Neglected Tropical Diseases: Epidemiology and Global Burden
Abstract
:1. Introduction
2. Epidemiology and Risk Factors
2.1. Buruli Ulcer
2.2. Chikungunya
2.3. Chagas Disease
2.4. Dengue Fever
2.5. Dracunculiosis (or Guinea Worm Disease)
2.6. Human African Trypanosomiasis (or Sleeping Sickness)
2.7. Leishmaniasis
2.8. Leprosy
2.9. Lymphatic Filariasis
2.10. Onchocerciasis (or River Blindness)
2.11. Rabies
2.12. Scabies
2.13. Schistosomiasis
2.14. Soil-Transmitted Helminthiasis
2.15. Trachoma
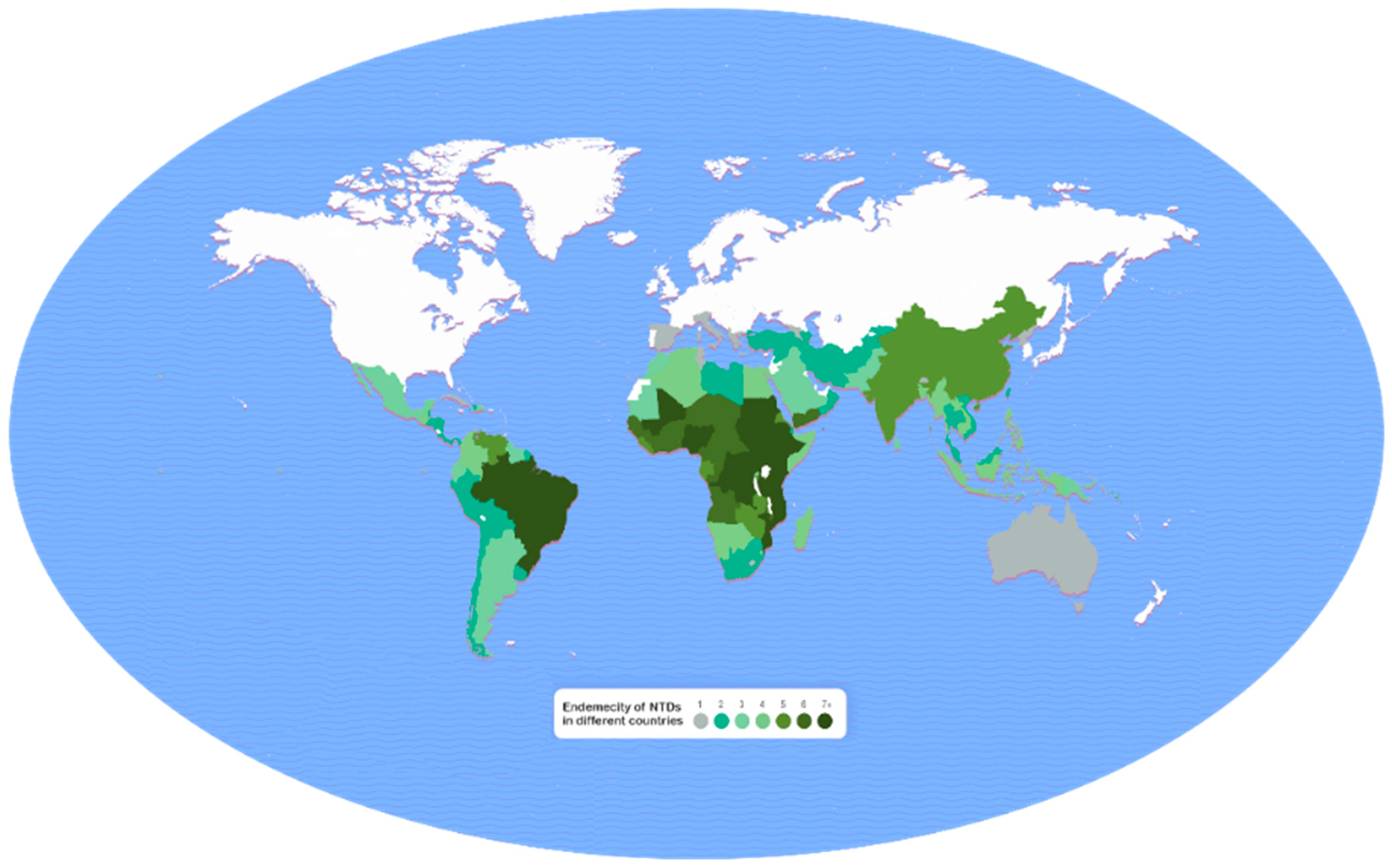
3. Global Burden
4. Recommendations
- Community awareness and early diagnosis of Buruli ulcer were found to be effective tools in reducing complications of the disease in rural areas of West and Central Africa [16], but more studies are needed to elucidate the mechanism by which M. ulcerans is transmitted from the environment to humans.
- Integrated vector management through the elimination of breeding sites, use of anti-adult and anti-larval measures and personal protection will help to prevent outbreaks of several vector-borne diseases including chikungunya, dengue fever, and HAT.
- Because the efficacy of medicines in Chagas disease decreases with increased chronicity of the disease, early intervention and treatment of the acute phase of infection would be efficacious than treatment of the chronic stage of the disease. There is a need for more effective, safer, and easier-to-use medicines for both phases of Chagas disease.
- Over 200 countries so far have been certified free of dracunculiasis, or guinea worm disease. Health education and behavioral change are effective tools in disease prevention [25].
- Treatment with ivermectin remains a challenge in individuals with onchocerciasis, especially those with high rate of parasitemia, because of the side effects and poor compliance of the drug in such cases [56]. Similarly, ivermectin is not recommended universally in the treatment of scabies. Future studies should aim at finding newer and safer medicines.
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hotez, P.J.; Kamath, A. Neglected tropical diseases in sub-Saharan Africa: Review of their prevalence, distribution, and disease burden. PLoS Negl. Trop. Dis. 2009, 3, e412. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Alvarado, M.; Basáñez, M.-G.; Bolliger, I.; Bourne, R.; Boussinesq, M.; Brooker, S.J.; Brown, A.S.; Buckle, G.; Budke, C.M.; et al. The global burden of disease study 2010: Interpretation and implications for the neglected tropical diseases. PLoS Negl. Trop. Dis. 2014, 8, e2865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Neglected Tropical Diseases. Program. 2017. Available online: http://www.who.int/neglected_diseases/en/ (accessed on 18 July 2017).
- Rosenberg, M.; Utzinger, J.; Addiss, D.G. Preventive chemotherapy versus innovative and intensified disease management in neglected tropical diseases: A distinction whose shelf life has expired. PLoS Negl. Trop. Dis. 2016, 10, e0004521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molyneux, D.H. The ‘Neglected Tropical Diseases’: Now a brand identity, responsibilities, context and promise. Parasites Vectors 2012, 5. [Google Scholar] [CrossRef] [PubMed]
- Githeko, A.K.; Lindsay, S.W.; Confalonieri, U.E.; Patz, J.A. Climate change and vector-borne diseases: A regional analysis. Bull. World Health Organ. 2000, 78, 1136–1147. [Google Scholar] [PubMed]
- Alonso, P.; Engels, D.; Reeder, J. Renewed push to strengthen vector control globally. 2017. Available online: http://www.who.int/mediacentre/commentaries/strengthen-vector-control/en/ (accessed on 13 June 2017).
- World Intellectual Property Organization (WIPO) Re:Search: New Plan for Fight Against Neglected Tropical Diseases, Malaria and TB. 2017. Available online: http://www.wipo.int/pressroom/en/articles/2017/article_0005.html (accessed on 15 June 2017).
- The Carter Center. Health Programs. Available online: https://www.cartercenter.org/health/index.html (accessed on 16 June 2017).
- Centers for Disease Control and Prevention. Neglected Tropical Diseases. 2017. Available online: https://www.cdc.gov/globalhealth/ntd/diseases/index.html (accessed on 15 June 2017).
- London Declaration on Neglected Tropical Diseases. 2012. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/67443/NTD_20Event_20-_20London_20Declaration_20on_20NTDs.pdf (accessed on 16 June 2017).
- Ampah, K.A.; Asare, P.; Binnah, D.D.-G.; Maccaulley, S.; Opare, W.; Röltgen, K.; Pluschke, G.; Yeboah-Manu, D. Burden and historical trend of Buruli ulcer prevalence in selected communities along the Offin River of Ghana. PloS Negl. Trop. Dis. 2016, 10, e0004603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- N’krumah, R.T.A.S.; Koné, B.; Tiembre, I.; Cissé, G.; Pluschke, G.; Tanner, M.; Utzinger, J. Socio-environmental factors associated with the risk of contracting Buruli ulcer in Tiassalé, South Côte d’Ivoire: A case-control study. PLoS Negl. Trop. Dis. 2016, 10, e0004327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sears, A.V.; Hay, R.J. Buruli ulcer—A rapidly changing scene. Acta Derm. Venereol. 2015, 95, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Ahorlu, C.K.; Koka, E.; Yeboah-Manu, D.; Lamptey, I.; Ampadu, W. Enhancing Buruli ulcer control in Ghana through social interventions: A case study from the Obom sub-district. BMC Public Health 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Sanyaolu, A.; Okorie, C.; Badaru, O.; Wynveen, E.; White, S.; Wallace, W. Chikungunya epidemiology: A global perspective. SM J. Public Health Epidemiol. 2016, 2, 1028. [Google Scholar]
- Cardona-Ospina, J.A.; Villamil-Gomez, W.E.; Jimenez-Canizales, C.E.; Castaneda-Hernandez, D.M.; Rodrıguez-Morales, A.J. Estimating the burden of disease and the economic cost attributable to chikungunya, Colombia, 2014. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Thiboutot, M.M.; Kannan, S.; Kawalekar, O.U.; Shedlock, D.J.; Khan, A.S.; Sarangan, G.; Srikanth, P.; Weiner, D.B.; Muthumani, K. Chikungunya: A potentially emerging epidemic? PLoS Negl. Trop. Dis. 2010, 4, e623. [Google Scholar] [CrossRef] [PubMed]
- Dhimal, M.; Gautam, I.; Joshi, H.D.; O’Hara, R.B.; Ahrens, B.; Kuch, U. Risk factors for the presence of chikungunya and dengue vectors (Aedes aegypti and Aedes albopictus), their altitudinal distribution and climatic determinants of their abundance in central Nepal. PLoS Negl. Trop. Dis. 2015, 9, e0003545. [Google Scholar] [CrossRef] [PubMed]
- Nakkhara, P.; Chongsuvivatwong, V.; Thammapalo, S. Risk factors for symptomatic and asymptomatic chikungunya infection. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Chikungunya Virus: Symptoms, Diagnosis & Treatment. Available online: https://www.cdc.gov/chikungunya/symptoms/index.html (accessed on 18 July 2017).
- Bern, C. Chagas’ disease. N. Engl. J. Med. 2015, 373, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Shikanai-Yasuda, M.A.; Carvalho, N.B. Oral transmission of Chagas disease. Clin. Infect. Dis. 2012, 54, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Houweling, T.A.J.; Karim-Kos, H.E.; Kulik, M.C.; Stolk, W.A.; Haagsma, J.A.; Lenk, E.J.; Richardus, J.H.; de Vlas, S.J. Socioeconomic inequalities in neglected tropical diseases: A systematic review. PLoS Negl. Trop. Dis. 2016, 10, e0004546. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Health Topics. Available online: http://www.who.int/topics/en/ (accessed on 19 July 2017).
- Murray, N.E.; Quam, M.B.; Wilder-Smith, A. Epidemiology of dengue: Past, present and future prospects. Clin. Epidemiol. 2013, 5, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Dhar-Chowdhury, P.; Paul, K.K.; Haque, C.E.; Hossain, S.; Lindsay, L.R.; Dibernardo, A.; Abdullah, W.; Drebot, M.A. Dengue seroprevalence, seroconversion and risk factors in Dhaka, Bangladesh. PLoS Negl. Trop. Dis. 2017, 11, e0005475. [Google Scholar] [CrossRef] [PubMed]
- Banu, S.; Hu, W.; Hurst, C.; Guo, Y.; Islam, M.Z.; Tong, S. Space-time clusters of dengue fever in Bangladesh. Trop. Med. Int. Health 2012, 17, 1086–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart-Ibarra, A.M.; Muñoz, A.G.; Ryan, S.J.; Ayala, E.B.; Borbor-Cordova, M.J.; Finkelstein, J.L.; Mejía, R.; Ordoñez, T.; Recalde-Coronel, G.C.; Rivero, K. Spatiotemporal clustering, climate periodicity, and social-ecological risk factors for dengue during an outbreak in Machala, Ecuador, in 2010. BMC Infect. Dis. 2014, 14, 610. [Google Scholar] [CrossRef] [PubMed]
- Toan, D.T.; Hoat, L.N.; Hu, W.; Wright, P. Risk factors associated with an outbreak of dengue fever/dengue haemorrhagic fever in Hanoi, Vietnam. Epidemiol. Infect. 2015, 143, 1594–1598. [Google Scholar] [CrossRef] [PubMed]
- Biswas, G.; Sankara, D.P.; Agua-Agum, J.; Maiga, A. Dracunculiasis (guinea worm disease): Eradication without a drug or a vaccine. Philos. Trans. R. Soc. B 2013, 368, 20120146. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, D.; Sankara, D.P. Guinea worm eradication: Progress and challenges—should we beware of the dog? PLoS Negl. Trop. Dis. 2017, 11, e0005495. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, D.R.; Ruiz-Tiben, E.; Eberhard, M.L.; Roy, S.L.; Weiss, A.J. Progress toward global eradication of dracunculiasis—January 2015–June 2016. MMWR 2016, 65, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Al-Awadi, A.R.; Al-Kuhlani, A.; Breman, J.G.; Doumbo, O.; Eberhard, M.L.; Guiguemde, R.T.; Magnussen, P.; Molyneux, D.H.; Nadim, A. Guinea worm (dracunculiasis) eradication: Update on progress and endgame challenges. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 249–251. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dracunculiosis. 2017. Available online: http://www.who.int/dracunculiasis/disease/disease_more/en/ (accessed on 19 July 2017).
- Fe`vre, E.M.; Wissmann, B.V.; Welburn, S.C.; Lutumba, P. The burden of human African trypanosomiasis. PLoS Negl. Trop. Dis. 2008, 2, e333. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, C.S.; Yukich, J.; Goeree, R.; Tediosi, F. A literature review of economic evaluations for a neglected tropical disease: Human African trypanosomiasis (“sleeping sickness”). PLoS Negl. Trop. Dis. 2015, 9, e0003397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malvy, D.; Chappuis, F. Sleeping sickness. Clin. Microbiol. Infect. 2011, 17, 986–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, J.R.; Simarro, P.P.; Diarra, A.; Jannin, J.G. Epidemiology of human African trypanosomiasis. Clin. Epidemiol. 2014, 6, 257–275. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention. Parasite—African trypanosomiasis (also known as sleeping sickness). Available online: https://www.cdc.gov/parasites/sleepingsickness/prevent.html (accessed on 19 July 2017).
- Lumbala, C.; Simarro, P.P.; Cecchi, P.P.; Paone, M.; Franco, J.R.; Betu Ku Mesu, V.K.; Makabuza, J.; Diarra, A.; Chansy, S.; Priotto, G.; et al. Human African trypanosomiasis in the Democratic Republic of the Congo: Disease distribution and risk. Int. J. Health Geogr. 2015, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Mondal, D.; Chowdhury, V.; Faria, S.; Alvar, J.; Nabi, S.G.; Boelaert, M.; Dash, A.P. How far are we from visceral leishmaniasis elimination in Bangladesh? An assessment of epidemiological surveillance data. PLoS Negl. Trop. Dis. 2014, 8, e3020. [Google Scholar] [CrossRef] [PubMed]
- Picado, A.; Ostyn, B.; Singh, S.P.; Uranw, S.; Hasker, E.; Rijal, S.; Boelaert, M.; Chappuis, F. Risk factors for visceral leishmaniasis and asymptomatic Leishmania donovani infection in India and Nepal. PLoS ONE 2014, 9, e87641. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Parasite—Leishmaniasis. 2013. Available online: https://www.cdc.gov/parasites/leishmaniasis/treatment.html (accessed on 19 July 2017).
- World Health Organization. Kala-azar Elimination Programme, Report of a WHO Consultation of Partners, Geneva, Switzerland. 2015. Available online: http://apps.who.int/iris/bitstream/10665/185042/1/9789241509497_eng.pdf (accessed on 11 June 2017).
- Kerr-Pontes, L.; Barreto, M.L.; Evangelista, C.; Rodrigues, L.C.; Heukelbach, J.; Feldmeier, H. Socioeconomic, environmental, and behavioural risk factors for leprosy in Northeast Brazil: Results of a case–control study. Int. J. Epidemiol. 2006, 35, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Reibel, F.; Cambau, E.; Aubry, A. Update on the epidemiology, diagnosis, and treatment of leprosy. Med. Mal. Infect. 2015, 45, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Feenstra, S.G.; Nahar, Q.; Pahan, D.; Oskam, L.; Richardus, J.H. Recent food shortage is associated with leprosy disease in Bangladesh: A case-control study. PLoS Negl. Trop. Dis. 2011, 5, e1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegazy, A.A.; Abdel-Hamid, I.A.; Ahmed, E.-S.F.; Hammad, S.M.; Hawas, S.A. Leprosy in a high-prevalence Egyptian village: Epidemiology and risk factors. Int. J. Dermatol. 2002, 41, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Lymphatic Filariasis: Epidemiology and Risk Factors. 2013. Available online: https://www.cdc.gov/parasites/lymphaticfilariasis/epi.html (accessed on 11 June 2017).
- Obindo, J.; Abdulmalik, J.; Nwefoh, E.; Agbir, M.; Nwoga, C.; Armiya’u, A.; Davou, F.; Maigida, K.; Otache, E.; Ebiloma, A.; et al. Prevalence of depression and associated clinical and socio-demographic factors in people living with lymphatic filariasis in Plateau State, Nigeria. PLoS Negl. Trop. Dis. 2017, 11, e0005567. [Google Scholar] [CrossRef] [PubMed]
- Chesnais, C.B.; Missamou, F.; Pion, S.D.; Bopda, J.; Louya, F.; Majewski, A.C.; Fischer, P.U.; Weil, G.J.; Boussinesq, M.A. A case study of risk factors for lymphatic filariasis in the Republic of Congo. Parasites Vectors 2014, 7, 300. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Parasites—onchocerciasis (also known as river blindness). Epidemiology and risk factors. Available online: https://www.cdc.gov/parasites/onchocerciasis/epi.html (accessed on 19 July 2017).
- Kim, Y.E.; Remme, J.H.F.; Steinmann, P.; Stolk, W.A.; Roungou, J.-B.; Tediosi, F. Control, elimination, and eradication of river blindness: Scenarios, timelines, and ivermectin treatment needs in Africa. PLoS Negl. Trop. Dis. 2015, 9, 0003664. [Google Scholar] [CrossRef] [Green Version]
- Kelly-Hope, L.A.; Unnasch, T.R.; Stanton, M.C.; Molyneux, D.H. Hypo-endemic onchocerciasis hotspots: Defining areas of high risk through micro-mapping and environmental delineation. Infect. Dis. Poverty 2015, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Njim, T.; Aminde, L.N. An appraisal of the neglected tropical disease control program in Cameroon: The case of the national program against onchocerciasis. BMC Public Health 2017, 17, 103. [Google Scholar] [CrossRef] [PubMed]
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar]
- Kakkar, M.; Venkataramanan, V.; Krishnan, S.; Chauhan, R.S.; Abbas, S.S. Moving from rabies research to rabies control: Lessons from India. PLoS Negl. Trop. Dis. 2012, 6, e1748. [Google Scholar] [CrossRef] [PubMed]
- Devleesschauwer, B.; Aryal, A.; Sharma, B.K.; Ale, A.; Declercq, A.; Depraz, S.; Gaire, T.N.; Gongal, G.; Karki, S.; Pandey, B.D. Epidemiology, impact and control of rabies in Nepal: A systematic review. PLoS Negl. Trop. Dis. 2016, 10, e0004461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talukder, K.; Talukder, M.Q.K.; Farooque, M.G.; Khairul, M.; Sharmin, F.; Jerin, I.; Rahman, M.A. Controlling scabies in madrasahs (Islamic religious schools) in Bangladesh. Public Health 2013, 127, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Peterson, G.M.; Walton, S.F.; Carson, C.F.; Naunton, M.; Baby, K.E. Scabies: An ancient global disease with a need for new therapies. BMC Infect Dis 2015, 15, 250. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. The burden of schistosomiasis (schisto, bilharzia, snail fever). 2011. Available online: https://www.cdc.gov/globalhealth/ntd/diseases/schisto_burden.html (accessed on 17 June 2017).
- Knowles, S.C.L.; Webster, B.L.; Garba, A.; Sacko, M.; Diaw, O.T.; Fenwick, A.; Rollinson, D.; Webster, J.P. Epidemiological interactions between urogenital and intestinal human schistosomiasis in the context of praziquantel treatment across three west African countries. PLoS Negl. Trop. Dis. 2015, 9, e0004019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adenowo, A.F.; Oyinloye, B.E.; Ogunyinka, B.I.; Kappo, A.P. Impact of human schistosomiasis in sub-Saharan Africa. Braz. J. Infect. Dis. 2015, 19, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Mazigo, H.D.; Nuwaha, F.; Kinung’hi, S.M.; Morona, D.; de Moira, A.P.; Wilson, S.; Wilson, S.; Heukelbach, J.; Dunne, D.W. Epidemiology and control of human schistosomiasis in Tanzania. Parasites Vectors 2012, 5, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Atlas of Helminth Infection (GAHI). Soil-transmitted helminths. Available online: http://www.thiswormyworld.org/maps/soil-transmitted-helminths (accessed on 14 June 2017).
- Steinbaum, L.; Kwong, L.H.; Ercumen, A.; Negash, M.S.; Lovely, A.J.; Njenga, S.M.; Boehm, A.B.; Pickering, A.J.; Nelson, K.L. Detecting and enumerating soil-transmitted helminth eggs in soil: New method development and results from field testing in Kenya and Bangladesh. PLoS Negl. Trop. Dis. 2017, 11, e0005522. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, P.M.; Montresor, A.; Walson, J.L. Building on the success of soil-transmitted helminth control—The future of deworming. PLoS Negl. Trop. Dis. 2017, 11, e0005497. [Google Scholar] [CrossRef] [PubMed]
- Ramadhani, A.M.; Derrick, T.; Holland, M.J.; Burton, M.J. Blinding trachoma: Systematic review of rates and risk factors for progressive disease. PLoS Negl. Trop. Dis. 2016, 10, e0004859. [Google Scholar] [CrossRef] [PubMed]
- Emerson, P.M.; Hooper, P.J.; Sarah, V. Progress and projections in the program to eliminate trachoma. PLoS Negl. Trop. Dis. 2017, 11, e0005402. [Google Scholar] [CrossRef] [PubMed]
- Muluneh, E.K.; Zewotir, T.; Bekele, Z. Rural children active trachoma risk factors and their interactions. Pan Afr. Med. J. 2016, 24, 128. [Google Scholar] [CrossRef]
- Ngondi, J.; Matthews, F.; Reacher, M.; Onsarigo, A.; Matende, I.; Baba, S.; Brayne, C.; Zingeser, J.; Emerson, P. Prevalence of risk factors and severity of active trachoma in southern Sudan: An ordinal analysis. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 432–438. [Google Scholar] [CrossRef] [PubMed]
- United to Combat. Burden Map—Neglected tropical diseases. Available online: http://unitingtocombatntds.org/resource/burden-map-neglected-tropical-diseases (accessed on 10 June 2017).
- World Health Organization. Health Statistics and Information Systems. Estimates for 2000–2015. Disease Burden. Available online: http://www.who.int/healthinfo/global_burden_disease/estimates/en/index2.html (accessed on 18 June 2017).
- Bhutta, Z.A.; Sommerfeld, J.; Lassi, Z.S.; Salam, R.A.; Das, J.K. Global burden, distribution, and interventions for infectious diseases of poverty. Infect. Dis. Poverty 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.D.; Ezzati, M.; Lopez, A.D. Measuring the burden of neglected tropical diseases: The global burden of disease framework. PLoS Negl. Trop. Dis. 2007, 1, e114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnamoorthy, K.; Harichandrakumar, K.T.; Kumari, A.K.; Das, L.K. Burden of chikungunya in India: Estimates of disability adjusted life years (DALY) lost in 2006 epidemic. J. Vector Borne Dis. 2009, 46, 26–35. [Google Scholar] [PubMed]
| Disease | CDC | WHO |
|---|---|---|
| Buruli ulcer (Mycobacterium ulcerans infection) | + | + |
| Chikungunya a | – | + |
| Chagas disease | + | + |
| Cysticercosis | + | + |
| Dengue fever | + | + |
| Dracunculiosis (or guinea worm disease) b | + | + |
| Echinococcosis | + | + |
| Fascioliasis | + | + |
| Foodborne trematodiasis a | – | + |
| Human African trypanosomiasis (or sleeping sickness) | + | + |
| Leishmaniasis (or kala-azar) | + | + |
| Leprosy | + | + |
| Lymphatic filariasis b | + | + |
| Mycetoma | + | + |
| Onchocerciasis (or river blindness) b | + | + |
| Rabies | + | + |
| Schistosomiasis b | + | + |
| Soil-transmitted helminthiasis b | + | + |
| Trachoma b | + | + |
| Yaws | + | + |
| Disease | 2015 Data a | 2010 Data b | ||
|---|---|---|---|---|
| YLL (thousand) | YLD (thousand) | DALY = YLL + YDL (thousand) | DALY (thousand) b | |
| Soil-transmitted helminthiasis | 449.50 | 3993.97 | 4443.47 | 5043 |
| Ascariasis | 225.30 | 869.37 | 1094.67 | 1254 |
| Trichuriasis c | - | 542.80 | 542.80 | 630 |
| Hookworm c | - | 1739.58 | 1739.58 | 3159 |
| Schistosomiasis | 1042.20 | 2471.65 | 3513.85 | 3971 |
| Dengue fever | 1848.79 | 761.29 | 2610.08 | 1243 |
| Lymphatic filariasis c | - | 2070.85 | 2070.85 | 2740 |
| Cysticercosis | 1258.27 | 598.09 | 1856.36 | 503 |
| Rabies | 1672.03 | 0.14 | 1672.17 | 2297 |
| Leishmaniasis | 1310.74 | 45.72 | 1356.46 | 3754 |
| Onchocerciasis c | - | 1135.57 | 1135.57 | 564 |
| Foodborne trematodiasis | 224.12 | 842.22 | 1066.34 | 665 |
| Echinococcosis | 568.20 | 73.23 | 641.43 | 600 |
| Leprosy | 457.67 | 30.97 | 488.64 | 215 |
| Human African trypanosomiasis | 368.68 | 2.97 | 371.65 | 1346 |
| Trachoma c | - | 278.97 | 278.97 | 308 |
| Chagas disease | 189.65 | 63.05 | 252.70 | 499 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitra, A.K.; Mawson, A.R. Neglected Tropical Diseases: Epidemiology and Global Burden. Trop. Med. Infect. Dis. 2017, 2, 36. https://doi.org/10.3390/tropicalmed2030036
Mitra AK, Mawson AR. Neglected Tropical Diseases: Epidemiology and Global Burden. Tropical Medicine and Infectious Disease. 2017; 2(3):36. https://doi.org/10.3390/tropicalmed2030036
Chicago/Turabian StyleMitra, Amal K., and Anthony R. Mawson. 2017. "Neglected Tropical Diseases: Epidemiology and Global Burden" Tropical Medicine and Infectious Disease 2, no. 3: 36. https://doi.org/10.3390/tropicalmed2030036





