The Understanding of Ebola Virus Disease (EVD) Among Medical Practitioners of Karachi, Pakistan
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Duration
2.2. Study Population and Eligibility Criteria
2.3. Sample Size
2.4. Sampling Technique
2.5. Research Instrument Development and Piloting
2.6. Data Collection and Analysis
2.7. Statement of Consent and Ethical Consideration
3. Results
3.1. Demographic Information:
3.2. Knowledge Regarding EVD
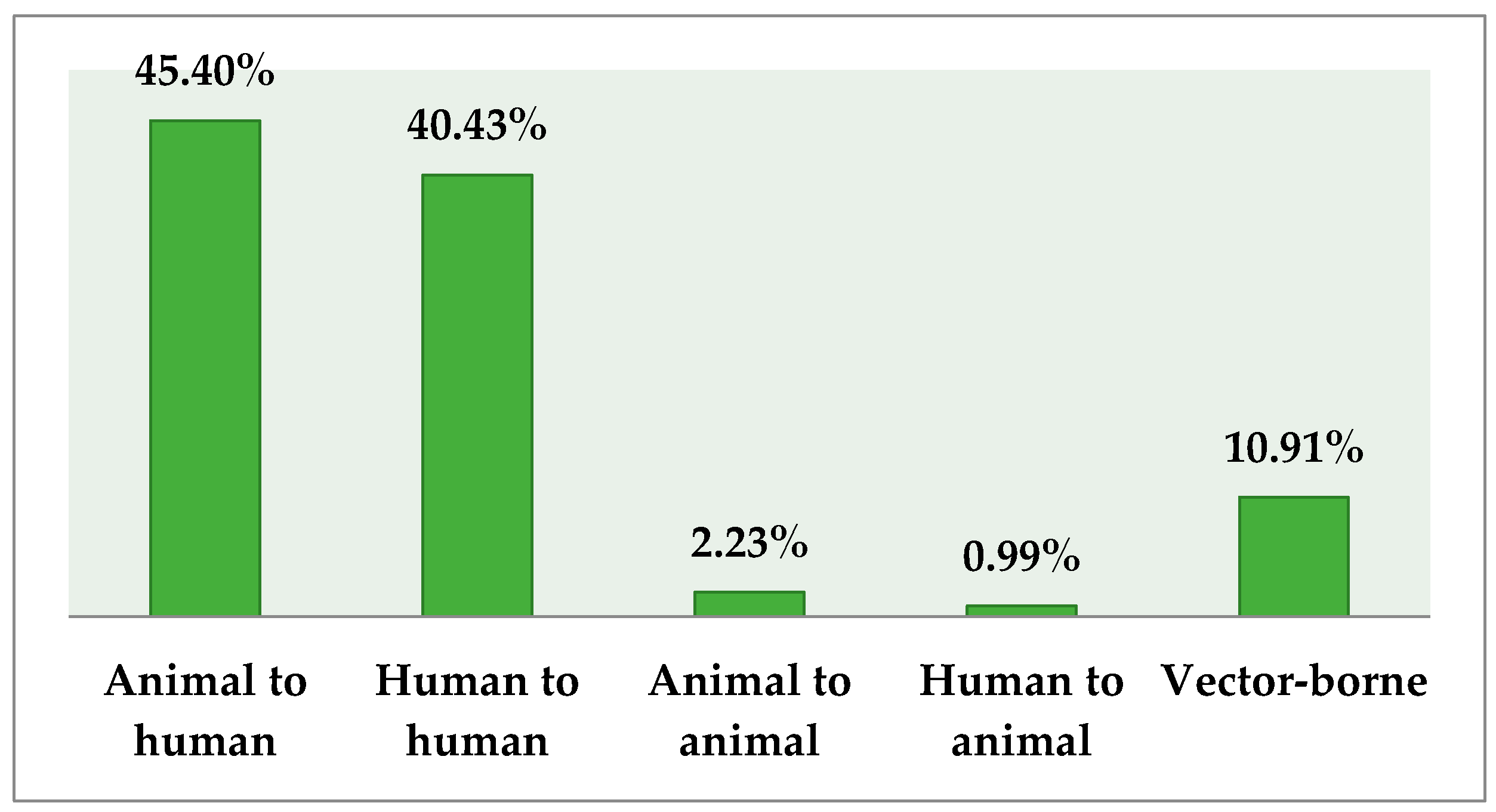
3.3. Knowledge about Transmission and Source of Infection
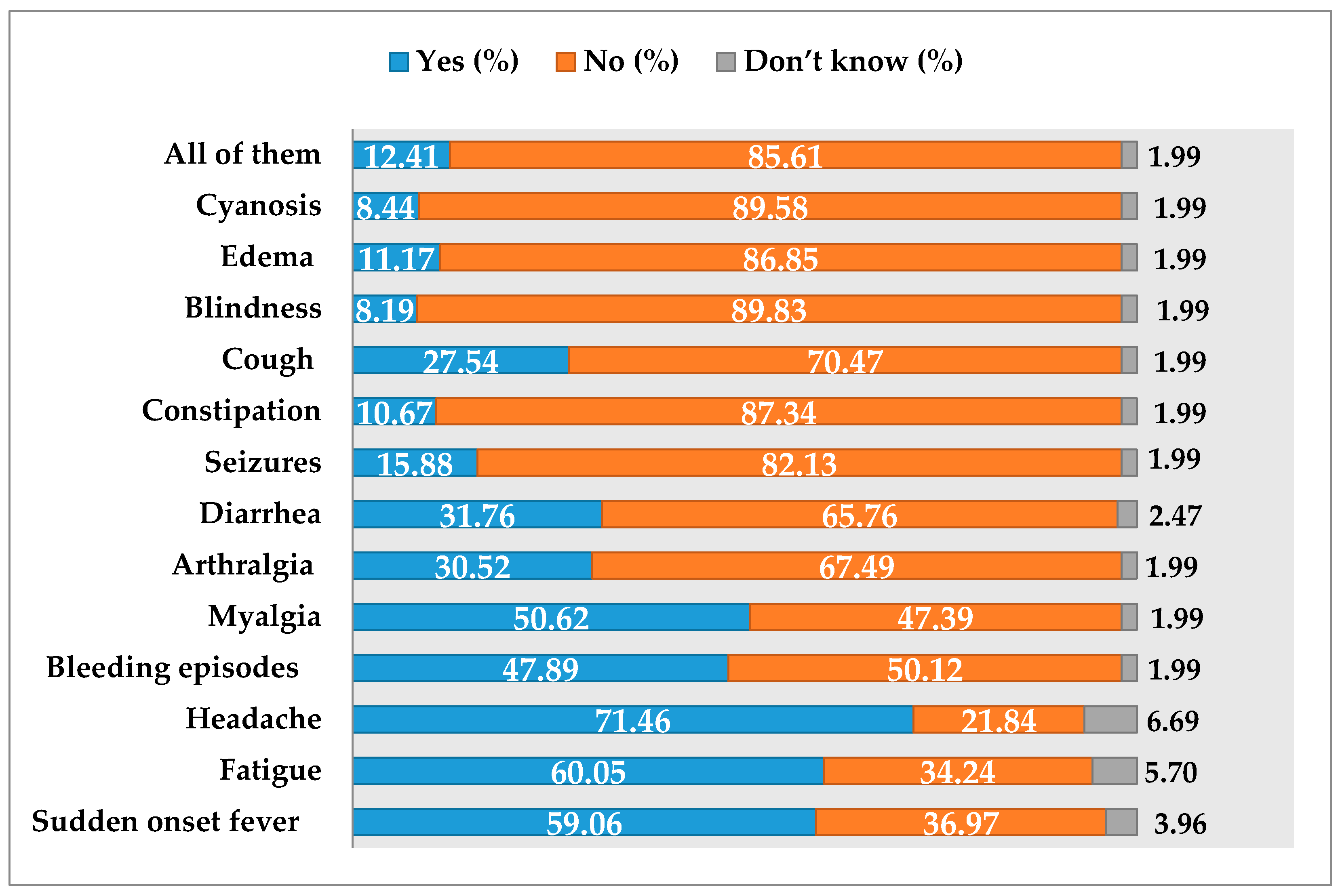
3.4. Knowledge about Clinical Features of EVD
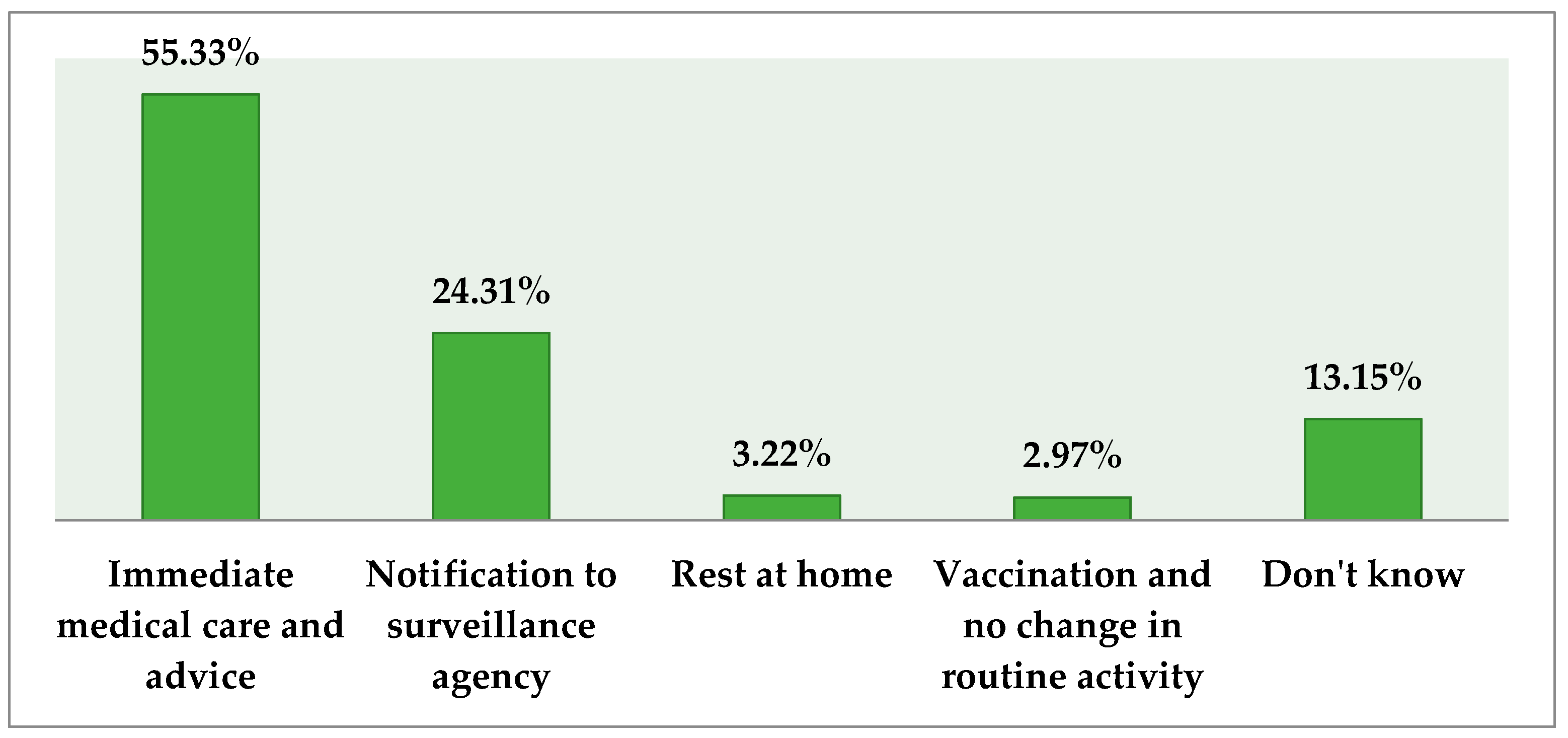
3.5. Respondents’ Opinion about the Management Strategies
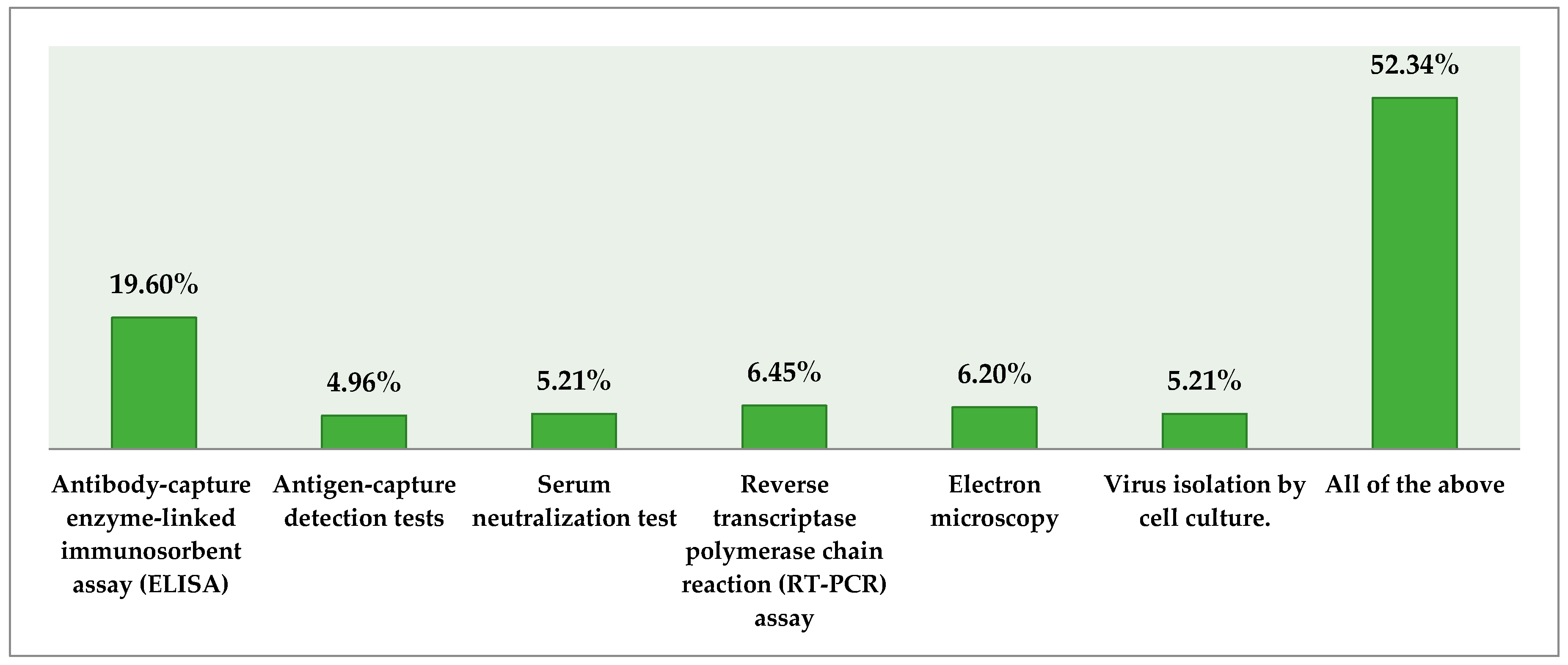
3.6. Knowledge about Diagnosis of EVD
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Knipe, D.; Howley, P.; Griffin, D.; Lamb, R.; Martin, M. Filoviridae: Marburg and Ebola viruses. In Fields Virology, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; pp. 1410–1448. [Google Scholar]
- Drosten, C.; Göttig, S.; Schilling, S.; Asper, M.; Panning, M.; Schmitz, H.; Günther, S. Rapid detection and quantification of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift Valley fever virus, dengue virus, and yellow fever virus by real-time reverse transcription-PCR. J. Clin. Microbiol. 2002, 40, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Wiwanitkit, V. Ebola virus infection: What should be known? N. Am. J. Med. Sci. 2014, 6, 549. [Google Scholar] [CrossRef] [PubMed]
- Muyembe-Tamfum, J.J.; Mulangu, S.; Masumu, J.; Kayembe, J.; Kemp, A.; Paweska, J.T. Ebola virus outbreaks in Africa: Past and present. Onderstepoort J. Vet. Res. 2012, 79, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Rewar, S.; Mirdha, D. Transmission of Ebola virus disease: An overview. Ann. Glob. Health 2014, 80, 444–451. [Google Scholar]
- Tambo, E.; Ugwu, E.C.; Ngogang, J.Y. Need of surveillance response systems to combat Ebola outbreaks and other emerging infectious diseases in African countries. Infect. Dis. Poverty 2014, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- WHO Ebola Response Team. Ebola virus disease in West Africa—The first 9 months of the epidemic and forward projections. N. Engl. J. Med. 2014, 371, 1481–1495. [Google Scholar]
- Bausch, D.G.; Towner, J.S.; Dowell, S.F.; Kaducu, F.; Lukwiya, M.; Sanchez, A.; Nichol, S.T.; Ksiazek, T.G.; Rollin, P.E. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. JID 2007, 196 (Suppl. 2), S142–S147. [Google Scholar] [CrossRef] [PubMed]
- Gire, S.K.; Goba, A.; Andersen, K.G.; Sealfon, R.S.; Park, D.J.; Kanneh, L.; Jalloh, S.; Momoh, M.; Fullah, M.; Dudas, G.; et al. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science 2014, 345, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Briand, S.; Bertherat, E.; Cox, P.; Formenty, P.; Kieny, M.P.; Myhre, J.K.; Roth, C.; Shindo, N.; Dye, C. The international Ebola emergency. N. Engl. J. Med. 2014, 371, 1180–1183. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Mehta, N.; Gupta, P.; Arora, V.; Setia, P. Knowledge regarding Ebola hemorrhagic fever among private dental practitioners in Tricity, India: A cross-sectional questionnaire study. Niger. Med. J. 2015, 56, 138. [Google Scholar] [PubMed]
- Khan, H.; Ahmad, I. Threat of Ebola virus disease for Pakistan. Gomal J. Med. Sci. 2015, 12, 127–128. [Google Scholar]
- Yazdani, N.; Abbas, A. Pakistan adopts defensive strategies against Ebola contagion. Arch. Pharma. Pract. 2015, 6, 13. [Google Scholar] [CrossRef]
- Pakistan Medical and Dental Council (PM&DC). Available online: http://www.pmdc.org.pk/PractitionerSearch/tabid/177/Default.aspx (accessed on 2 June 2017).
- Kilmarx, P.H.; Clarke, K.R.; Dietz, P.M.; Hamel, M.J.; Husain, F.; McFadden, J.D.; Park, B.J.; Sugerman, D.E.; Bresee, J.S.; Mermin, J.; et al. Ebola virus disease in health care workers—Sierra Leone. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 1168–1171. [Google Scholar] [PubMed]
- Ahmad, A.; Khan, M.U.; Jamshed, S.Q.; Kumar, B.D.; Kumar, G.S.; Reddy, P.G.; Ajmera, S. Are healthcare workers ready for Ebola? An assessment of their knowledge and attitude in a referral hospital in South India. J. Infect. Dev. Ctries. 2016, 10, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.; Bhalla, S.; Singh, D.; Rasania, S.; Singh, S. Knowledge, attitude and practice (KAP) on dengue fever: A hospital based study. Indian J. Commun. Med. 2006, 31, 185–186. [Google Scholar]
- Dunklin, R.; Thompson, S. ER Doctor discusses role in Ebola patient’s initial misdiagnosis. Dallas Morning News. 2014. Available online: https://www.dallasnews.com/news/news/2014/12/06/er-doctor-discusses-role-in-ebola-patients-initial-misdiagnosis (accessed on 4 June 2017).
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Leroy, E.M.; Baize, S.; Volchkov, V.; Fisher-Hoch, S.; Georges-Courbot, M.; Lansoud-Soukate, J.; Capron, M.; Debré, P.; McCormick, J.B.; Georges, A.J. Human asymptomatic Ebola infection and strong inflammatory response. Lancet 2000, 355, 2210–2215. [Google Scholar] [CrossRef]
- Suchitra, J.; Devi, N.L. Impact of education on knowledge, attitudes and practices among various categories of health care workers on nosocomial infections. Indian J. Med. Microbiol. 2007, 25, 181. [Google Scholar] [CrossRef] [PubMed]
- Munnell, A.H.; Sass, S.A.; Soto, M. Employer attitudes towards older workers: Survey results. Work Oppor. Older Am. Ser. 2006, 3, 1–14. [Google Scholar]
- Rahnavardi, M.; Rajaeinejad, M.; Pourmalek, F.; Mardani, M.; Holakouie-Naieni, K.; Dowlatshahi, S. Knowledge and attitude toward Crimean–Congo haemorrhagic fever in occupationally at-risk Iranian healthcare workers. J. Hosp. Infect. 2008, 69, 77–85. [Google Scholar] [CrossRef] [PubMed]

| Demographic Information | Frequency (%) |
|---|---|
| Gender | |
| Male | 173 (42.92) |
| Female | 230 (57.07) |
| Age | |
| 25–30 years | 205 (50.86) |
| 31–35 years | 47 (11.66) |
| 36–40 years | 36 (8.93) |
| 41–50 years | 38 (9.42) |
| 51 and above | 77 (19.09) |
| Organization | |
| Government | 223 (55.32) |
| Private | 177 (43.92) |
| Designation | |
| General practitioner | 89 (22.08) |
| Chief medical officer | 54 (13.39) |
| Head of department | 10 (2.48) |
| Resident medical officer | 52 (12.90) |
| Faculty | 198 (49.11) |
| Experience | |
| Less than 5 years | 185 (45.90) |
| 5–10 years | 73 (18.11) |
| 10–15 years | 39 (9.67) |
| 15–20 years | 62 (15.38) |
| 20 and above | 44 (10.91) |
| Average number of patients per day | |
| 1–30 | 114 (55.3) |
| 31–60 | 68 (33.0) |
| 61–90 | 20 (9.7) |
| Locality of Practice | |
| Urban | 207 (51.36) |
| Peri-urban | 196 (48.63) |
| Demographics | Knowledge Regarding EVD | |||
|---|---|---|---|---|
| Gender | No Knowledge | No Adequate Knowledge | Somewhat Knowledgeable | Adequate Knowledge |
| Male | 15 (23.9) | 28 (21) | 78 (76.6) | 4 (4.4) |
| Female | 50 (41.1) | 28 (36) | 130 (131.4) | 8 (7.6) |
| Designation | ||||
| General practitioner | 21 (13.3) | 10 (14.8) | 51 (56.2) | 7 (4.7) |
| Chief medical officer | 5 (6) | 3 (6.6) | 29 (25.3) | 3 (2.1) |
| Head of department | 2 (1.5) | 1 (1.7) | 5 (6.3) | 2 (0.5) |
| Resident medical officer | 5 (7.8) | 13 (8.6) | 33 (32.8) | 1 (2.7) |
| Professor | 0 (1.2) | 2 (1.3) | 6 (5.1) | 0 (0.4) |
| Lecturer | 4 (7.2) | 12 (8) | 32 (30.3) | 0 (2.5) |
| Experience | ||||
| Less than 5 years | 43 (34.3) | 32 (29.7) | 90 (97.7) | 3 (6.3) |
| Between 5 to 10 years | 8 (9.8) | 10 (8.5) | 28 (27.9) | 2 (1.8) |
| Between 11 to 15 years | 6 (3.5) | 3 (3) | 7 (9.9) | 1 (0.6) |
| Between 16 to 20 years | 2 (7.8) | 6 (6.7) | 24 (22.1) | 5 (1.4) |
| Above 20 years | 0 (4.7) | 1 (4.1) | 22 (13.4) | 0 (0.9) |
| Case Management Strategies Available | No. (%) |
|---|---|
| Supportive treatment | 270 (66.93) |
| Approved vaccine | 88 (21.83) |
| Immunoglobulin | 45 (11.16) |
| Personal Protection Among Health Care Workers | |
| Hand hygiene | 31 (7.69) |
| Personal protective equipment | 66 (16.37) |
| Proper handling of sharps and needles | 25 (6.20) |
| All of them | 281 (69.72) |
| Personal Protective Equipment Recommended by WHO | |
| Double examination gloves | 40 (9.92) |
| Impermeable gown | 15 (3.72) |
| Medical mask/face shield | 21 (5.21) |
| Shoe covers | 2 (0.49) |
| Head cover | 1 (0.24) |
| All of them | 324 (80.39) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakeel, S.; Iffat, W.; Naqvi, A.A.; Ahmed, F.; Usmani, S.; Mazhar, M.; Nisar, A. The Understanding of Ebola Virus Disease (EVD) Among Medical Practitioners of Karachi, Pakistan. Trop. Med. Infect. Dis. 2017, 2, 16. https://doi.org/10.3390/tropicalmed2020016
Shakeel S, Iffat W, Naqvi AA, Ahmed F, Usmani S, Mazhar M, Nisar A. The Understanding of Ebola Virus Disease (EVD) Among Medical Practitioners of Karachi, Pakistan. Tropical Medicine and Infectious Disease. 2017; 2(2):16. https://doi.org/10.3390/tropicalmed2020016
Chicago/Turabian StyleShakeel, Sadia, Wajiha Iffat, Atta Abbas Naqvi, Fouzia Ahmed, Shugufta Usmani, Manahil Mazhar, and Ayesha Nisar. 2017. "The Understanding of Ebola Virus Disease (EVD) Among Medical Practitioners of Karachi, Pakistan" Tropical Medicine and Infectious Disease 2, no. 2: 16. https://doi.org/10.3390/tropicalmed2020016






