Can Contrast-Response Functions Indicate Visual Processing Levels?
Abstract
:1. Introduction
2. The Relevance of Contrast Response Functions (CRFs)
2.1. Interrelatedness of Naturalistic Distributions of Local Contrasts, Cortical CRFs, and the Distinction between Perceptual and Preperceptual Vision
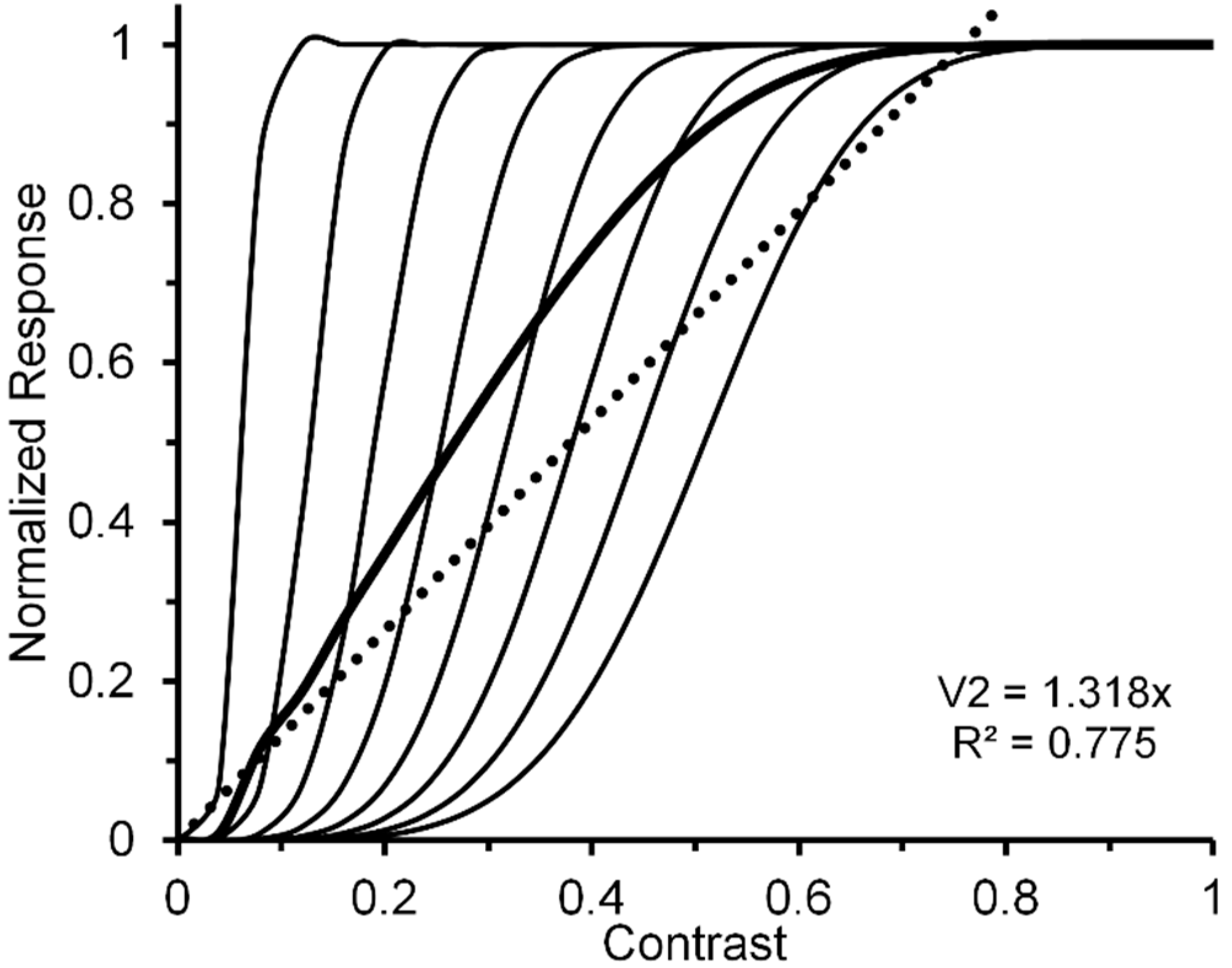
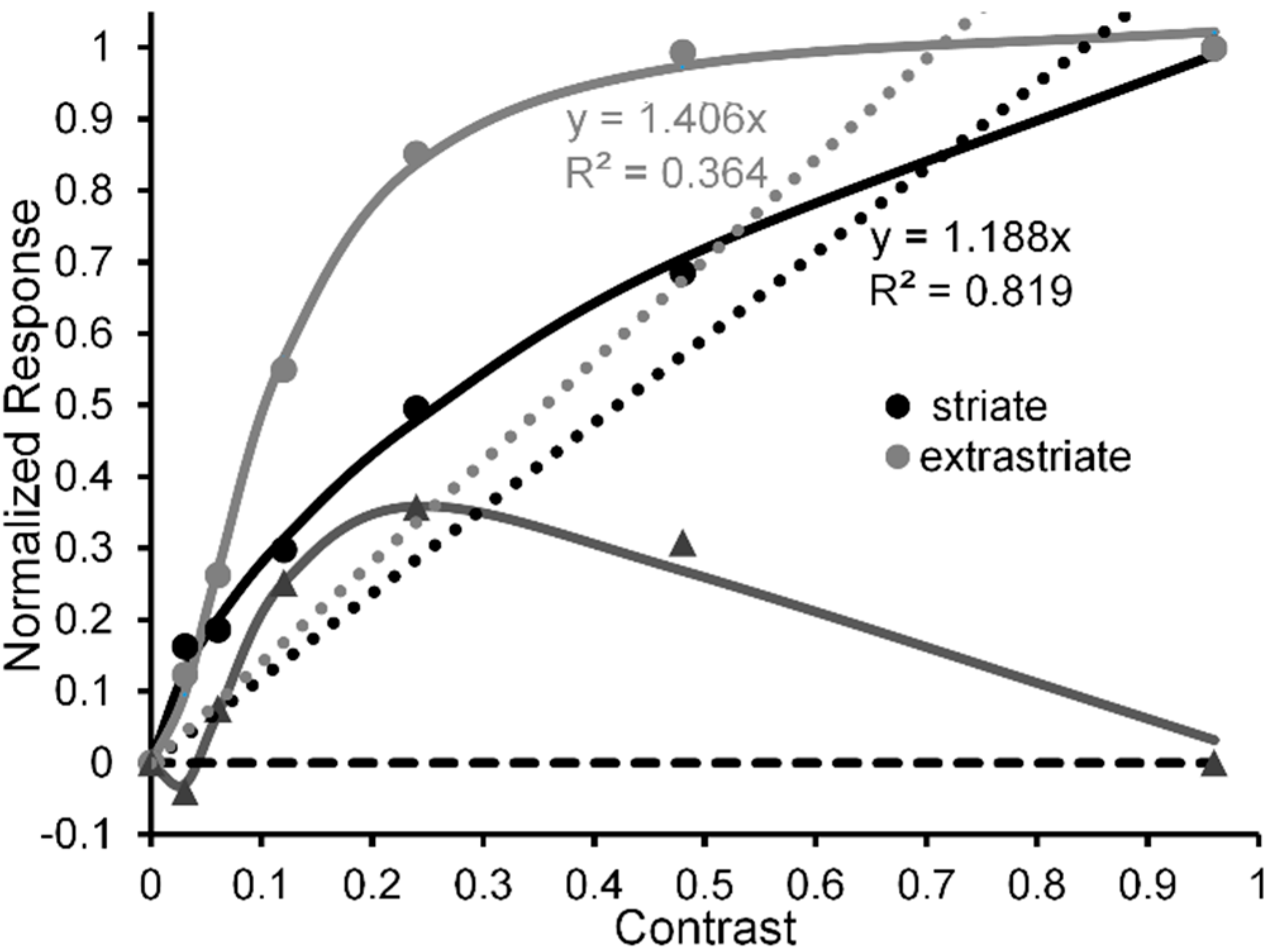
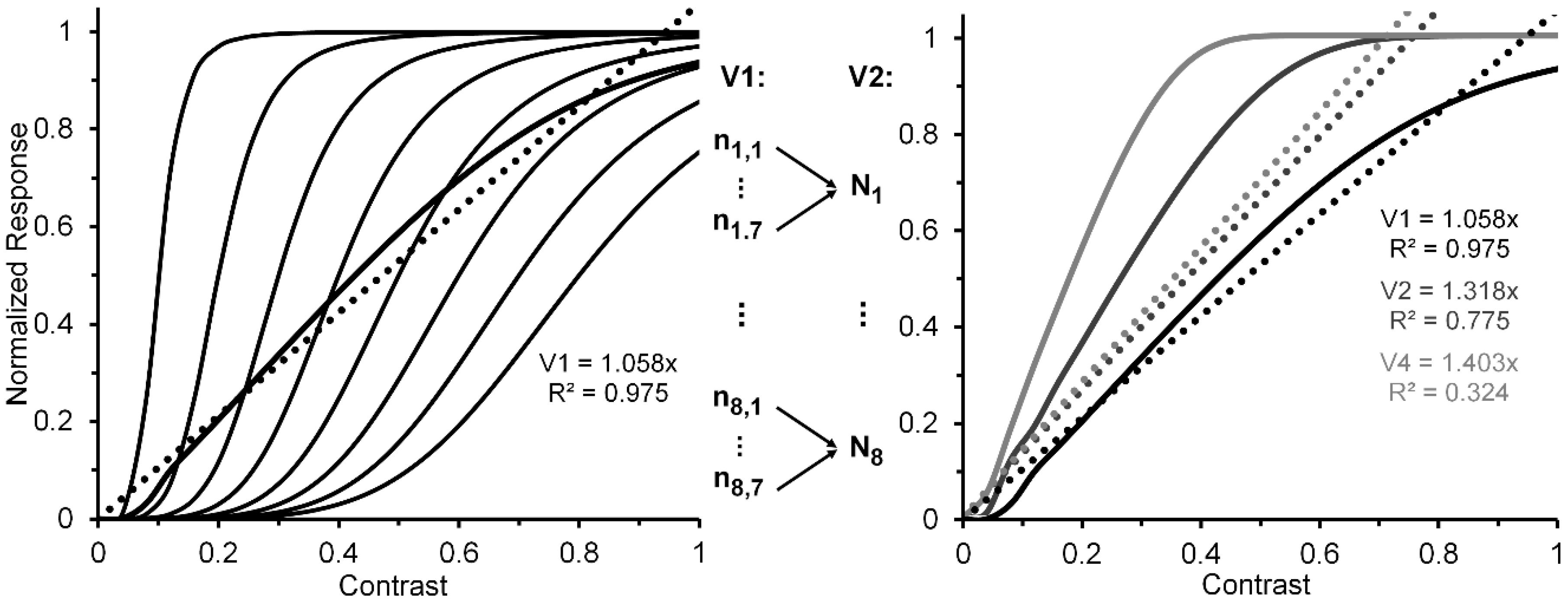
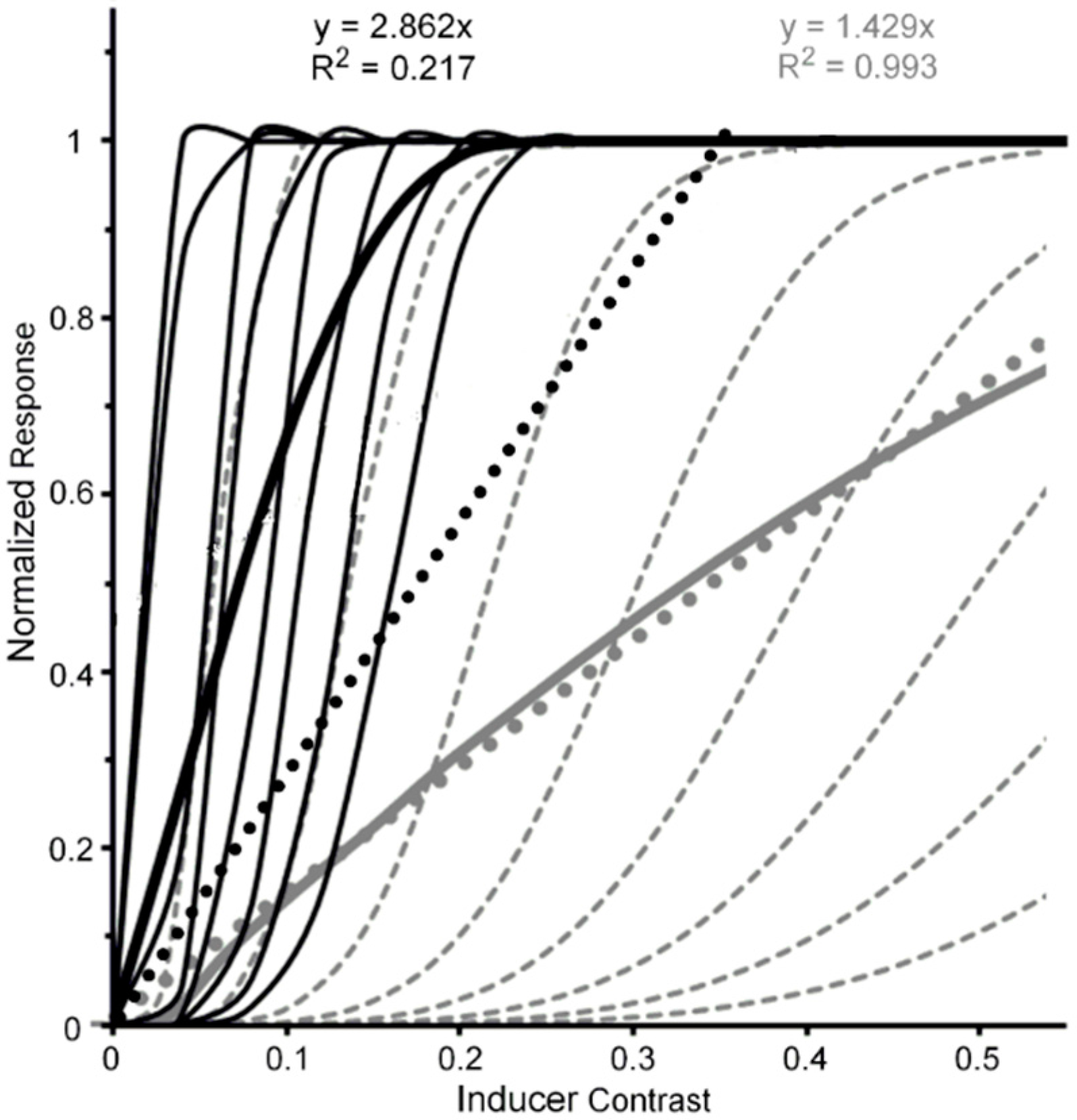
2.2. Relating CRFs of Individual Neurons to CRFs of Neural Ensembles
3. Contrast-Dependent Effects in Visual Illusions, Crowding, and Pedestal Masking
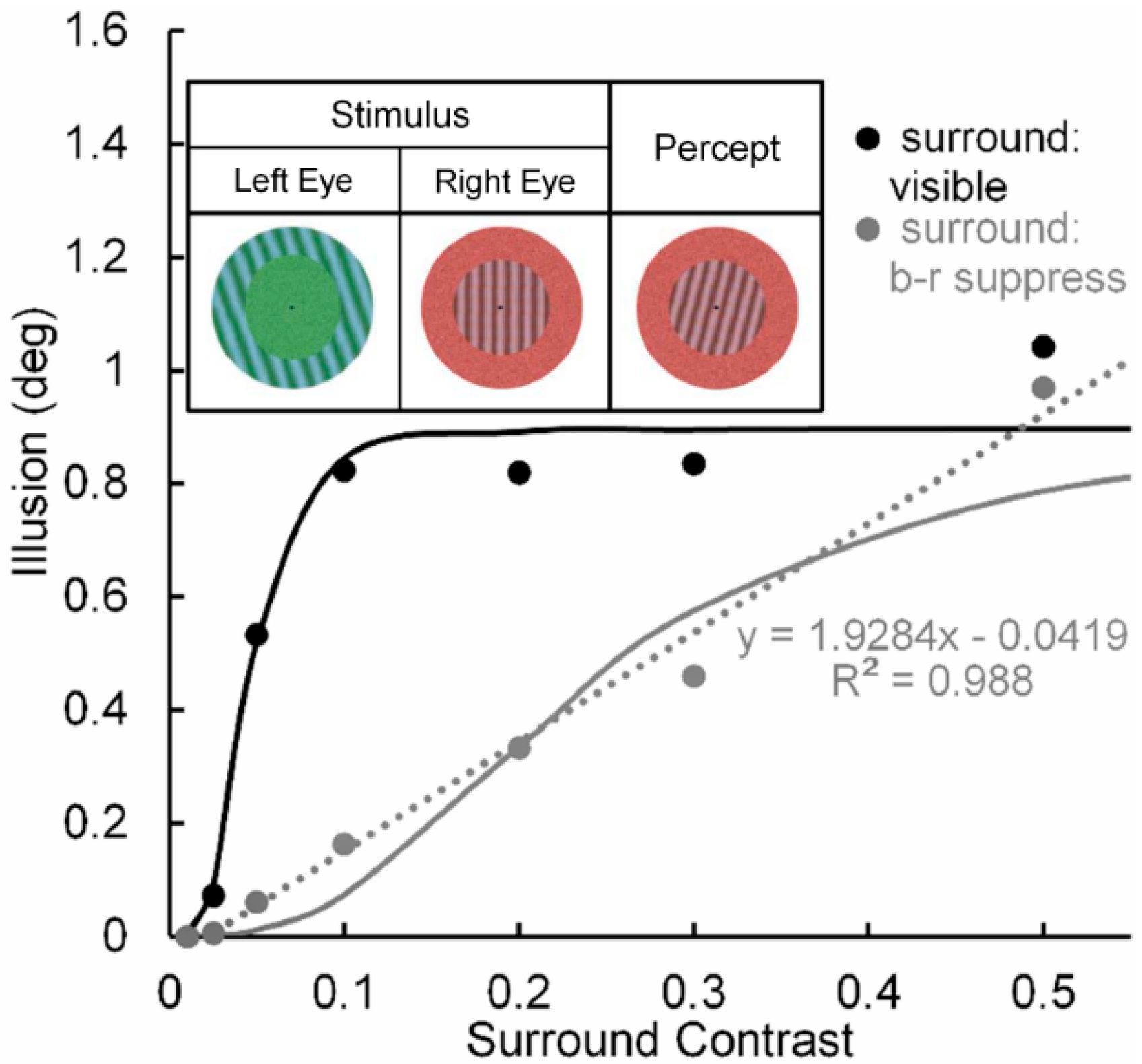
3.1. Visual Illusions
3.2. Visual Crowding
3.3. Pedestal Masking
4. Discussion, Implications, and Directions for Further Research
4.1. A Distinction between Functional and Anatomic Levels of Processing
4.2. Interpretations of Crowding Studies
4.3. The Problem of Residual Nonlinearities
4.4. Extensions to Other Visual Phenomena
5. Summary and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References and Notes
- Breitmeyer, B.G.; Koç, A.; Öğmen, H.; Ziegler, R. Functional hierarchies of nonconscious visual processing. Vis. Res. 2008, 48, 1509–1513. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthi, R.; Cavanagh, P. Recovery of a crowded object by masking the flankers: Determining the locus of feature integration. J. Vis. 2009, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Engel, S.A. Interocular rivalry revealed in the human cortical blind-spot representation. Nature 2001, 411, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Breitmeyer, B.G. Experimental “blinding” methods reveal a functional hierarchy of unconscious visual processing. Conscious. Cognit. 2015, 35, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Dakin, S.C.; Schwarzkopf, D.S.; Rees, G.; Greenwood, J.A. The neural correlates of crowding-induced changes in appearance. Curr. Biol. 2012, 22, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Dakin, S.C.; Greenwood, J.A.; Carlson, T.A.; Bex, P.J. Crowding is tuned for perceived (not physical) location. J. Vis. 2011, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Maus, G.W.; Fischer, J.; Whitney, D. Perceived positions determine crowding. PLoS ONE 2011, 6, e19796. [Google Scholar] [CrossRef] [PubMed]
- Wallis, T.S.A.; Bex, P.J. Visual crowding is correlated with awareness. Curr. Biol. 2011, 21, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Kovács, I.; Papathomas, T.V.; Yang, M.; Fehér, A. When the brain changes its mind: Interocular grouping during binocular rivalry. Proc. Natl. Acad. Sci. USA 1996, 93, 15508–15511. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Blake, R. A fresh look at interocular grouping during binocular rivalry. Vis. Res. 2004, 44, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Meng, M.; Blake, R. Neural bases of binocular rivalry. Trends Cognitive Sci. 2006, 10, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.D.; Deichmann, R.; Rees, G. Eye-specific effects of binocular rivalry in the human lateral geniculate nucleus. Nature 2005, 438, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, K.; Schneider, K.A.; Kastner, S. Neural correlates of binocular rivalry in the human lateral geniculate nucleus. Nat. Neurosci. 2005, 8, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Nakayama, K.; Vaughan, J.T.; Kanwisher, N. Binocular rivalry and visual awareness in human extrastriate cortex. Neuron 1998, 21, 753–759. [Google Scholar] [CrossRef]
- Haynes, J.D.; Driver, J.; Rees, G. Visibility reflects dynamic changes of effective connectivity between V1 and fusiform cortex. Neuron 2005, 46, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Tse, P.U.; Martinez-Conde, S.; Schlegel, A.A.; Macknik, S.L. Visibility, visual awareness, and visual masking of simple unattended targets are confined to areas in the occipital cortex beyond human V1/V2. Proc. Natl. Acad. Sci. USA 2005, 102, 17178–17183. [Google Scholar] [CrossRef] [PubMed]
- Carlson, T.A.; Rauschenberger, R.; Verstraten, F.A.J. No representation without awareness in the lateral occipital cortex. Psychol. Sci. 2007, 18, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.; Donner, T.H.; Heeger, D.J. Inter-area correlations in the ventral visual pathway reflect feature integration. J. Vis. 2011, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Millin, R.; Arman, A.C.; Chung, S.T.; Tjan, B.S. Visual crowding in V1. Cereb. Cortex 2014, 24, 3107–3115. [Google Scholar] [CrossRef] [PubMed]
- Chirimuuta, M.; Clatworthy, P.L.; Tolhurst, D.J. Coding of the contrasts in natural images by visual cortex (V1) neurons: A Bayesian approach. J. Opt. Soc. Am. A 2003, 20, 1253–1260. [Google Scholar] [CrossRef]
- Chirimuuta, M.; Tolhurst, D.J. Does a Bayesian model of V1 contrast coding offer a neurophysiological account of human contrast discrimination? Vis. Res. 2005, 45, 2943–2959. [Google Scholar] [CrossRef] [PubMed]
- Tadmor, Y.; Tolhurst, D.J. Calculating the contrasts that retinal ganglion cells and LGN neurones encounter in natural scenes. Vis. Res. 2000, 40, 3145–3157. [Google Scholar] [CrossRef]
- Figure 1, Figure 3, Figure 5, Figure 6, Figure 7 and Figure 8 of the present paper were redrawn using data values extracted with DataThief software (available at datathief.org) from originally published figures. Consequently, there may be very small differences between the data-point values used to plot the original figures and the values extracted by us. Moreover, although some of the original figures had contrasts displayed on a logarithmic scale along their abscissae, in Figure 1, Figure 3 and Figure 5, Figure 6, Figure 7, Figure 8 all contrasts are displayed on a linear scale along their abscissae. Except for two studies [24,66] that did not specify mean luminance of the visual display, the remaining data were obtained from studies [51,59,60,65,68,70,83] specifying mean display luminances ranging from 10. 7 (low photopic) to 120 cd/m2 (medium photopic). This is an important consideration, since parvocellular and magnocellular neurons respectively yield linear and nonlinear CRFs at photopic mean-luminance levels, whereas both types of neurons tend to yield linear CRFs at scotopic mean-luminance levels [102].
- Hall, S.D.; Holliday, I.E.; Hillebrand, A.; Furlong, P.L.; Singh, K.D.; Barnes, G.R. Distinct contrast response functions in striate and extra-striate regions of visual cortex revealed with magnetoencephalography (MEG). Clin. Neurophysiol. 2005, 116, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.D.; Roe, A.W. Optical imaging of contrast response in macaque monkey V1 and V2. Cereb. Cortex 2007, 17, 2675–2695. [Google Scholar] [CrossRef] [PubMed]
- Sani, I.; Santandrea, E.; Golzar, A.; Morrone, M.C.; Chelazzi, L. Selective tuning for contrast in macaque area V4. J. Neurosci. 2013, 33, 18583–18596. [Google Scholar] [CrossRef] [PubMed]
- Tootell, R.B.H.; Hadjikhani, N.K.; Vanduffel, W.; Liu, A.K.; Mendola, J.D.; Sereno, M.I.; Dale, A.M. Functional analysis of primary visual cortex (V1) in humans. Proc. Natl. Acad. Sci. USA 1998, 95, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Williford, T.; Maunsell, J.H.R. Effects of spatial attention on contrast response functions in macaque area V4. J. Neurophysiol. 2006, 96, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Although two investigations [26,28] reported an overall monotonic nonlinear increase (with amplification in the low-contrast range) of neural CRFs, they also found that a subset of neurons showed contrast selectivity (peak response not at a contrast of 1.0, but at lower contrasts) when the monkeys’ attention was directed toward the stimuli falling in their receptive fields. This selectivity for contrast makes sense, since besides the selective chromatic contrasts giving rise to distinguishable colors, selective achromatic contrasts giving rise to distinguishable perceptual grey levels also are important features of visual stimuli [34].
- Avidan, G.; Harel, M.; Hendler, T.; Ben-Bashat, D.; Zohary, E.; Malach, R. Contrast sensitivity in human visual areas and its relationship to object recognition. J. Neurophysiol. 2002, 87, 3102–3116. [Google Scholar] [CrossRef] [PubMed]
- Leopold, D.A.; Logothetis, N.K. Activity changes in early visual cortex reflect monkey’s percept during binocular rivalry. Nature 1996, 379, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, N.; Schall, J.D. Neuronal correlates of subjective visual perception. Science 1989, 245, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Sheinberg, D.L.; Logothetis, N.K. The role of temporal cortical areas in perceptual organization. Proc. Natl. Acad. Sci. USA 1997, 94, 3408–3413. [Google Scholar] [CrossRef] [PubMed]
- Pashler, H.; Dobkins, K.; Huang, L. Is contrast just another feature for visual selective attention? Vis. Res. 2004, 44, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.G.; Hamilton, D.B. Striate cortex of monkey and cat: Contrast response function. J. Neurophysiol. 1882, 48, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.B.; Solomon, J.A. Model of visual contrast gain control and pattern masking. J. Opt. Soc. Am. A 1997, 14, 2379–2390. [Google Scholar] [CrossRef]
- The Naka-Rushton equation is given by where C is the variable contrast; Rmax is the maximal response; C0.5 is the contrast producing half of the maximal response; and α is a free-parameter exponent.
- Felleman, D.J.; Burkhalter, A.; van Essen, D.C. Cortical connections of areas V3 and VP of macaque monkey extrastriate visual cortex. J. Compart. Neurol. 1997, 379, 21–47. [Google Scholar] [CrossRef]
- Salin, P.-A.; Bullier, J. Corticocortical connections in the visual system: Structure and function. Physiol. Rev. 1995, 75, 107–154. [Google Scholar] [CrossRef] [PubMed]
- Shipp, S.; Zeki, S. The organization of connections between areas V5 and V2 in macaque monkey visual cortex. Eur. J. Neurosci. 1989, 1, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Bullier, J. Integrated model of visual processing. Brain Res. Rev. 2001, 36, 96–107. [Google Scholar] [CrossRef]
- Hochstein, S.; Ahissar, M. View from the top: Hierarchies and reverse hierarchies in the visual system. Neuron 2002, 36, 791–804. [Google Scholar] [CrossRef]
- Wyatte, D.; Jilk, D.J.; O’Reilly, R.C. Early recurrent feedback facilitates visual object recognition under challenging conditions. Front. Psychol. 2014, 5, 674. [Google Scholar] [CrossRef] [PubMed]
- Cardin, V.; Friston, K.J.; Zeki, S. Top-down modulations in the visual form pathway revealed with dynamic causal modeling. Cereb. Cortex 2011, 21, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Hupé, J.M.; James, A.C.; Payne, B.R.; Lomber, S.G.; Girard, P.; Bullier, J. Cortical feedback improves discrimination between figure and background by V1, V2 andV3 neurons. Nature 1998, 394, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.W.; Maffei, L. The tilt aftereffect: A fresh look. Vis. Res. 1971, 11, 833–840. [Google Scholar] [CrossRef]
- Morant, R.B.; Harris, J.R. Two different after-effects of exposure to visual tilts. Am. J. Psychol. 1965, 78, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Tomassini, A.; Solomon, J.A. Awareness is the key to attraction: Dissociating the tilt illusions via conscious perception. J. Vis. 2014, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Clifford, C.W. The tilt illusion: Phenomenology and functional implications. Vis. Res. 2014, 104, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, C.; Carpenter, R.H.S.; Georgeson, M.A. Lateral inhibition between orientation detectors in the human visual system. Nature 1970, 228, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.; Clifford, C.W.G. Suppressed patterns alter vision during binocular rivalry. Curr. Biol. 2005, 15, 2142–2148. [Google Scholar] [CrossRef] [PubMed]
- A similar finding [91] has been reported when the surrounding tilt-inducing grating is rendered invisible by adaption-induced blindness (AIB). Moreover, AIB, like eye-specific b-r suppression, most likely occurs at a low, striate level of processing.
- Lee, S.-H.; Blake, R.; Heeger, D. Travelling waves of activity in primary visual cortex during binocular rivalry. Nat. Neurosci. 2005, 8, 22–23. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, A.; Blake, R.; Braun, J.; Heeger, D.J. Neuronal activity in human primary visual cortex correlates with perception during binocular rivalry. Nat. Neurosci. 2000, 3, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Herzog, M.H.; Sayim, B.; Chicherov, V.; Manassi, M. Crowding, grouping, and object recognition: A matter of appearance. J. Vis. 2015, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Levi, D.M. Crowding—An essential bottleneck for object recognition: A mini-review. Vis. Res. 2008, 48, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Pelli, D.G.; Tillman, K.A. The uncrowded window of object recognition. Nat. Neurosci. 2008, 11, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Whitney, D.; Levi, D.M. Visual crowding: A fundamental limit on conscious perception and object recognition. Trends Cognitive Sci. 2011, 15, 160–168. [Google Scholar] [CrossRef] [PubMed]
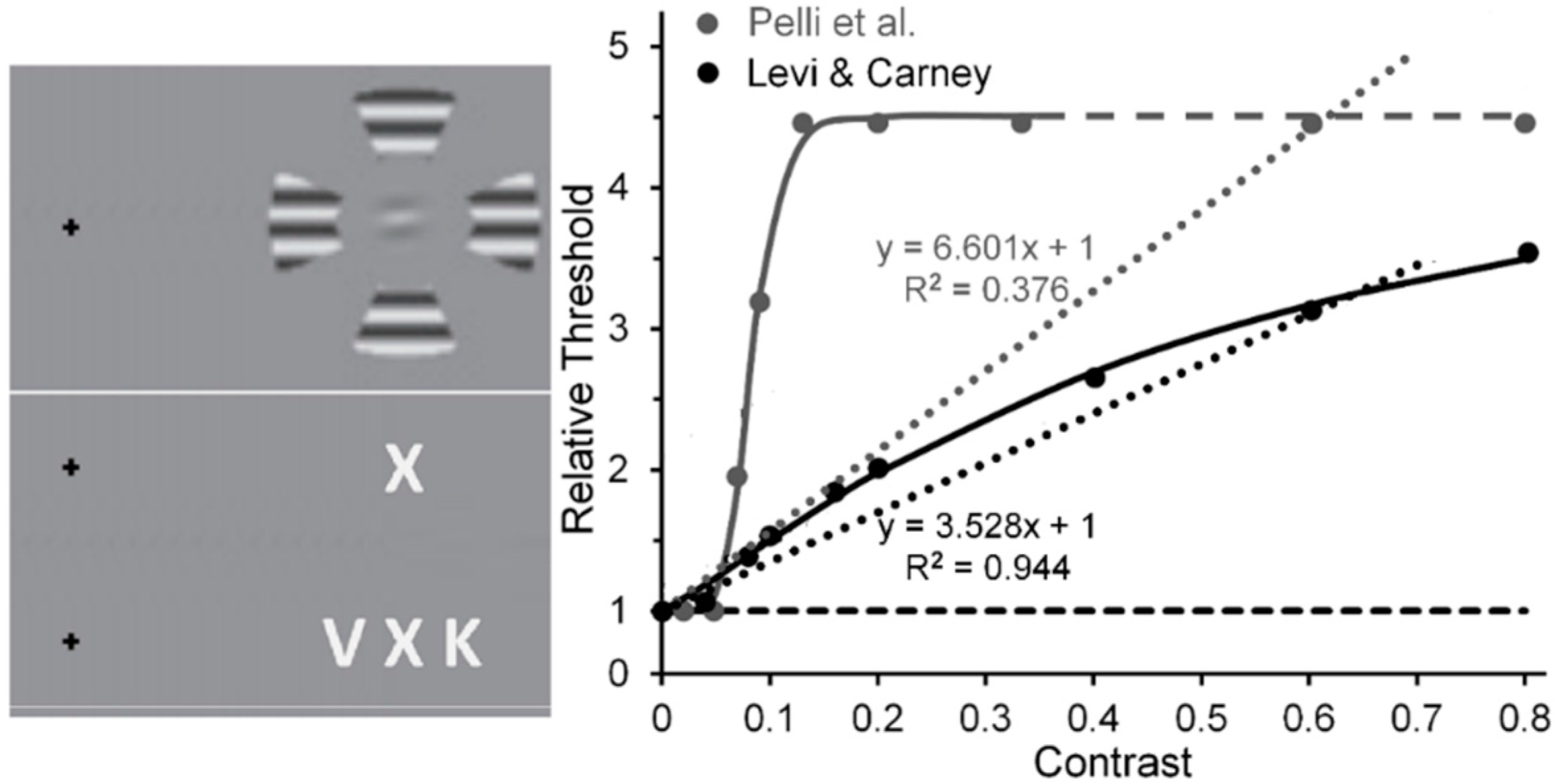
- Levi, D.M.; Carney, T. Crowding in peripheral vision: Why bigger Is better. Curr. Biol. 2009, 19, 1988–1993. [Google Scholar] [CrossRef] [PubMed]
- Pelli, D.G.; Palomares, M.; Majaj, N.J. Crowding is unlike ordinary masking: Distinguishing feature integration from detection. J. Vis. 2004, 4, 12. [Google Scholar] [CrossRef] [PubMed]
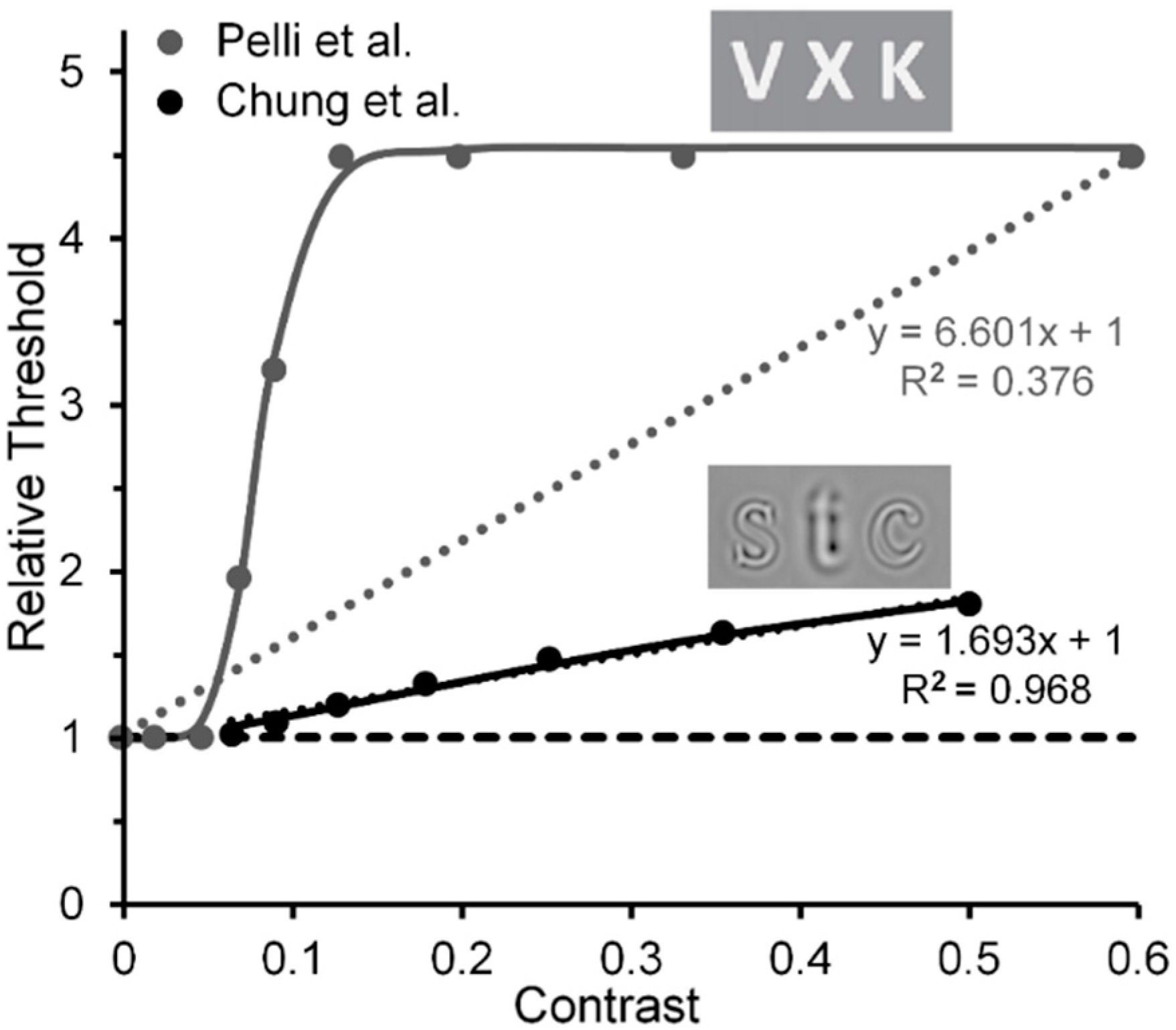
- Pelli et al. used Weber-contrast values to plot their data. We have converted them to Michelson-contrast values to make their results compatibly comparable with the Michelson contrast used by Levi and Carney. This resulted in Pelli et al.’s Weber-contrast range of 0 to 1 being compressed into a Michelson-contrast range of 0 to 0.33. For Michelson contrasts larger than 0.33, the relative thresholds for Pelli et al.’s data were assumed to be those extrapolated via the Naka-Rushton function.
- Using the same stimuli and methods, Levi & Carney [82] replicated this trend (shown in the right panel of their Figure 5) for normal (nonamblyopic) observers. However, Yeotikar et al. [103] failed to replicate the effects of flanker contrast variations on the strength of crowding. They ascribed the discrepancy of findings to differences in separation between the target and flanker Gabors in the Levi & Carney [59,82] studies and in their study. We believe that another possible source of the discrepancy is that in the Levi & Carney studies the carrier frequency of the target Gabors was 2.5 c/deg with a 1/f Gaussian standard deviation of 0.4, whereas in the Yeotikar et al. study the carrier frequency was 6 c/deg with a Gaussian standard deviation of 0.16. We suggest that the orientation of the off-horizontal, tilted Gabor patches used as targets by Levi and Carney may have been more difficult to discriminate from flanking horizontally oriented ones than the similar off-horizontally tilted target Gabors used by Yeotikar et al., which were more likely to “pop out” as different from the flanking horizontally tilted Gabor patches.
- The Pelli et al. results shown in Figure 5 were obtained from their Figure 11a by averaging results obtained with center-to-center target-flanker separations of 0.50 deg and those with center-to-center target-flanker separations of 0.75 deg, at which target and flankers did not overlap spatially. Since at these separations the 0.32-deg wide target and flanker letters used by Pelli et al. did not overlap spatially, ordinary masking with overlapping stimuli was not involved. Also, since the data of Pelli et al.’s Figure 11a were somewhat noisy, the data values extracted from it as well as from the less noisy data in Levi and Carney’s [59] Figure 3 were obtained from the best fitting functions found in both figures.
- Legge, G.E.; Foley, J.M. Contrast masking in human vision. J. Opt. Soc. Am. 1980, 70, 1458–1471. [Google Scholar] [CrossRef] [PubMed]
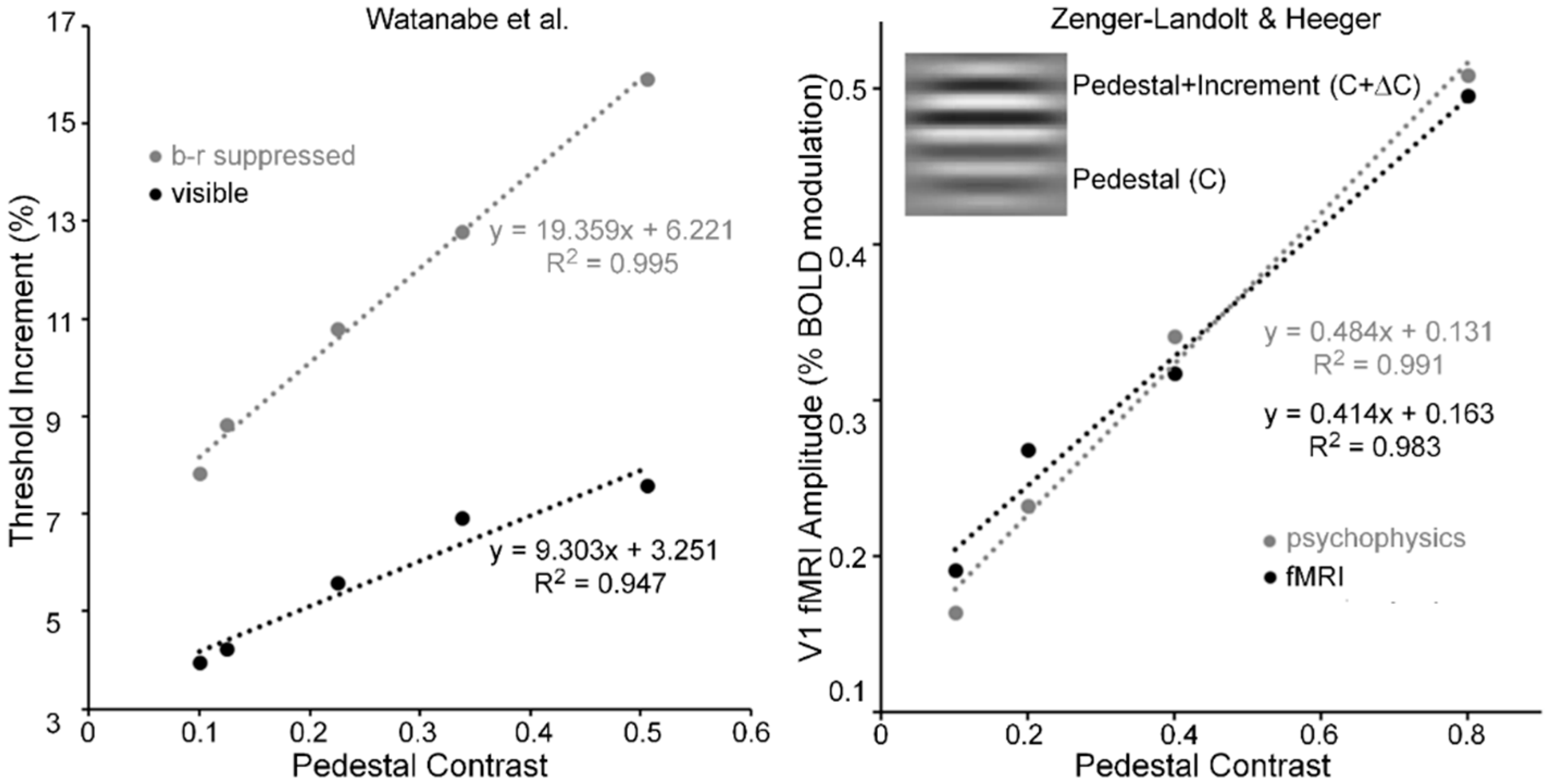
- Watanabe, K.; Paik, Y.; Blake, R. Preserved gain control for luminance contrast during binocular rivalry suppression. Vis. Res. 2004, 44, 3065–3071. [Google Scholar] [CrossRef] [PubMed]
- Zenger-Landoldt, B.; Heeger, D.J. Response suppression in V1 agrees with psychophysics of surround masking. J. Neurosci. 2003, 23, 6884–6893. [Google Scholar]
- The right panel of Figure 6 was derived from Zenger-Landolt and Heeger’s Figure 9. To show the tight coupling between fMRI amplitude and psychophysical increment thresholds, they scaled the increment thresholds to the range of fMRI amplitudes.
- Hu, Q.; Klein, S.A.; Carney, T. Can sinusoidal Vernier acuity be predicted by contrast discrimination? Vis. Res. 1993, 33, 1241–1258. [Google Scholar] [CrossRef]
- For each of the three pedestal spatial frequencies of 5, 10 and 20 c/deg, the TvC functions, averaged across the two Os showed strong linearities that accounting for 98, 97 and 96% of systematic variability, respectively.
- Greenlee, M.W.; Heitger, F. The functional role of contrast adaptation. Vis. Res. 1998, 28, 791–797. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Jiang, Y.; Sun, X.; He, S. Reduction of the crowding effect in spatially adjacent but cortically remote visual stimuli. Curr. Biol. 2009, 19, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Motter, B.C.; Simoni, D.A. The roles of cortical image separation and size in active visual search performance. J. Vis. 2007, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.P.; Cavanagh, P. The extent of crowding in peripheral vision does not scale with target size. Vis. Res. 2002, 42, 2357–2369. [Google Scholar] [CrossRef]
- The level at which post-striate feature conjunctions are processed [104,105,106,107] implicates also the level at which attentional effects may play a major role [108], this despite the fact the role of attention in crowding is in dispute [57].
- Kooi, F.L.; Toet, A.; Tripathy, S.P.; Levi, D.M. The effect of similarity and duration on spatial interaction in peripheral vision. Spat. Vis. 1994, 8, 255–279. [Google Scholar] [CrossRef] [PubMed]
- Bair, W.; Cavanaugh, J.R.; Movshon, J.A. Time course and time–distance relationships for surround suppression in macaque V1 neurons. J. Neurosci. 2003, 23, 7690–7701. [Google Scholar] [PubMed]
- Cavanaugh, J.R.; Bair, W.; Movshon, J.A. Nature and interaction of signals from the receptive field center and surround in macaque V1 neurons. J. Neurophysiol. 2002, 88, 2530–2546. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.S.; Dhruv, N.T.; Solomon, S.G.; Tailby, C.; Lennie, P. Early and late mechanisms of surround suppression in striate cortex of macaque. J. Neurosci. 2005, 25, 11666–11675. [Google Scholar] [CrossRef] [PubMed]
- Polat, U. Functional architecture of long-range perceptual interactions. Spat. Vis. 1999, 12, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Polat, U.; Sagi, D. Temporal asymmetry of collinear lateral interactions. Vis. Res. 2006, 46, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Polat, U.; Sterkin, A.; Yehezkel, O. Spatio-temporal low-level neural networks account for visual masking. Adv. Cognitive Psychol. 2007, 3, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Levi, D.M.; Carney, T. The effect of flankers on three tasks in central, peripheral, and amblyopic vision. J. Vis. 2011, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.T.; Levi, D.M.; Legge, G.E. Spatial-frequency and contrast properties of crowding. Vis. Res. 2001, 41, 1833–1850. [Google Scholar] [CrossRef]
- The Chung et al. results shown in our Figure 8 were derived from the left panel of their Figure 9, which shows threshold elevations, for target and flanker letters having a spatial frequency of 1.25 c/letter, which did not change with flanker contrasts below 0.06, but on a log-log scale increased linearly with a slope of 0.3 as flanker contrasts increased beyond a value of 0.06. Similar results, depicted in the right panel of their Figure 9, were obtained with a target and flanker spatial frequency of 2.5 d/deg, but here with a smaller slope of 0.13.
- Kaplan, E.; Shapley, R.M. The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proc. Natl. Acad. Sci. USA 1986, 83, 2755–2757. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.L.; Sun, P.; Waggoner, R.A.; Ueno, K.; Tanaka, K.; Cheng, K. Contrast adaptation and representation in human early visual cortex. Neuron 2005, 47, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, Z.-L.; Tjan, B.S.; Dosher, B.A.; Chu, W. Blood oxygenation level-dependent contrast response functions identify mechanisms of covert attention in early visual areas. Proc. Natl. Acad. Sci. USA 2008, 105, 6202–6207. [Google Scholar] [CrossRef] [PubMed]
- Schiller, P.H.; Logothetis, N.K. The color-opponent and broad-band channels of the primate visual system. Trends Neurosci. 1990, 13, 392–398. [Google Scholar] [CrossRef]
- Albert, M.K. Mechanisms of modal and amodal interpolation. Psychol. Rev. 2007, 114, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.M.; Foxe, D.M.; Javitt, D.C.; Foxe, J.J. Setting boundaries: Brain dynamics of modal and amodal illusory shape completion in humans. J. Neurosci. 2004, 24, 6898–6903. [Google Scholar] [CrossRef] [PubMed]
- Motoyoshi, I.; Hayakawa, S. Adaptation-induced blindness to sluggish stimuli. J. Vis. 2010, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Motter, B.C. Modulation of transient and sustained response components of V4 neurons by temporal crowding in flashed stimulus sequences. J. Neurosci. 2006, 26, 9683–9694. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Breitmeyer, B.G.; Hale, R.G.; Plummer, R.W. Contrast sensitivity indicates processing level of visual illusions. J. Exp. Psychol. 2018, in press. [Google Scholar]
- White, M. The effect of the nature of the surround on the perceived lightness of grey bars within square-wave test gratings. Perception 1981, 10, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Felleman, D.J.; Xiao, Y.; McClendon, E. Modular organization of occipito-temporal pathways: Cortical connections between visual area 4 and visual area 2 and posterior inferotemporal ventral area in macaque monkeys. J. Neurosci. 1997, 17, 3185–3200. [Google Scholar] [PubMed]
- Murray, M.M.; Wylie, G.R.; Higgins, B.A.; Javitt, D.C.; Schroeder, C.E.; Foxe, J.J. The spatiotemporal dynamics of illusory contour processing: Combined high-density electrical mapping, sources analysis, and functional magnetic resonance imaging. J. Neurosci. 2002, 22, 5055–5073. [Google Scholar] [PubMed]
- McCourt, M.E.; Blakeslee, B. Contrast-matching analysis of grating induction and suprathreshold contrast perception. J. Opt. Soc. Am. A 1994, 11, 14–24. [Google Scholar] [CrossRef]
- Brown, J.M.; Gyoba, J.; May, J.G. Stationary phantoms and grating induction with oblique inducing gratings: Implications for different mechanisms underlying the two phenomena. Psychon. Bull. Rev. 2001, 8, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-Y.; Blake, R. Psychophysical magic: Rendering the visible ‘invisible’. Trends Cognitive Sci. 2005, 9, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Breitmeyer, B.G.; Öğmen, H. Visual Masking: Time Slices through Conscious and Unconscious Vision; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- The near linearity of the normalized V1 neural-ensemble response remains robust over variations not only of (as demonstrated above) but also over variations of α and of Rmax. In fact, it remains intact when all three are varied independently and randomly. We ran four simulations in which Rmax could vary randomly in steps of 0.05 from 0.65 to 1.0, randomly in steps of 0.1 from 0.1 to 0.8, and α randomly in steps of 0.4 from 3.6 to 6.4. The four best linear fits accounted for 95.6, 99.3, 96.7, and 90.9% of the systematic variability. The best linear fit of the average of the four V1 ensemble responses accounted for 98.2% of systematic variability.
- Purpura, K.; Kaplan, E.; Shapley, R.M. Background light and the contrast gain of primate P and M retinal ganglion cells. Proc. Natl. Acad. Sci. USA 1988, 85, 4534–4537. [Google Scholar] [CrossRef] [PubMed]
- Yeotikar, N.S.; Khuu, S.K.; Asper, L.J.; Suttle, C.M. Configuration specificity of crowding in peripheral vision. Vis. Res. 2011, 51, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Anzai, A.; Peng, X.; Van Essen, D.C. Neurons in monkey visual area V2 encode combinations of orientations. Nat. Neurosci. 2007, 10, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Komatsu, H. Representation of angles embedded within contour stimuli in area V2 of macaque monkeys. J. Neurosci. 2004, 24, 3313–3324. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, A.; Connor, C.E. Population coding of shape in area V4. Nat. Neurosci. 2002, 5, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, K.; Yamane, Y.; Nishizaki, M.; Tanifuji, M. Complex objects are represented in macaque inferotemporal cortex by the combination of feature columns. Nat. Neurosci. 2001, 4, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.C. Binding, spatial attention and perceptual awareness. Nat. Neurosci. Rev. 2003, 4, 93–102. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breitmeyer, B.G.; Tripathy, S.P.; Brown, J.M. Can Contrast-Response Functions Indicate Visual Processing Levels? Vision 2018, 2, 14. https://doi.org/10.3390/vision2010014
Breitmeyer BG, Tripathy SP, Brown JM. Can Contrast-Response Functions Indicate Visual Processing Levels? Vision. 2018; 2(1):14. https://doi.org/10.3390/vision2010014
Chicago/Turabian StyleBreitmeyer, Bruno G., Srimant P. Tripathy, and James M. Brown. 2018. "Can Contrast-Response Functions Indicate Visual Processing Levels?" Vision 2, no. 1: 14. https://doi.org/10.3390/vision2010014




