Human Monkeypox: Current State of Knowledge and Implications for the Future
Abstract
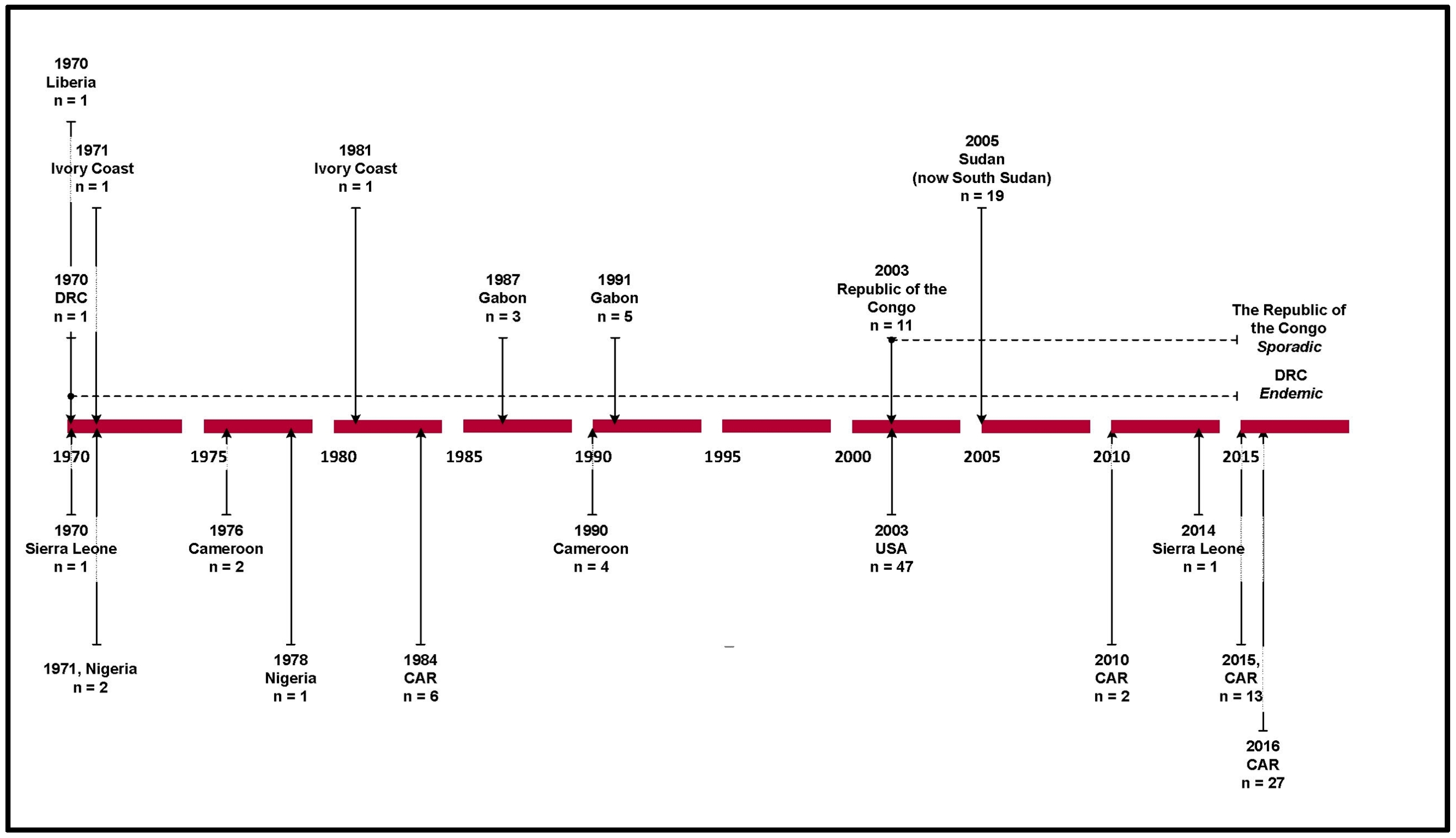
:1. Introduction
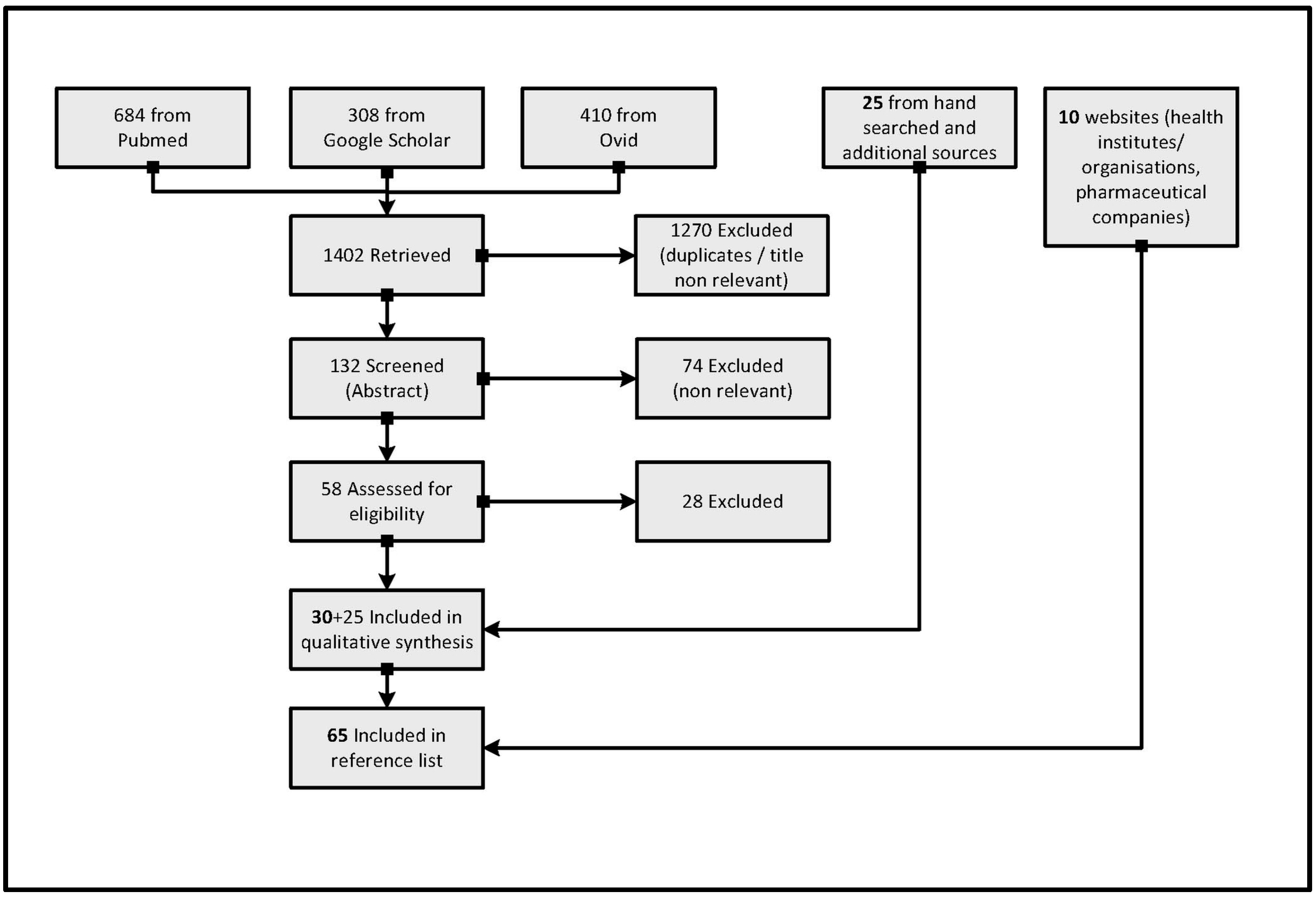
2. Materials and Methods
3. Results
3.1. Clinical Features
3.2. Reservoir Host
3.3. Diagnosis
3.4. Transmission
3.5. Prevention and Treatment
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hutson, C.L.; Nakazawa, Y.J.; Self, J.; Olson, V.A.; Regnery, R.L.; Braden, Z.; Weiss, S.; Malekani, J.; Jackson, E.; Tate, M.; et al. Laboratory investigations of African pouched rats (Cricetomys gambianus) as a potential reservoir host species for monkeypox virus. PLoS Negl. Trop. Dis. 2015, 9, e0004013. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Smallpox. Available online: www.who.int/topics/smallpox/en/ (accessed on 6 September 2016).
- Macneil, A.; Reynolds, M.G.; Braden, Z.; Carroll, D.S.; Bostik, V.; Karem, K.; Smith, S.K.; Davidson, W.; Li, Y.; Moundeli, A.; et al. Transmission of atypical varicella-zoster virus infections involving palm and sole manifestations in an area with monkeypox endemicity. Clin. Infect. Dis. 2009, 48, e6–e8. [Google Scholar] [CrossRef] [PubMed]
- Radonic, A.; Metzger, S.; Dabrowski, P.W.; Couacy-Hymann, E.; Schuenadel, L.; Kurth, A.; Matz-Rensing, K.; Boesch, C.; Leendertz, F.H.; Nitsche, A. Fatal monkeypox in wild-living sooty mangabey, Cote d’Ivoire, 2012. Emerg. Infect. Dis. 2014, 20, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, D.B.; Eckburg, P.B. Human monkeypox: An emerging zoonosis. Lancet Infect. Dis. 2004, 4, 15–25. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Available online: http://www.cdc.gov/poxvirus/monkeypox/index.html (accessed on 13 May 2016).
- Jezek, Z.; Grab, B.; Szczeniowski, M.V.; Paluku, K.M.; Mutombo, M. Human monkeypox: Secondary attack rates. Bull. World Health Organ. 1988, 66, 465–470. [Google Scholar] [PubMed]
- Hammarlund, E.; Lewis, M.W.; Carter, S.V.; Amanna, I.; Hansen, S.G.; Strelow, L.I.; Wong, S.W.; Yoshihara, P.; Hanifin, J.M.; Slifka, M.K. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat. Med. 2005, 11, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, H.A.; Fuller, T.; Asefi-Najafabady, S.; Shiplacoff, J.A.; Mulembakani, P.M.; Blumberg, S.; Johnston, S.C.; Kisalu, N.K.; Kinkela, T.L.; Fair, J.N.; et al. Pathogen-host associations and predicted range shifts of human monkeypox in response to climate change in central Africa. PLoS ONE 2013, 8, e66071. [Google Scholar] [CrossRef] [PubMed]
- Jezek, Z.; Marennikova, S.S.; Mutumbo, M.; Nakano, J.H.; Paluku, K.M.; Szczeniowski, M. Human monkeypox: A study of 2510 contacts of 214 patients. J. Infect. Dis. 1986, 154, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.; Buller, R.M. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Futur. Virol. 2013, 8, 129–157. [Google Scholar] [CrossRef] [PubMed]
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar] [PubMed]
- McCollum, A.M.; Damon, I.K. Human monkeypox. Clin. Infect. Dis. 2014, 58, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.; Nuara, A.; Buller, R.M.; Schultz, D.A. Human monkeypox: An emerging zoonotic disease. Future Microbiol. 2007, 2, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Sale, T.A.; Melski, J.W.; Stratman, E.J. Monkeypox: an epidemiologic and clinical comparison of African and US disease. J. Am. Acad. Dermatol. 2006, 55, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Nolen, L.D.; Osadebe, L.; Katomba, J.; Likofata, J.; Mukadi, D.; Monroe, B.; Doty, J.; Hughes, C.M.; Kabamba, J.; Malekani, J.; et al. Extended human-to-human transmission during a monkeypox outbreak in the Democratic Republic of the Congo. Emerg. Infect. Dis. 2016, 22, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Monkeypox. Available online: http://www.who.int/mediacentre/factsheets/fs161/en/ (accessed on 11 December 2016).
- Guarner, J.; Johnson, B.J.; Paddock, C.D.; Shieh, W.J.; Goldsmith, C.S.; Reynolds, M.G.; Damon, I.K.; Regnery, R.L.; Zaki, S.R.; Veterinary Monkeypox Virus Working Group. Monkeypox transmission and pathogenesis in prairie dogs. Emerg. Infect. Dis. 2004, 10, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Hutson, C.L.; Lee, K.N.; Abel, J.; Carroll, D.S.; Montgomery, J.M.; Olson, V.A.; Li, Y.; Davidson, W.; Hughes, C.; Dillon, M.; et al. Monkeypox zoonotic associations: Insights from laboratory evaluation of animals associated with the multi-state US outbreak. Am. J. Trop. Med. Hyg. 2007, 76, 757–768. [Google Scholar] [PubMed]
- Formenty, P.; Muntasir, M.O.; Damon, I.; Chowdhary, V.; Opoka, M.L.; Monimart, C.; Mutasim, E.M.; Manuguerra, J.C.; Davidson, W.B.; Karem, K.L.; et al. Human monkeypox outbreak caused by novel virus belonging to Congo Basin clade, Sudan, 2005. Emerg. Infect. Dis. 2010, 16, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Learned, L.A.; Reynolds, M.G.; Wassa, D.W.; Li, Y.; Olson, V.A.; Karem, K.; Stempora, L.L.; Braden, Z.H.; Kline, R.; Likos, A.; et al. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am. J. Trop. Med. Hyg. 2005, 73, 428–434. [Google Scholar] [PubMed]
- International Federation of Red Cross and Red Crescent Societies Emergency Plan of Action (EPoA) CAR Monkey-Pox Epidemic Outbreak. Available online: http://reliefweb.int/sites/reliefweb.int/files/resources/MDRCF020.pdf (accessed on 20 May 2016).
- Damon, I.K.; Roth, C.E.; Chowdhary, V. Discovery of monkeypox in Sudan. N. Engl. J. Med. 2006, 355, 962–963. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Li, G.; Liszewski, M.K.; Atkinson, J.P.; Jahrling, P.B.; Feng, Z.; Schriewer, J.; Buck, C.; Wang, C.; Lefkowitz, E.J.; et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology 2005, 340, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Hutson, C.L.; Carroll, D.S.; Self, J.; Weiss, S.; Hughes, C.M.; Braden, Z.; Olson, V.A.; Smith, S.K.; Karem, K.L.; Regnery, R.L.; et al. Dosage comparison of Congo Basin and West African strains of monkeypox virus using a prairie dog animal model of systemic orthopoxvirus disease. Virology 2010, 402, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; Frace, A.M.; Li, Y.; Olsen-Rasmussen, M.; Davidson, W.; Galloway, R.; Khristova, M.L.; Reynolds, M.G.; et al. A tale of two clades: monkeypox viruses. J. Gen. Virol. 2005, 86 Pt 10, 2661–2672. [Google Scholar] [CrossRef] [PubMed]
- Bavari, S.; Whitehouse, C.A. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin. Infect. Dis. 2005, 41, 1765–1771. [Google Scholar]
- Reynolds, M.G.; Yorita, K.L.; Kuehnert, M.J.; Davidson, W.B.; Huhn, G.D.; Holman, R.C.; Damon, I.K. Clinical manifestations of human monkeypox influenced by route of infection. J. Infect. Dis. 2006, 194, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Karem, K.L.; Reynolds, M.; Braden, Z.; Lou, G.; Bernard, N.; Patton, J.; Damon, I.K. Characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin. Diagn. Lab. Immunol. 2005, 12, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Jezek, Z.; Szczeniowski, M.; Paluku, K.M.; Mutombo, M. Human monkeypox: Clinical features of 282 patients. J. Infect. Dis. 1987, 156, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Nolen, L.D.; Osadebe, L.; Katomba, J.; Likofata, J.; Mukadi, D.; Monroe, B.; Doty, J.; Kalemba, L.; Malekani, J.; Kabamba, J.; et al. Introduction of monkeypox into a community and household: Risk factors and zoonotic reservoirs in the Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 2015, 93, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Breman, J.G. Monkeypox: An emerging infection for humans? In Emerging Infections 4; American Society of Microbiology: Washington, DC, USA, 2000; pp. 45–67. [Google Scholar]
- Huhn, G.D.; Bauer, A.M.; Yorita, K.; Graham, M.B.; Sejvar, J.; Likos, A.; Damon, I.K.; Reynolds, M.G.; Kuehnert, M.J. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin. Infect. Dis. 2005, 41, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Essbauer, S.; Meyer, H. Genus Orthopoxvirus: Monkeypox virus. In Poxviruses; Birkhäuser: Basel, Switzerland, 2007; pp. 65–73. [Google Scholar]
- Weese, J.S.; Fulford, M. Viral diseases. In Companion Animal Zoonoses; Wiley-Blackwell: Oxford, Uk, 2011; pp. 241–274. [Google Scholar]
- Lewis, M.W.; Graham, M.B.; Hammarlund, E.; Hanifin, J.; Slifka, M.K. Monkeypox without exanthem. N. Engl. J. Med. 2007, 356, 2112–2114. [Google Scholar] [CrossRef] [PubMed]
- Roess, A.A.; Monroe, B.P.; Kinzoni, E.A.; Gallagher, S.; Ibata, S.R.; Badinga, N.; Molouania, T.M.; Mabola, F.S.; Mombouli, J.V.; Carroll, D.S.; et al. Assessing the effectiveness of a community intervention for monkeypox prevention in the Congo basin. PLoS Negl. Trop. Dis. 2011, 5, e1356. [Google Scholar] [CrossRef] [PubMed]
- Breman, J.G.; Kalisa, R.; Steniowski, M.V.; Zanotto, E.; Gromyko, A.I.; Arita, I. Human monkeypox, 1970–79. Bull. World Health Organ. 1980, 58, 165–182. [Google Scholar] [PubMed]
- World Health Organization Technical Advisory Group on Human Monkeypox. Report of a WHO Meeting. Geneva, Switzerland, 11–12 January 1999; Available online: http://www.who.int/csr/resources/publications/viral/whocdscsraph995.pdf?ua=1 (accessed on 17 May 2016).
- Damon, I.K. Status of human monkeypox: Clinical disease, epidemiology and research. Vaccine 2011, 29 (Suppl. 4), D54–D59. [Google Scholar] [CrossRef] [PubMed]
- Jezek, Z.; Grab, B.; Paluku, K.M.; Szczeniowski, M.V. Human monkeypox: Disease pattern, incidence and attack rates in a rural area of northern Zaire. Trop. Geogr. Med. 1988, 40, 73–83. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention. Human monkeypox—Kasai Oriental, Democratic Republic of Congo, February 1996–October 1997. MMWR Morb. Mortal. Wkly. Rep. 1997, 46, 1168–1171. [Google Scholar]
- Fuller, T.; Thomassen, H.A.; Mulembakani, P.M.; Johnston, S.C.; Lloyd-Smith, J.O.; Kisalu, N.K.; Lutete, T.K.; Blumberg, S.; Fair, J.N.; Wolfe, N.D.; et al. Using remote sensing to map the risk of human monkeypox virus in the Congo Basin. Ecohealth 2011, 8, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Khodakevich, L.; Jezek, Z.; Kinzanzka, K. Isolation of monkeypox virus from wild squirrel infected in nature. Lancet 1986, 1, 98–99. [Google Scholar] [CrossRef]
- World Health Organization. Human Monkeypox (MPX). Available online: http://www.who.int/csr/disease/monkeypox/en/ (accessed on 10 May 2016).
- Townsend, M.B.; MacNeil, A.; Reynolds, M.G.; Hughes, C.M.; Olson, V.A.; Damon, I.K.; Karem, K.L. Evaluation of the Tetracore Orthopox BioThreat(R) antigen detection assay using laboratory grown orthopoxviruses and rash illness clinical specimens. J. Virol. Methods 2013, 187, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.; Perrichot, M.; Stemmler, M.; Emmerich, P.; Schmitz, H.; Varaine, F.; Shungu, R.; Tshioko, F.; Formenty, P. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J. Clin. Microbiol. 2002, 40, 2919–2921. [Google Scholar] [CrossRef] [PubMed]
- Rimoin, A.W.; Mulembakani, P.M.; Johnston, S.C.; Smith, J.O.L.; Kisalu, N.K.; Kinkela, T.L.; Blumberg, S.; Thomassen, H.A.; Pike, B.L.; Fair, J.N.; et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. USA 2010, 107, 16262–16267. [Google Scholar] [CrossRef] [PubMed]
- Nakouné, E.; Kazanji, M. Monkeypox detection in maculopapular lesions in two young Pygmies in the Central African Republic. Int. J. Infect. Dis. 2012, 16, e266–e267. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Prevention, Update: Multistate outbreak of monkeypox —Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb. Mortal. Wkly. Rep. 2003, 52, 589–590. [Google Scholar]
- Avert HIV and Aids in Sub-Saharan Africa Regional Overview. Available online: http://www.avert.org/professionals/hiv-around-world/sub-saharan-africa/overview (accessed on 29 October 2016).
- Jordan, R.; Leeds, J.M.; Tyavanagimatt, S.; Hruby, D.E. Development of ST-246(R) for treatment of poxvirus infections. Viruses 2010, 2, 2409–2435. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Progressive vaccinia in a military smallpox vaccinee – United States, 2009. MMWR Morb. Mortal. Wkly. Rep. 2009, 58, 532–536. [Google Scholar]
- Petersen, B.W.; Damon, I.K.; Pertowski, C.A.; Meaney-Delman, D.; Guarnizo, J.T.; Beigi, R.H.; Edwards, K.M.; Fisher, M.C.; Frey, S.E.; Lynfield, R.; et al. Clinical guidance for smallpox vaccine use in a post-event vaccination program. MMWR Recomm. Rep. 2015, 64, 1–26. [Google Scholar] [PubMed]
- Nalca, A.; Zumbrun, E.E. ACAM2000: The new smallpox vaccine for United States Strategic National Stockpile. Drug Des. Dev. Ther. 2010, 4, 71–79. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Summary Report on First, Second and Third Generation Smallpox Vaccines. Available online: http://www.who.int/immunization/sage/meetings/2013/november/2_Smallpox_vaccine_review_updated_11_10_13.pdf (accessed on 1 November 2016).
- Greenberg, R.N.; Overton, E.T.; Haas, D.W.; Frank, I.; Goldman, M.; von Krempelhuber, A.; Virgin, G.; Badeker, N.; Vollmar, J.; Chaplin, P. Safety, immunogenicity, and surrogate markers of clinical efficacy for modified vaccinia Ankara as a smallpox vaccine in HIV-infected subjects. J. Infect. Dis. 2013, 207, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention Advisory Committee on Immunization Practices (ACIP) Statement on Smallpox Preparedness and Vaccination. Available online: https://emergency.cdc.gov/agent/smallpox/vaccination/acipjun2003.asp (accessed on 31 October 2016).
- Damon, I.K.; Damaso, C.R.; McFadden, G. Are we there yet? The smallpox research agenda using variola virus. PLoS Pathog. 2014, 10, e1004108. [Google Scholar] [CrossRef] [PubMed]
- SIGA Technologies Product Candidates — Smallpox. Available online: https://www.siga.com/product-pipeline/ (accessed on 31 October 2016).
- Mucker, E.M.; Goff, A.J.; Shamblin, J.D.; Grosenbach, D.W.; Damon, I.K.; Mehal, J.M.; Holman, R.C.; Carroll, D.; Gallardo, N.; Olson, V.A.; et al. Efficacy of tecovirimat (ST-246) in nonhuman primates infected with variola virus (smallpox). Antimicrob. Agents Chemother. 2013, 57, 6246–6253. [Google Scholar] [CrossRef] [PubMed]
- Hutin, Y.J.; Williams, R.J.; Malfait, P.; Pebody, R.; Loparev, V.N.; Ropp, S.L.; Rodriguez, M.; Knight, J.C.; Tshioko, F.K.; Khan, A.S.; et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2001, 7, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, Y.; Mauldin, M.R.; Emerson, G.L.; Reynolds, M.G.; Lash, R.R.; Gao, J.; Zhao, H.; Li, Y.; Muyembe, J.J.; Kingebeni, P.M.; et al. A phylogeographic investigation of African monkeypox. Viruses 2015, 7, 2168–2184. [Google Scholar] [CrossRef] [PubMed]

| Specific Symptoms | Primary Research 1 | Secondary Research 2 |
|---|---|---|
| Fever and Fatigue | Huhn et al. (2005) [35] Reed et al. (2004) [19] Reynolds et al. (2006) [29] Formenty et al. (2010) [21] Jezek et al. (1987) [32] | Bavari & Whitehouse (2005) [28] Macneil et al. (2009) [3] Sale et al. (2006) [15] Di Giulio & Eckberg (2004) [5] Nolen et al. (2015) [33] Parker et al. (2007) [14] |
| Rash | Huhn et al. (2005) [35] Reed et al. (2004) [19] Reynolds et al. (2006) [29] Formenty et al. (2010) [21] Jezek et al. (1987) [32] | Bavari & Whitehouse (2005) [28] Macneil et al. (2009) [3] Sale et al. (2006) [15] Di Giulio & Eckberg (2004) [5] Nolen et al. (2015) [33] Parker et al. (2007) [14] Breman (2000) [34] |
| Lymphadenopathy | Huhn et al. (2005) [35] Reed et al. (2004) [19] Reynolds et al. (2006) [29] Formenty et al. (2010) [21] Jezek et al. (1987) [32] | Bavari & Whitehouse (2005) [28] Macneil et al. (2009) [3] Sale et al. (2006) [15] Di Giulio & Eckberg (2004) [5] Nolen et al. (2015) [33] Parker et al. (2007) [14] |
| Lesions (including palms of hands and soles of feet) | Huhn et al. (2005) [35] Reed et al. (2004) [19] Reynolds et al. (2006) [29] Formenty et al. (2010) [21] Jezek et al. (1987) [32] | Bavari & Whitehouse (2005) [28] Macneil et al. (2009) [3] Sale et al. (2006) [15] Di Giulio & Eckberg (2004) [5] Nolen et al. (2015) [33] Parker et al. (2007) [14] |
| Respiratory symptoms | Reed et al. (2004) [19] Reynolds et al. (2006) [29] Formenty et al. (2010) [21] Jezek et al. (1987) [32] | Parker et al. (2007) [14] Di Giulio & Eckberg (2004) |
| Date and Location | 1970–1979 Central and West Africa | 1981–1986 DRC | 1996–1998 DRC | 2003 USA | 2005 South Sudan |
|---|---|---|---|---|---|
| Case Fatality Rate (%) | 17 [40] | 9.8 1 [41,42,43] | 1.5 2 [44] | No recorded deaths | No recorded deaths |
| Suspected Reservoir Host | Primary Research | Secondary Research |
|---|---|---|
| Rope squirrel (Funisciurus sp.) | Fuller et al. (2011) [45] Thomassen et al. (2013) [9] Khodakevich et al. (1986) [46] | Guarner et al. (2004) [18] Sale et al. (2006) [15] Di Giulio & Eckberg (2004) [5] Parker & Buller (2013) [11] |
| Gambian pouched rat (Cricetomys gambianus) | Hutson et al. (2015) [1] | Parker & Buller (2013) [11] Sale et al. (2006) [15] Fuller et al. (2011) [45] Di Giulio & Eckberg (2004) [5] Formenty et al. (2010) [21] |
| Sooty mangabey monkey (Cercocebus atys) | Radonic et al. (2014) [4] | Nolen et al. (2015) [33] |
| Test | Description |
|---|---|
| Viral culture/isolation | Live virus is grown and characterised from a patient specimen |
| Electron microscopy | Clear image of a brick-shaped particle for visual classification of a poxvirus |
| Immunohistochemistry | Tests for the presence of OPXV specific antigens |
| PCR (including real-time PCR) | Tests for the presence of MPXV specific DNA signatures |
| Anti-OPXV IgG | Tests for the presence of OPXV antibodies |
| Anti-OPXV IgM | Tests for the presence of OPXV antibodies |
| Tetracore OrthopoxBioThreat | Alert test for the presence of OPXV antigens |
| Transmission | Primary Research | Secondary Research |
|---|---|---|
| Direct contact with infected humans or animals | Guarner et al. (2004) [18] Jezek et al. (1988) [7] Meyer et al. (2002) [49] Reed et al. (2004) [19] Reynolds et al. (2006) [29] Learned et al. (2005) [22] Formenty et al. (2010) [21] | Rimoin et al. (2010) [50] Parker & Buller (2013) [11] Sale et al. (2006) [15] Hammarlund et al. (2005) [8] Hutson et al. (2015) [1] McCollum & Damon (2014) [13] |
| Respiratory | Guarner et al. (2004) [18] Hammarlund et al. (2005) [8] Reynolds et al. (2006) [29] | Parker & Buller (2013) [11] Hutson et al. (2015) [1] |
| Fomites | Hammarlund et al. (2005) [8] Nolen et al. (2015) [33] Reynolds et al. (2006) [29] Formenty et al. (2010) [21] | Parker & Buller (2013) [11] |
| Consuming infected meats | Meyer et al. (2002) [49] Nakouné E, Kazanji M (2012) [51] | Parker & Buller (2013) [11] Sale et al. (2006) [15] Thomassen et al. (2013) [9] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, K.; Leggat, P.A. Human Monkeypox: Current State of Knowledge and Implications for the Future. Trop. Med. Infect. Dis. 2016, 1, 8. https://doi.org/10.3390/tropicalmed1010008
Brown K, Leggat PA. Human Monkeypox: Current State of Knowledge and Implications for the Future. Tropical Medicine and Infectious Disease. 2016; 1(1):8. https://doi.org/10.3390/tropicalmed1010008
Chicago/Turabian StyleBrown, Katy, and Peter A. Leggat. 2016. "Human Monkeypox: Current State of Knowledge and Implications for the Future" Tropical Medicine and Infectious Disease 1, no. 1: 8. https://doi.org/10.3390/tropicalmed1010008







