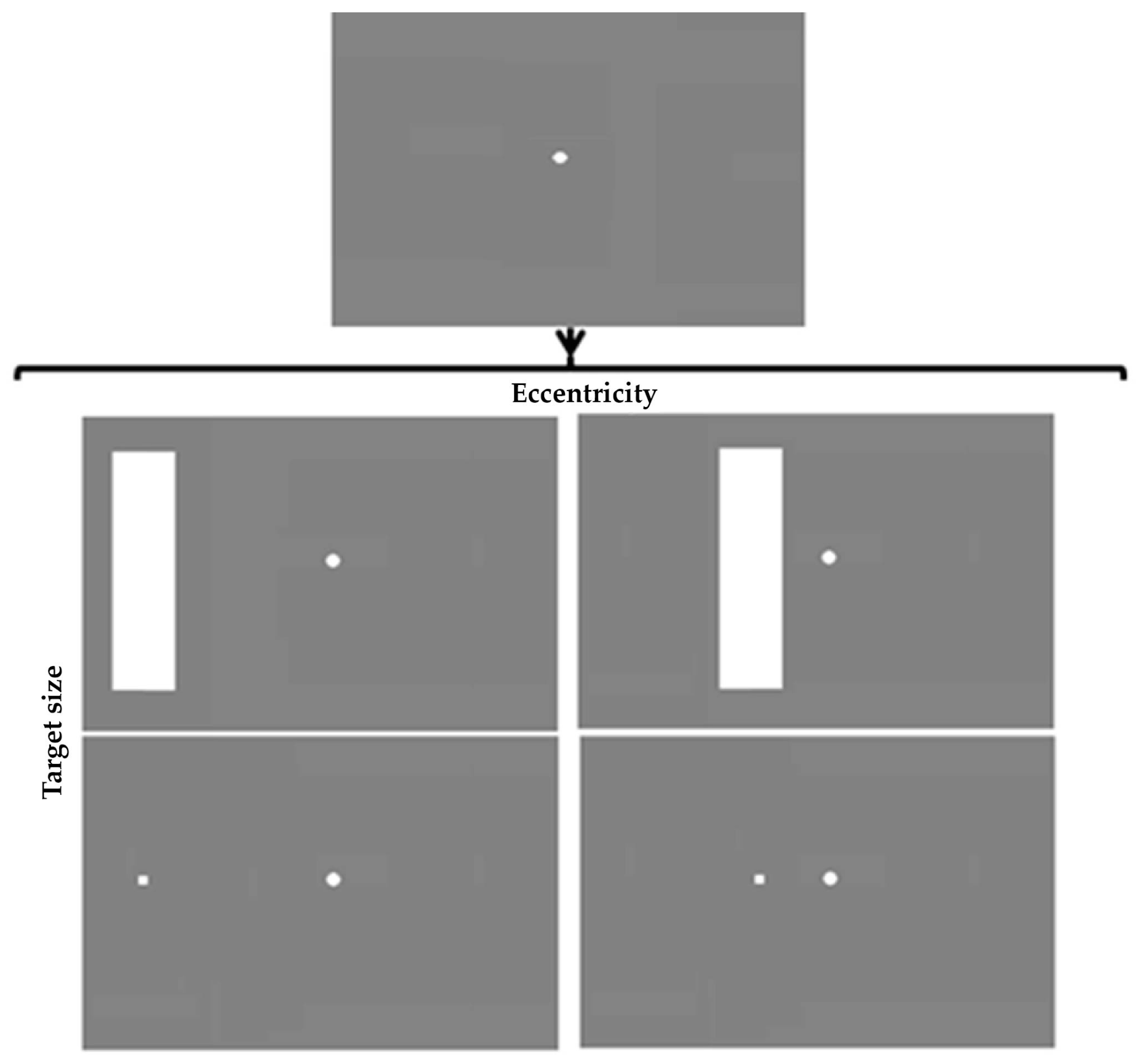
4.2 Design and Stimuli
4.3 Procedure
4.4 Gaze-Contingent Eye-Tracking
4.5 Eye-Tracking Data Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wass, S.; Smith, T.J.; Johnson, M.H. Parsing eye-tracking data of variable quality to provide accurate fixation duration estimates in infants and adults. Behav. Res. Methods 2012, 45, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Gredebäck, G.; Johnson, S.P.; von Hofsten, C. Eye tracking in infancy research. Dev. Neuropsychol. 2009, 35, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.; Braddick, O.; Anker, S.; Curran, W.; Andrew, R.; Wattam-Bell, J.; Braddick, F. Neurobiological models of visuospatial cognition in children with williams syndrome: Measures of dorsal-stream and frontal function. Dev. Neuropsychol. 2003, 23, 139–172. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.; Braddick, O.; Anker, S.; Nardini, M.; Birtles, D.; Rutherford, M.A.; Mercuri, E.; Dyet, L.; Edwards, A.D.; Cowan, F.M. Cortical vision, mri and developmental outcome in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F292–F297. [Google Scholar] [CrossRef] [PubMed]
- Elsabbagh, M.; Volein, A.; Holmboe, K.; Tucker, L.; Csibra, G.; Baron-Cohen, S.; Bolton, P.; Charman, T.; Baird, G.; Johnson, M.H. Visual orienting in the early broader autism phenotype: Disengagement and facilitation. J. Child Psychol. Psychiatry 2009, 50, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Gliga, T.; Jones, E.J.; Bedford, R.; Charman, T.; Johnson, M.H. From early markers to neuro-developmental mechanisms of autism. Dev. Rev. 2014, 34, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Atkinson, J.; Braddick, O.; Anker, S.; Cowan, F.; Rutherford, M.; Pennock, J.; Dubowitz, L. Visual function in full-term infants with hypoxic-ischaemic encephalopathy. Neuropediatrics 1997, 28, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Haataja, L.; Guzzetta, A.; Anker, S.; Cowan, F.; Rutherford, M.; Andrew, R.; Braddick, O.; Cioni, G.; Dubowitz, L. Visual function in term infants with hypoxic-ischaemic insults: Correlation with neurodevelopment at 2 years of age. Arch. Dis. Child. Fetal Neonatal Ed. 1999, 80, F99–F104. [Google Scholar] [CrossRef] [PubMed]
- Braddick, O.; Atkinson, J.; Hood, B.; Harkness, W.; Jackson, G.; Vargha-Khademt, F. Possible blindsight in infants lacking one cerebral hemisphere. Nature 1992, 360, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.; Hood, B.; Braddick, O.; Wattam-Bell, J. Infants control of fixation shifts with single and competing targets-mechanisms of shifting attention. Perception 1988, 17, 367–368. [Google Scholar]
- Atkinson, J.; Hood, B.; Wattam-Bell, J.; Braddick, O. Changes in infants’ ability to switch visual attention in the first three months of life. Perception 1992, 21, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Hood, B.; Atkinson, J. Disengaging visual attention in the infant and adult. Infant Behav. Dev. 1993, 16, 405–422. [Google Scholar] [CrossRef]
- Atkinson, J.; Braddick, O. Visual attention in the first years: Typical development and developmental disorders. Dev. Med. Child Neurol. 2012, 54, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Butcher, P.R.; Kalverboer, A.F.; Geuze, R.H. Infants’ shifts of gaze from a central to a peripheral stimulus: A longitudinal study of development between 6 and 26 weeks. Infant Behav. Dev. 2000, 23, 3–21. [Google Scholar] [CrossRef]
- Kulke, L.; Atkinson, J.; Braddick, O. Automatic detection of attention shifts in infancy: Eye tracking in the fixation shift paradigm. PLoS ONE 2015, 10, e0142505. [Google Scholar] [CrossRef] [PubMed]
- Kulke, L.; Atkinson, J.; Braddick, O. Neural mechanisms of attention become more specialised during infancy: Insights from combined eye tracking and EEG. Dev. Psychobiol. 2017, 59, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Kulke, L.; Atkinson, J.; Braddick, O. Neural differences between covert and overt attention studied using eeg with simultaneous remote eye tracking. Front. Hum. Neurosci. 2016, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Farroni, T.; Simion, F.; Umiltà, C.; Barba, B.D. The gap effect in newborns. Dev. Sci. 1999, 2, 174–186. [Google Scholar] [CrossRef]
- Matsuzawa, M.; Shimojo, S. Infants’ fast saccades in the gap paradigm and development of visual attention. Infant Behav. Dev. 1997, 20, 449–455. [Google Scholar] [CrossRef]
- Colombo, J. The development of visual attention in infancy. Annu. Rev. Psychol. 2001, 52, 337–367. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.H.; Posner, M.I.; Rothbart, M.K. Components of visual orienting in early infancy: Contingency learning, anticipatory looking, and disengaging. J. Cogn. Neurosci. 1991, 3, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Elsabbagh, M.; Fernandes, J.; Jane Webb, S.; Dawson, G.; Charman, T.; Johnson, M.H. Disengagement of visual attention in infancy is associated with emerging autism in toddlerhood. Biol. Psychiatry 2013, 74, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.; Braddick, O. Early development of the control of visual attention. Perception 1985, 14, A25. [Google Scholar]
- Hunnius, S.; Geuze, R.H. Gaze shifting in infancy: A longitudinal study using dynamic faces and abstract stimuli. Infant Behav. Dev. 2004, 27, 397–416. [Google Scholar] [CrossRef]
- Aslin, R.N.; Salapatek, P. Saccadic localization of visual targets by the very young human infant. Percept. Psychophys. 1975, 17, 293–302. [Google Scholar] [CrossRef]
- Kulke, L. Cortical Mechanisms of Visual Attention in Typically Developing Infants and Adults; University College London: London, UK, 2015. [Google Scholar]
- Csibra, G.; Johnson, M.H.; Tucker, L.A. Attention and oculomotor control: A high-density erp study of the gap effect. Neuropsychologia 1997, 35, 855–865. [Google Scholar] [CrossRef]
- Kulke, L.; Wattam-Bell, J. Combining event-related potentials and eye-tracking to assess the effect of attention on cortical response. Perception 2013, 42, 219. [Google Scholar]
- Tripathy, S.P.; Cavanagh, P. The extent of crowding in peripheral vision does not scale with target size. Vis. Res. 2002, 42, 2357–2369. [Google Scholar] [CrossRef]
- Johnson, C.A.; Keltner, J.L.; Balestrery, F. Effects of target size and eccentricity on visual detection and resolution. Vis. Res. 1978, 18, 1217–1222. [Google Scholar] [CrossRef]
- Bles, M.; Schwarzbach, J.; De Weerd, P.; Goebel, R.; Jansma, B.M. Receptive field size-dependent attention effects in simultaneously presented stimulus displays. Neuroimage 2006, 30, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.E.; Ross, L.E. Saccade latency in children and adults: Effects of warning interval and target eccentricity. J. Exp. Child Psychol. 1977, 23, 539–549. [Google Scholar] [CrossRef]
- Hodgson, T.L. The location marker effect. Exp. Brain Res. 2002, 145, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Dick, S.; Ostendorf, F.; Kraft, A.; Ploner, C.J. Saccades to spatially extended targets: The role of eccentricity. Neuroreport 2004, 15, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.C.; Hoyt, W.F. The representation of the visual field in human striate cortex: A revision of the classic holmes map. Arch. Ophthalmol. 1991, 109, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Hubel, D.H.; Wiesel, T.N. Uniformity of monkey striate cortex: A parallel relationship between field size, scatter, and magnification factor. J. Comp. Neurol. 1974, 158, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Schiller, P.H.; Tehovnik, E.J. Neural mechanisms underlying target selection with saccadic eye movements. Prog. Brain Res. 2005, 149, 157–171. [Google Scholar] [PubMed]
- Collins, C.E.; Lyon, D.C.; Kaas, J.H. Distribution across cortical areas of neurons projecting to the superior colliculus in new world monkeys. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2005, 285, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Neggers, S.; Raemaekers, M.; Lampmann, E.; Postma, A.; Ramsey, N. Cortical and subcortical contributions to saccade latency in the human brain. Eur. J. Neurosci. 2005, 21, 2853–2863. [Google Scholar] [CrossRef] [PubMed]
- Heinze, H.J.; Mangun, G.R.; Burchert, W.; Hinrichs, H.; Scholz, M.; Munte, T.F.; Gos, A.; Scherg, M.; Johannes, S.; Hundeshagen, H.; et al. Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature 1994, 372, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Hillyard, S.A.; Anllo-Vento, L. Event-related brain potentials in the study of visual selective attention. Proc. Natl. Acad. Sci. USA 1998, 95, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Sommer, M.A.; Wurtz, R.H. What the brain stem tells the frontal cortex. Ii. Role of the sc-md-fef pathway in corollary discharge. J. Neurophysiol. 2004, 91, 1403–1423. [Google Scholar] [CrossRef] [PubMed]
- Guitton, D.; Buchtel, H.A.; Douglas, R. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp. Brain Res. 1985, 58, 455–472. [Google Scholar] [CrossRef] [PubMed]
- Pierrot-Deseilligny, C.; Rivaud, S.; Gaymard, B.; Agid, Y. Cortical control of reflexive visually-guided saccades. Brain 1991, 114, 1473–1485. [Google Scholar] [CrossRef] [PubMed]
- Rafal, R.D.; Machado, L.; Ro, T.; Ingle, H. Looking forward to looking: Saccade preparation and control of the visual grasp reflex. In Attention & Performance XVIII; Monsell, S., Driver, J., Eds.; MIT Press: Cambridge, MA, USA, 2000; Volume XVIII, pp. 155–174. [Google Scholar]
- Miller, E.K. The neural basis of top-down control of visual attention in the prefrontal cortex. In Control of Cognitive Processes: Attention and Performance; Monsell, S., Driver, J., Eds.; MIT Press: Cambridge, MA, USA, 2000; Volume XVIII, p. 511. [Google Scholar]
- Henik, A.; Rafal, R.D.; Rhodes, D. Endogenously generated and visually guided saccades after lesions of the human frontal eye fields. J. Cogn. Neurosci. 1994, 6, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Peelen, M.V.; Heslenfeld, D.J.; Theeuwes, J. Endogenous and exogenous attention shifts are mediated by the same large-scale neural network. Neuroimage 2004, 22, 822–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shipp, S. The brain circuitry of attention. Trends Cogn. Sci. 2004, 8, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Kimchi, R. Perceptual organization and visual attention. Progress Brain Res. 2009, 176, 15–33. [Google Scholar]
- Morey, R.D.; Rouder, J.N. Bayesfactor: Computation of bayes factors for common designs, version 0.9.12-2; Available online: https://cran.r-project.org/web/packages/BayesFactor/index.html (accessed on 7 December 2017).
- R Development Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Liang, F.; Paulo, R.; Molina, G.; Clyde, M.A.; Berger, J.O. Mixtures of g priors for bayesian variable selection. J. Am. Stat. Assoc. 2012, 103, 410–423. [Google Scholar] [CrossRef]
- Hanes, D.P.; Wurtz, R.H. Interaction of the frontal eye field and superior colliculus for saccade generation. J. Neurophysiol. 2001, 85, 804–815. [Google Scholar] [PubMed]
- Crowne, D.P. The frontal eye field and attention. Psychol. Bull. 1983, 93, 232. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.M. Eye movement control during visual object processing: Effects of initial fixation position and semantic constraint. Can. J. Exp. Psychol. 1993, 47, 79. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.W.; Kowler, E.; Sharma, A.; Chubb, C. Saccadic localization of random dot targets. Vis. Res. 1998, 38, 895–909. [Google Scholar] [CrossRef]
- Ploner, C.J.; Ostendorf, F.; Dick, S. Target size modulates saccadic eye movements in humans. Behav. Neurosci. 2004, 118, 237. [Google Scholar] [CrossRef] [PubMed]
- Wässle, H.; Grünert, U.; Röhrenbeck, J.; Boycott, B.B. Cortical magnification factor and the ganglion cell density of the primate retina. Nature 1989, 341, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Virsu, V.; Näsänen, R.; Osmoviita, K. Cortical magnification and peripheral vision. J. Opt. Soc. Am. A 1987, 4, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Cowey, A. Cortical maps and visual perception the grindley memorial lecture. Q. J. Exp. Psychol. 1979, 31, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yeshurun, Y.; Carrasco, M. Attention improves or impairs visual performance by enhancing spatial resolution. Nature 1998, 396, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.; Atienza, M.; Gomez, G.; Vazquez, M. Response latencies and event-related potentials during the gap paradigm using saccadic responses in human subjects. Int. J. Psychophysiol. 1996, 23, 91–99. [Google Scholar] [CrossRef]
| 12.9° Eccentricity | 5° Eccentricity | ||
|---|---|---|---|
| 0.33° target | Mean | 275 | 272 |
| SD | 37.9 | 52.1 | |
| 3.1° × 13.2° target | Mean | 261 | 290 |
| SD | 45.1 | 51.1 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulke, L. The Effect of Stimulus Size and Eccentricity on Attention Shift Latencies. Vision 2017, 1, 25. https://doi.org/10.3390/vision1040025
Kulke L. The Effect of Stimulus Size and Eccentricity on Attention Shift Latencies. Vision. 2017; 1(4):25. https://doi.org/10.3390/vision1040025
Chicago/Turabian StyleKulke, Louisa. 2017. "The Effect of Stimulus Size and Eccentricity on Attention Shift Latencies" Vision 1, no. 4: 25. https://doi.org/10.3390/vision1040025





