Soil-Transmitted Helminths in Tropical Australia and Asia
Abstract
:1. Introduction
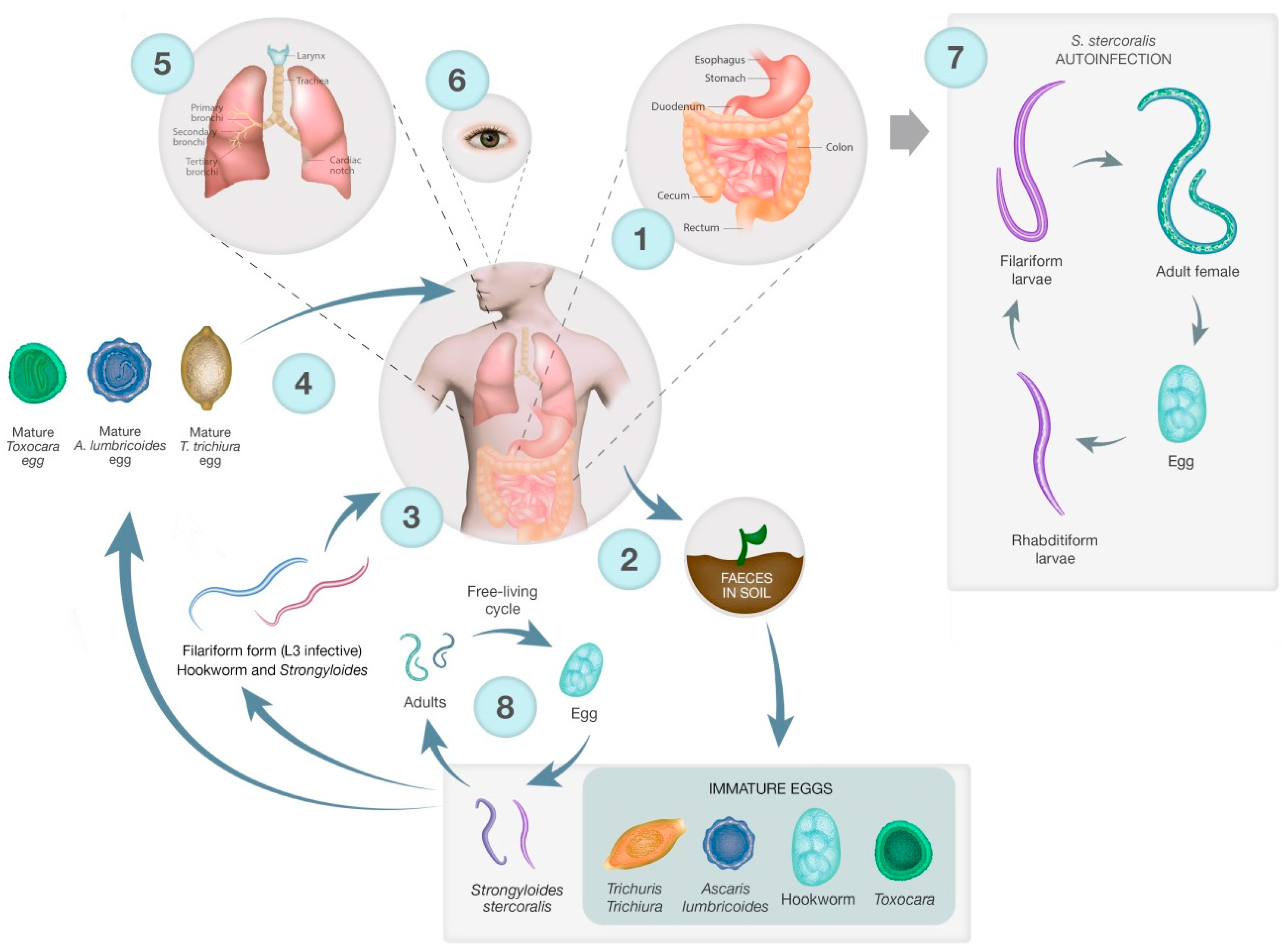
2. Lifecycles
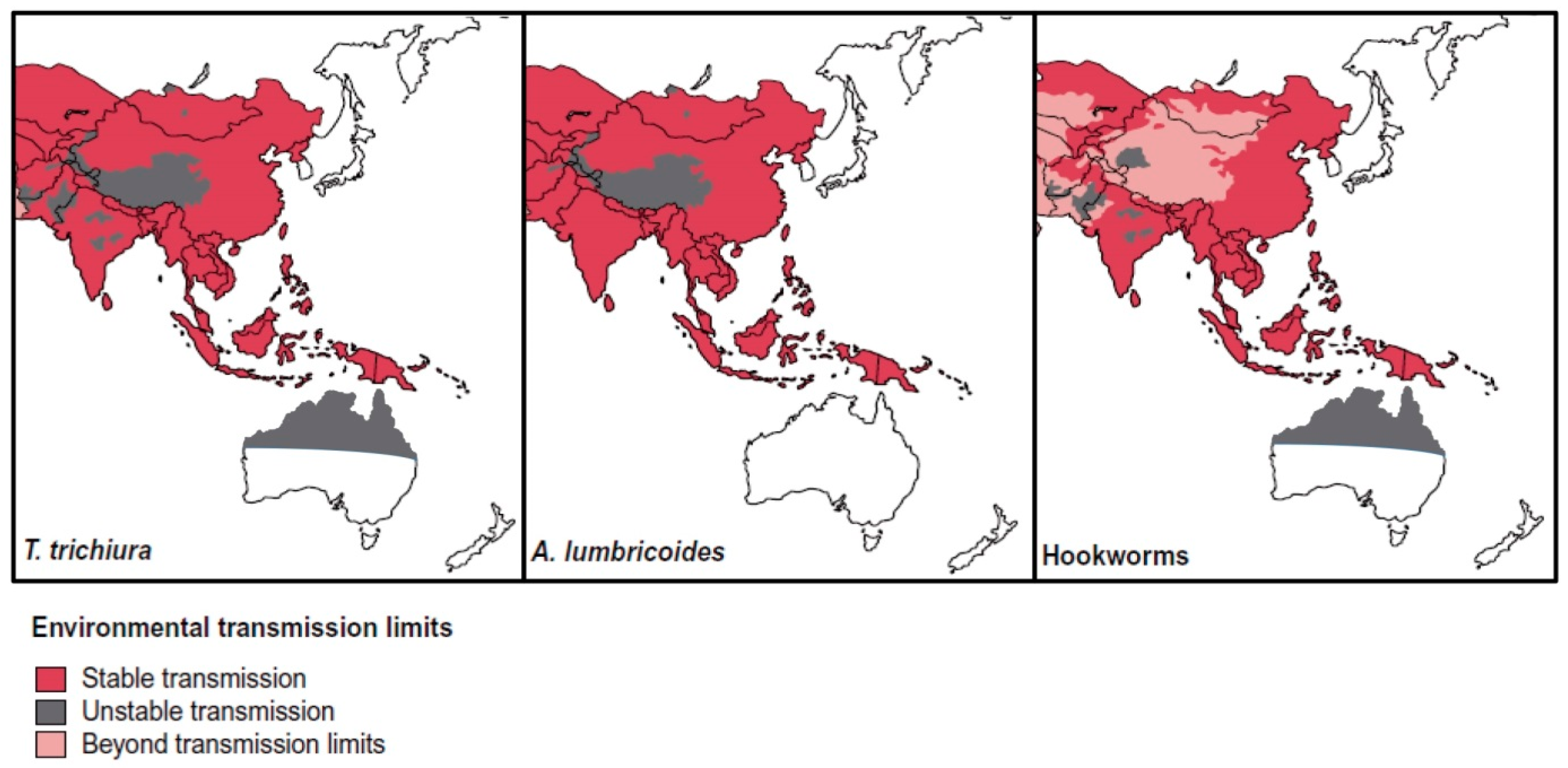
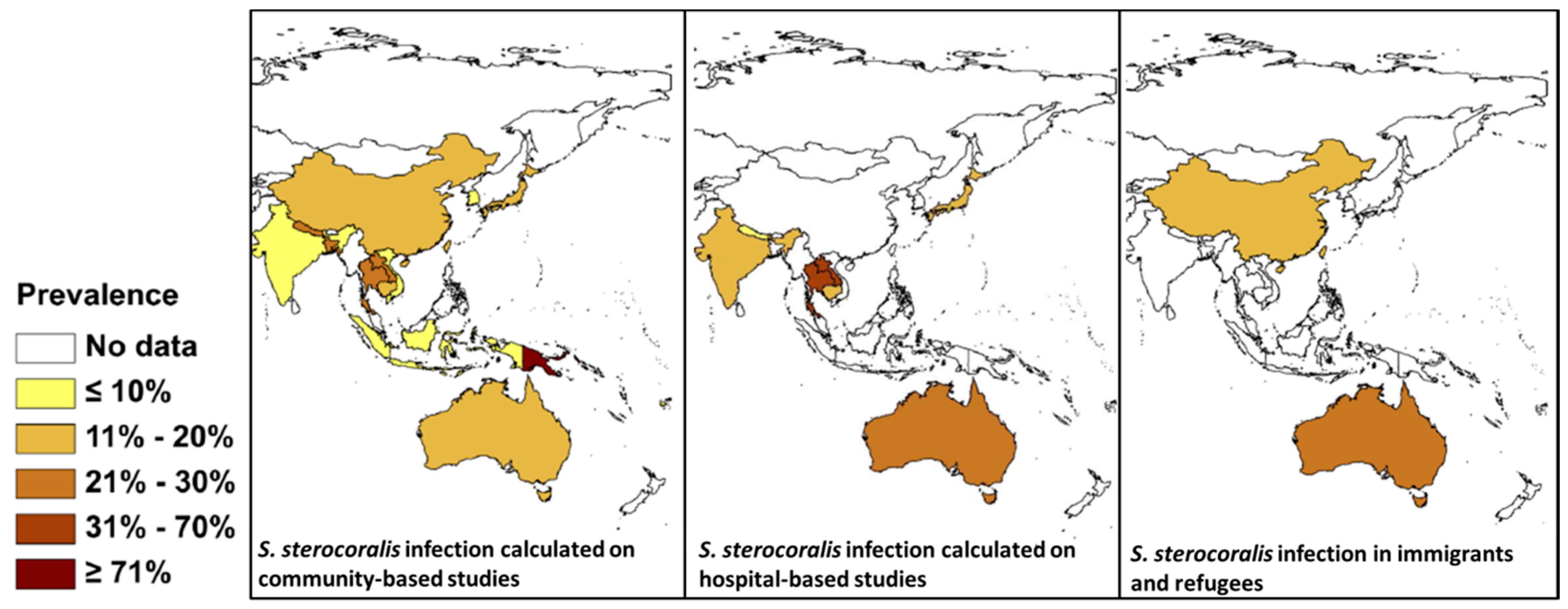
3. STH and Strongyloides in SEA
4. STH and Strongyloides in Australia
5. Immigration Screening in Australia
6. Returned Service Personnel
7. Diagnostics
Microscopy
8. Immunodiagnostics
9. Molecular Diagnostics
10. Costs of Diagnostics
11. Treatment and Mass Drug Administration (MDA) of STH Infections
12. Control Programs
13. Zoonotic Roundworms
Zoonotic Hookworms
14. Toxocara
15. Ascaris suum
16. Trichuris suis and T. vulpis
17. Helminth Therapy
18. Discussion and Conclusion
Author Contributions
Conflicts of Interest
List of Abbreviations
| STH | soil transmitted helminths |
| SEA | South-East Asia |
| DNA | deoxyribonucleic acid |
| qPCR | quantitative real-time polymerase chain reaction |
| cPCR | conventional polymerase chain reaction |
| ddPCR | digital droplet polymerase chain reaction |
| RFLP-PCR | restriction fragment length polymorphism polymerase chain reaction |
| Cox 1 | cytochrome oxidase 1 |
| LAMP | Loop-mediated isothermal amplification |
| GIT | gastrointestinal tract |
| WASH | water, sanitation, and hygiene |
| MDA | mass drug administration |
| NTDs | neglected tropical diseases |
| DALYs | disability adjusted life years |
| YLDs | years lived with disability |
| HTLV-I | human T cell lymphotropic virus type I |
| CR | cure rate |
| IHCP | the integrated helminth control program |
| WHO | World Health Organization |
| IBD | inflammatory bowel disease |
| KK | Kato-Katz |
| ELISA | enzyme linked immunosorbent assay |
| DBS | dried blood spot |
| TOS | Trichuris suis eggs |
References
- Bethony, J.; Brooker, S.; Alboico, M.; Geirger, S.M.; Loukas, A.; Diemart, D.; Hotez, P.J. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet 2006, 367, 1521–1532. [Google Scholar] [CrossRef]
- Utzinger, J.; Keiser, J. Schistosomiasis and soil-transmitted helminthiasis: Common drugs for treatment and control. Expert Opin. Pharmacother. 2004, 5, 263–285. [Google Scholar] [CrossRef] [PubMed]
- WHO; UNICEF. Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Gordon, C.A.; McManus, D.P.; Jones, M.K.; Gray, D.J.; Gobert, G.N. The increase of exotic zoonotic helminth infections: The impact of urbanization, climate change and globalization. Adv. Parasitol. 2016, 91, 311–397. [Google Scholar] [PubMed]
- Areekul, P.; Putaporntip, C.; Pattanawong, U.; Sitthicharoenchai, P.; Jongwutiwes, S. Trichuris vulpis and T. trichiura infections among schoolchildren of a rural community in northwestern Thailand: The possible role of dogs in disease transmission. Asian Biomed. 2010, 4, 49–60. [Google Scholar]
- Arizono, N.; Yoshimura, Y.; Tohzaka, N.; Yamada, M.; Tegoshi, T.; Onishi, K.; Uchikawa, R. Ascariasis in Japan: Is pig-derived Ascaris infecting humans? Jpn. J. Infect. Dis. 2010, 63, 447–448. [Google Scholar] [PubMed]
- Betson, M.; Nejsum, P.; Bendall, R.P.; Deb, R.M.; Stothard, J.R. Molecular epidemiology of ascariasis: A global perspective on the transmission dynamics of Ascaris in people and pigs. J. Infect. Dis. 2014, 210, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Dutto, M.; Petrosillo, N. Hybrid ascaris suum/lumbricoides (ascarididae) infestation in a pig farmer: A rare case of zoonotic ascariasis. Cent. Eur. J. Public Health 2013, 21, 224–226. [Google Scholar] [PubMed]
- Cutillas, C.; Callejon, R.; de Rojas, M.; Tewes, B.; Ubeda, J.M.; Ariza, C.; Guevara, D.C. Trichuris suis and Trichuris trichiura are different nematode species. Acta Trop. 2009, 111, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.; Al-Jubury, A.; Hansen, T.V.; Olsen, A.; Christensen, H.; Thamsborg, S.M.; Nejsum, P. Genetic analysis of Trichuris suis and Trichuris trichiura recovered from humans and pigs in a sympatric setting in Uganda. Vet. Parasitol. 2012, 188, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Ngui, R.; Lim, Y.A.; Traub, R.; Mahmud, R.; Mistam, M.S. Epidemiological and genetic data supporting the transmission of Ancylostoma ceylanicum among human and domestic animals. PLoS Negl. Trop. Dis. 2012, 6, e1522. [Google Scholar] [CrossRef] [PubMed]
- Phosuk, I.; Intapan, P.M.; Thanchomnang, T.; Sanpool, O.; Janwan, P.; Laummaunwai, P.; Aamnart, W.; Morakote, N.; Maleewong, W. Molecular detection of Ancylostoma duodenale, Ancylostoma ceylanicum, and Necator americanus in humans in northeastern and southern Thailand. Korean J. Parasitol. 2013, 51, 747–749. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Geldhof, P.; Albonico, M.; Ame, S.M.; Bethony, J.M.; Engels, D.; Mekonnen, Z.; Montresor, A.; Hem, S.; Tchuem-Tchuente, L.A.; et al. The molecular speciation of soil-transmitted helminth eggs collected from school children across six endemic countries. Trans. R. Soc. Trop. Med. Hyg. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bahgat, M.A.; El Gindy, A.E.; Mahmoud, L.A.; Hegab, M.H.; Shahin, A.M. Evaluation of the role of Ancylostoma caninum in humans as a cause of acute and recurrent abdominal pain. J. Egypt. Soc. Parasitol. 1999, 29, 873–882. [Google Scholar] [PubMed]
- Leles, D.; Gardner, S.L.; Reinhard, K.; Iniguez, A.; Araujo, A. Are Ascaris lumbricoides and Ascaris suum a single species? Parasit. Vectors 2012, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.A.; Berk, S.L. Diagnosis of Strongyloides stercoralis infection. Clin. Infect. Dis. 2001, 33, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Genta, R.M. Global prevalence of strongyloidiasis: Critical review with epidemiologic insights into the prevention of disseminated disease. Rev. Infect. Dis. 1989, 11, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Magnaval, J.-F.; Glickman, L.T.; Dorchies, P.; Morassin, B. Highlights of human toxocariasis. Korean J. Parasitol. 2001, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- WHO. Metrics: Disability-Adjusted Life Year (DALY). Available online: http://www.who.int/healthinfo/global_burden_disease/metrics_daly/en/ (accessed on 6 July 2017).
- Pullan, R.L.; Smith, J.L.; Jasrasaria, R.; Brooker, S.J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit. Vectors 2014, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Murray, C.J.; Barber, R.M.; Foreman, K.J.; Ozgoren, A.A.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Abraham, J.P.; Abubakar, I.; Abu-Raddad, L.J.; et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: Quantifying the epidemiological transition. Lancet 2015. [Google Scholar] [CrossRef]
- Osiro, S.; Hamula, C.; Glaser, A.; Rana, M.; Dunn, D. A case of Strongyloides hyperinfection syndrome in the setting of persistent eosinophilia but negative serology. Diagn. Microbiol. Infect. Dis. 2017, 88, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, K.G. Anaesthesia and Ascaris pneumonia (Loeffler’s syndrome). Indian J. Anaesth. 2015, 59, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Cataño, J.C.; Pinzón, M.A. Strongyloides Pneumonia. Am. J. Trop. Med. Hyg. 2012, 87, 195. [Google Scholar] [CrossRef] [PubMed]
- Cheepsattayakorn, A.; Cheepsattayakorn, R. Parasitic pneumonia and lung involvement. BioMed Res. Int. 2014, 2014, 18. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.K.; Holland, C.V.; Loxton, K.; Barghouth, U. Cerebral toxocariasis: Silent progression to neurodegenerative disorders? Clin. Microbiol. Rev. 2015, 28, 663–686. [Google Scholar] [CrossRef] [PubMed]
- Keiser, P.B.; Nutman, T.B. Strongyloides stercoralis in the immunocompromised population. Clin. Microbiol. Rev. 2004, 17, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Einsiedel, L.; Fernandes, L. Strongyloides stercoralis: A cause of morbidity and mortality for indigenous people in Central Australia. Intern. Med. J. 2008, 38, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Jaleta, T.G.; Zhou, S.; Bemm, F.M.; Schar, F.; Khieu, V.; Muth, S.; Odermatt, P.; Lok, J.B.; Streit, A. Different but overlapping populations of Strongyloides stercoralis in dogs and humans - dogs as a possible source for zoonotic strongyloidiasis. PLoS Negl. Trop. Dis. 2017, 11, e0005752. [Google Scholar] [CrossRef] [PubMed]
- Kouassi, R.Y.; McGraw, S.W.; Yao, P.K.; Abou-Bacar, A.; Brunet, J.; Pesson, B.; Bonfoh, B.; N’Goran, E.K.; Candolfi, E. Diversity and prevalence of gastrointestinal parasites in seven non-human primates of the Tai National Park, Cote d’Ivoire. Parasite 2015, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Thanchomnang, T.; Intapan, P.M.; Sanpool, O.; Rodpai, R.; Tourtip, S.; Yahom, S.; Kullawat, J.; Radomyos, P.; Thammasiri, C.; Maleewong, W. First molecular identification and genetic diversity of Strongyloides stercoralis and Strongyloides fuelleborni in human communities having contact with long-tailed macaques in Thailand. Parasitol. Res. 2017, 116, 1917–1923. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, R.; Yang, G.J.; Knopp, S.; Utzinger, J.; Tanner, M. Surveillance and response: Tools and approaches for the elimination stage of neglected tropical diseases. Acta Trop. 2015, 141, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Nagayasu, E.; Aung, M.; Hortiwakul, T.; Hino, A.; Tanaka, T.; Higashiarakawa, M.; Olia, A.; Taniguchi, T.; Win, S.M.T.; Ohashi, I.; et al. A possible origin population of pathogenic intestinal nematodes, Strongyloides stercoralis, unveiled by molecular phylogeny. Sci. Rep. 2017, 7, 4844. [Google Scholar] [CrossRef] [PubMed]
- Pet Ownership Statistics; Animal Medicines Australia: Barton ACT, Australia, 2016.
- Caruana, S.R.; Kelly, H.A.; Ngeow, J.Y.; Ryan, N.J.; Bennett, C.M.; Chea, L.; Nuon, S.; Bak, N.; Skull, S.A.; Biggs, B.A. Undiagnosed and potentially lethal parasite infections among immigrants and refugees in Australia. J. Travel Med. 2006, 13, 233–239. [Google Scholar] [CrossRef] [PubMed]
- De Silva, S.; Saykao, P.; Kelly, H.; MacIntyre, C.R.; Ryan, N.; Leydon, J.; Biggs, B.A. Chronic Strongyloides stercoralis infection in Laotian immigrants and refugees 7–20 years after resettlement in Australia. Epidemiol. Infect. 2002, 128, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Einsiedel, L.; Spelman, D. Strongyloides stercoralis: Risks posed to immigrant patients in an Australian tertiary referral centre. Intern. Med. J. 2006, 36, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Speare, R.; Bradbury, R.S.; Croese, J. A case of Ancylostoma ceylanicum infection occurring in an Australian soldier returned from Solomon Islands. Korean J. Parasitol. 2016, 54, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, H.; MacFarlane, A.C.; Rowland, K.E.; Einsiedel, L.J.; Neuhaus, S.J. Seroprevalence of Strongyloides stercoralis in a South Australian Vietnam veteran cohort. Aust. N. Z. J. Public Health 2015, 39, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.A.; Speare, R. Strongyloidiasis in personnel of the Regional Assistance Mission to Solomon Islands (RAMSI). Med. J. Aust. 2008, 189, 203–206. [Google Scholar] [PubMed]
- Crowe, A.L.; Smith, P.; Ward, L.; Currie, B.J.; Baird, R. Decreasing prevalence of Trichuris trichiura (whipworm) in the Northern Territory from 2002 to 2012. Med. J. Aust. 2014, 200, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Majumdar, S.S.; Forbes, R.T.; Smith, P.; Currie, B.J.; Baird, R.W. Hookworm in the Northern Territory: Down but not out. Med. J. Aust. 2013, 198, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.J.; Gibney, K.B.; Leder, K.; O’Brien, D.P.; Marshall, C.; Biggs, B.-A. Screening practices for infectious diseases among Burmese refugees in Australia. Emerg. Infect. Dis. 2009, 15, 1769–1772. [Google Scholar] [CrossRef] [PubMed]
- Biggs, B.A.; Caruana, S.; Mihrshahi, S.; Jolley, D.; Leydon, J.; Chea, L.; Nuon, S. Management of chronic strongyloidiasis in immigrants and refugees: Is serologic testing useful? Am. J. Trop. Med. Hyg. 2009, 80, 788–791. [Google Scholar] [PubMed]
- Mounsey, K.; Kearns, T.; Rampton, M.; Llewellyn, S.; King, M.; Holt, D.; Currie, B.J.; Andrews, R.; Nutman, T.; McCarthy, J. Use of dried blood spots to define antibody response to the Strongyloides stercoralis recombinant antigen NIE. Acta Trop. 2014, 138, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Shield, J.; Aland, K.; Kearns, T.; Gongdjalk, G.; Holt, D.; Currie, B.; Prociv, P. Intestinal parasites of children and adults in a remote Aboriginal community of the Northern Territory, Australia, 1994. West. Pac. Surveill. Response J. 2015, 6, 44–51. [Google Scholar] [CrossRef]
- Lim, L.; Biggs, B.A. Fatal disseminated strongyloidiasis in a previously-treated patient. Med. J. Aust. 2001, 174, 355–356. [Google Scholar] [PubMed]
- Koehler, A.V.; Bradbury, R.S.; Stevens, M.A.; Haydon, S.R.; Jex, A.R.; Gasser, R.B. Genetic characterization of selected parasites from people with histories of gastrointestinal disorders using a mutation scanning-coupled approach. Electrophoresis 2013, 34, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Traub, R.J. Ancylostoma ceylanicum, a re-emerging but neglected parasitic zoonosis. Int. J. Parasitol. 2013, 43, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Traub, R.J.; Inpankaew, T.; Sutthikornchai, C.; Sukthana, Y.; Thompson, R.C.A. PCR-based coprodiagnostic tools reveal dogs as reservoirs of zoonotic ancylostomiasis caused by Ancylostoma ceylanicum in temple communities in Bangkok. Vet. Parasitol. 2008, 155, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Schär, F.; Inpankaew, T.; Traub, R.J.; Khieu, V.; Dalsgaard, A.; Chimnoi, W.; Chhoun, C.; Sok, D.; Marti, H.; Muth, S.; et al. The prevalence and diversity of intestinal parasitic infections in humans and domestic animals in a rural Cambodian village. Parasitol. Int. 2014, 63, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, R.; Traub, R.J. Hookworm Infection in Oceania. In Neglected Tropical Diseases—Oceania; Loukas, A., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 33–68. [Google Scholar]
- Ngui, R.; Mahdy, M.A.; Chua, K.H.; Traub, R.; Lim, Y.A. Genetic characterization of the partial mitochondrial cytochrome oxidase c subunit I (cox 1) gene of the zoonotic parasitic nematode, Ancylostoma ceylanicum from humans, dogs and cats. Acta Trop. 2013, 128, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Hookworm biology. Available online: https://www.cdc.gov/parasites/hookworm/biology.html (accessed on 18 August 2017).
- Whipworm biology. Available online: https://www.cdc.gov/parasites/whipworm/biology.html (accessed on 18 August 2017).
- Ascariasis biology. Available online: https://www.cdc.gov/parasites/ascariasis/biology.html (accessed on 18 August 2017).
- Strongyloidiasis biology. Available online: https://www.cdc.gov/dpdx/strongyloidiasis/index.html (accessed on 18 August 2017).
- Yamada, M.; Matsuda, S.; Nakazawa, M.; Arizono, N. Species-specific differences in heterogonic development of serially transferred free-living generations of Strongyloides planiceps and Strongyloides stercoralis. J. Parasitol. 1991, 77, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Viney, M.E.; Lok, J.B. The biology of Strongyloides spp. WormBook Online Rev. C Elegans Biol. 2015, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Toxocariasis biology. Available online: https://www.cdc.gov/parasites/toxocariasis/biology.html (accessed on 18 August 2017).
- Quattrocchi, G.; Nicoletti, A.; Marin, B.; Bruno, E.; Druet-Cabanac, M.; Preux, P.M. Toxocariasis and epilepsy: Systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2012, 6, e1775. [Google Scholar] [CrossRef] [PubMed]
- Jex, A.R.; Lim, Y.A.; Bethony, J.M.; Hotez, P.J.; Young, N.D.; Gasser, R.B. Soil-transmitted helminths of humans in Southeast Asia - towards integrated control. Adv. Parasitol. 2011, 74, 231–265. [Google Scholar] [PubMed]
- Brooker, S.J.; Bundy, D.A.P. 55—Soil-transmitted Helminths (Geohelminths). In Manson’s Tropical Infectious Diseases (Twenty-Third Edition); Farrar, J., Hotez, P.J., Junghanss, T., Kang, G., Lalloo, D., White, N.J., Eds.; W.B. Saunders: London, UK, 2014. [Google Scholar]
- De Silva, N.R.; Brooker, S.; Hotez, P.J.; Montresor, A.; Engels, D.; Savioli, L. Soil-transmitted helminth infections: Updating the global picture. Trends Parasitol. 2003, 19, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.A.; McManus, D.P.; Acosta, L.P.; Olveda, R.; Williams, M.; Ross, A.G.; Gray, D.J.; Gobert, G.N. Multiplex real-time PCR monitoring of intestinal helminths in humans reveals widespread polyparasitism in Northern Samar, the Philippines. Int. J. Parasitol. 2015, 45, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Laymanivong, S.; Hangvanthong, B.; Keokhamphavanh, B.; Phommasansak, M.; Phinmaland, B.; Sanpool, O.; Maleewong, W.; Intapan, P.M. Current status of human hookworm infections, ascariasis, trichuriasis, schistosomiasis mekongi and other trematodiases in Lao People’s Democratic Republic. Am. J. Trop. Med. Hyg. 2014, 90, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Rim, H.-J.; Chai, J.-Y.; Min, D.-Y.; Cho, S.; Eom, K.S.; Hong, S.; Sohn, W.; Yong, T.; Deodato, G.; Standgaard, H.; et al. Prevalence of intestinal parasite infections on a national scale among primary schoolchildren in Laos. Parasitol. Res. 2003, 91, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Conlan, J.V.; Khamlome, B.; Vongxay, K.; Elliot, A.; Pallant, L.; Sripa, B.; Blacksell, S.D.; Fenwick, S.; Thompson, R.C. Soil-transmitted helminthiasis in Laos: A community-wide cross-sectional study of humans and dogs in a mass drug administration environment. Am. J. Trop. Med. Hyg. 2012, 86, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Meurs, L.; Polderman, A.M.; Vinkeles Melchers, N.V.; Brienen, E.A.; Verweij, J.J.; Groosjohan, B.; Mendes, F.; Mechendura, M.; Hepp, D.H.; Langenberg, M.C.; et al. Diagnosing polyparasitism in a high-prevalence setting in Beira, Mozambique: Detection of intestinal parasites in fecal samples by microscopy and real-time PCR. PLoS Negl. Trop. Dis. 2017, 11, e0005310. [Google Scholar] [CrossRef] [PubMed]
- Elyana, F.N.; Al-Mekhlafi, H.M.; Ithoi, I.; Abdulsalam, A.M.; Dawaki, S.; Nasr, N.A.; Atroosh, W.M.; Abd-Basher, M.H.; Al-Areeqi, M.A.; Sady, H.; et al. A tale of two communities: Intestinal polyparasitism among Orang Asli and Malay communities in rural Terengganu, Malaysia. Parasit. Vectors 2016, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Fleming, F.M.; Brooker, S.; Geiger, S.M.; Caldas, I.R.; Correa-Oliveira, R.; Hotez, P.J.; Bethony, J.M. Synergistic associations between hookworm and other helminth species in a rural community in Brazil. Trop. Med. Int. Health 2006, 11, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.S.; Rodriguez, A.; Chico, M.; Guadalupe, I.; Broncano, N.; Sandoval, C.; Tupiza, F.; Mitre, E.; Cooper, P.J. Maternal geohelminth infections are associated with an increased susceptibility to geohelminth infection in children: A case-control study. PLoS Negl. Trop. Dis. 2012, 6, e1753. [Google Scholar] [CrossRef] [PubMed]
- Menzies, S.K.; Rodriguez, A.; Chico, M.; Sandoval, C.; Broncano, N.; Guadalupe, I.; Cooper, P.J. Risk Factors for soil-transmitted helminth infections during the first 3 years of life in the tropics; findings from a birth cohort. PLoS Negl. Trop. Dis. 2014, 8, e2718. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.; van Lieshout, L.; Marti, H.; Polderman, T.; Polman, K.; Steinmann, P.; Stothard, R.; Thybo, S.; Verweij, J.J.; Magnussen, P. Strongyloidiasis--the most neglected of the neglected tropical diseases? Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Ngui, R.; Halim, N.A.; Rajoo, Y.; Lim, Y.A.; Ambu, S.; Rajoo, K.; Chang, T.S.; Woon, L.C.; Mahmud, R. Epidemiological characteristics of strongyloidiasis in inhabitants of indigenous communities in Borneo island, Malaysia. Korean J. Parasitol. 2016, 54, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Requena-Mendez, A.; Chiodini, P.; Bisoffi, Z.; Buonfrate, D.; Gotuzzo, E.; Munoz, J. The laboratory diagnosis and follow up of strongyloidiasis: A systematic review. PLoS Negl. Trop. Dis. 2013, 7, e2002. [Google Scholar] [CrossRef] [PubMed]
- Verweij, J.J.; Canales, M.; Polman, K.; Ziem, J.; Brienen, E.A.T.; Polderman, A.M.; van Lieshout, L. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Intapan, P.M.; Maleewong, W.; Wongsaroj, T.; Singthong, S.; Morakote, N. Comparison of the quantitative formalin ethyl acetate concentration technique and agar plate culture for diagnosis of human strongyloidiasis. J. Clin. Microbiol. 2005, 43, 1932–1933. [Google Scholar] [CrossRef] [PubMed]
- Boonjaraspinyo, S.; Boonmars, T.; Kaewsamut, B.; Ekobol, N.; Laummaunwai, P.; Aukkanimart, R.; Wonkchalee, N.; Juasook, A.; Sriraj, P. A cross-sectional study on intestinal parasitic infections in rural communities, northeast Thailand. Korean J. Parasitol. 2013, 51, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Kling, K.; Kuenzli, E.; Blum, J.; Neumayr, A. Acute strongyloidiasis in a traveller returning from South East Asia. Travel Med. Infect. Dis. 2016, 14, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Cheong, F.W.; Liew, J.W.; Lau, Y.L. Seroprevalence of fascioliasis, toxocariasis, strongyloidiasis and cysticercosis in blood samples diagnosed in Medic Medical Center Laboratory, Ho Chi Minh City, Vietnam in 2012. Parasit. Vectors 2016, 9, 486. [Google Scholar] [CrossRef] [PubMed]
- Nontasut, P.; Muennoo, C.; Sa-nguankiat, S.; Fongsri, S.; Vichit, A. Prevalence of Strongyloides in Northern Thailand and treatment with ivermectin vs albendazole. Southeast Asian J. Trop. Med. Public Health 2005, 36, 442–444. [Google Scholar] [PubMed]
- Schär, F.; Trostdorf, U.; Giardina, F.; Khieu, V.; Muth, S.; Marti, H.; Vounatsou, P.; Odermatt, P. Strongyloides stercoralis: Global distribution and risk factors. PLoS Negl. Trop. Dis. 2013, 7, e2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smout, F.A.; Skerratt, L.F.; Butler, J.R.A.; Johnson, C.N.; Congdon, B.C.; Thompson, R.C.A. The hookworm Ancylostoma ceylanicum: An emerging public health risk in Australian tropical rainforests and Indigenous communities. One Health (Amsterdam, Netherlands) 2017, 3, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Smout, F.A.; Thompson, R.C.A.; Skerratt, L.F. First report of Ancylostoma ceylanicum in wild canids. Int. J. Parasitol. Parasites Wildl. 2013, 2, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Lee, A. Internal parasites of pigs. Available online: https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0019/433018/internal-parasites-of-pigs.pdf (accessed on 4 July 2017).
- Prociv, P.; Luke, R.A. The changing epidemiology of human hookworm infection in Australia. Med. J. Aust. 1995, 162, 150–154. [Google Scholar] [PubMed]
- Health, V. Ascariasis (roundworm infection). Available online: https://www2.health.vic.gov.au/public-health/infectious-diseases/disease-information-advice/ascariasis-roundworm-infection (accessed on 27 July 2017).
- Johnston, F.H.; Morris, P.S.; Speare, R.; McCarthy, J.; Currie, B.; Ewald, D.; Page, W.; Dempsey, K. Strongyloidiasis: A review of the evidence for Australian practitioners. Aust. J. Rural Health 2005, 13, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.I. Intestinal parasite infections in Western Australian Aborigines. Med. J. Aust. 1980, 2, 375–380. [Google Scholar] [PubMed]
- Prociv, P.; Luke, R. Observations on strongyloidiasis in Queensland aboriginal communities. Med. J. Aust. 1993, 158, 160–163. [Google Scholar] [PubMed]
- Speare, R.; Miller, A.; Page, W.A. Strongyloidiasis: A case for notification in Australia? Med. J. Aust. 2015, 202, 523–524. [Google Scholar] [CrossRef] [PubMed]
- Page, W.; Speare, R. Chronic strongyloidiasis—Don’t look and you won’t find. Aust. Fam. Physician 2016, 45, 40–44. [Google Scholar] [PubMed]
- Hsu, Y.; Lin, J. Intestinal Infestation with Ancylostoma ceylanicum. N. Engl. J. Med. 2012, 366. [Google Scholar] [CrossRef] [PubMed]
- Inpankaew, T.; Schar, F.; Dalsgaard, A.; Khieu, V.; Chimnoi, W.; Chhoun, C.; Sok, D.; Marti, H.; Muth, S.; Odermatt, P.; et al. High prevalence of Ancylostoma ceylanicum hookworm infections in humans, Cambodia, 2012. Emerg. Infect. Dis. 2014, 20, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, G.; Alsarakibi, M. The zoonotic risk of Ancylostoma ceylanicum isolated from stray dogs and cats in Guangzhou, South China. BioMed Res. Int. 2014, 2014, 208759. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, M.A.; Lim, Y.A.; Ngui, R.; Siti Fatimah, M.R.; Choy, S.H.; Yap, N.J.; Al-Mekhlafi, H.M.; Ibrahim, J.; Surin, J. Prevalence and zoonotic potential of canine hookworms in Malaysia. Parasit. Vectors 2012, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zain, S.N.; Sahimin, N.; Pal, P.; Lewis, J.W. Macroparasite communities in stray cat populations from urban cities in Peninsular Malaysia. Vet. Parasitol. 2013, 196, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Ngui, R.; Lim, Y.A.; Chua, K.H. Rapid detection and identification of human hookworm infections through high resolution melting (HRM) analysis. PLoS ONE 2012, 7, e41996. [Google Scholar] [CrossRef] [PubMed]
- Ngui, R.; Lim, Y.A.; Ismail, W.H.; Lim, K.N.; Mahmud, R. Zoonotic Ancylostoma ceylanicum infection detected by endoscopy. Am. J. Trop. Med. Hyg. 2014, 91, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Pa Pa Aung, W.; Htoon, T.T.; Tin, H.H.; Sanpool, O.; Jongthawin, J.; Sadaow, L.; Phosuk, I.; Ropai, R.; Intapan, P.M.; Maleewong, W. First molecular identifications of Necator americanus and Ancylostoma ceylanicum infecting rural communities in lower Myanmar. Am. J. Trop. Med. Hyg. 2017, 96, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.S.; Traub, R.J.; Robertson, I.D.; Hobbs, R.P.; Elliot, A.; While, L.; Rees, R.; Thompson, R.C. The veterinary and public health significance of hookworm in dogs and cats in Australia and the status of A. ceylanicum. Vet. Parasitol. 2007, 145, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Traub, R.J.; Pednekar, R.P.; Cuttell, L.; Porter, R.B.; Abd Megat Rani, P.A.; Gatne, M.L. The prevalence and distribution of gastrointestinal parasites of stray and refuge dogs in four locations in India. Vet. Parasitol. 2014, 205, 233–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traub, R.J.; Robertson, I.D.; Irwin, P.; Mencke, N.; Thompson, R.C. Application of a species-specific PCR-RFLP to identify Ancylostoma eggs directly from canine faeces. Vet. Parasitol. 2004, 123, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Tun, S.; Ithoi, I.; Mahmud, R.; Samsudin, N.I.; Kek Heng, C.; Ling, L.Y. Detection of helminth eggs and identification of hookworm species in stray cats, dogs and soil from Klang Valley, Malaysia. PLoS ONE 2015, 10, e0142231. [Google Scholar] [CrossRef] [PubMed]
- Kaya, D.; Yoshikawa, M.; Nakatani, T.; Tomo-Oka, F.; Fujimoto, Y.; Ishida, K.; Fujinaga, Y.; Aihara, Y.; Nagamatsu, S.; Matsuo, E.; et al. Ancylostoma ceylanicum hookworm infection in Japanese traveler who presented chronic diarrhea after return from Lao People’s Democratic Republic. Parasitol. Int. 2016, 65, 737–740. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Levecke, B.; Kattula, D.; Velusamy, V.; Roy, S.; Geldhof, P.; Sarkar, R.; Kang, G. Molecular identification of hookworm isolates in humans, dogs and soil in a tribal area in Tamil Nadu, India. PLoS Negl. Trop. Dis. 2016, 10, e0004891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pumidonming, W.; Salman, D.; Gronsang, D.; Abdelbaset, A.E.; Sangkaeo, K.; Kawazu, S.I.; Igarashi, M. Prevalence of gastrointestinal helminth parasites of zoonotic significance in dogs and cats in lower Northern Thailand. J. Vet. Med. Sci. 2017, 78, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.; Lemoine, J.P.; Lefebvre, N.; Denis, J.; Pfaff, A.W.; Abou-Bacar, A.; Traub, R.J.; Pesson, B.; Candolfi, E. Bloody diarrhea associated with hookworm infection in traveler returning to France from Myanmar. Emerg. Infect. Dis. 2015, 21, 1878–1879. [Google Scholar] [CrossRef] [PubMed]
- Ng-Nguyen, D.; Hii, S.F.; Nguyen, V.A.; Van Nguyen, T.; Van Nguyen, D.; Traub, R.J. Re-evaluation of the species of hookworms infecting dogs in Central Vietnam. Parasit. Vectors 2015, 8, 401. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Yu, X.G.; Wu, S.; Tan, L.P.; Song, M.R.; Abdulahi, A.Y.; Wang, Z.; Jiang, B.; Li, G.Q. Levels of Ancylostoma infections and phylogenetic analysis of cox 1 gene of A. ceylanicum in stray cat faecal samples from Guangzhou, China. J. Helminthol. 2016, 90, 392–397. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Kaliappan, S.P.; Kattula, D.; Roy, S.; Geldhof, P.; Kang, G.; Vercruysse, J.; Levecke, B. Identification of Ancylostoma ceylanicum in children from a tribal community in Tamil Nadu, India using a semi-nested PCR-RFLP tool. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Jiraanankul, V.; Aphijirawat, W.; Mungthin, M.; Khositnithikul, R.; Rangsin, R.; Traub, R.J.; Piyaraj, P.; Naaglor, T.; Taamasri, P.; Leelayoova, S. Incidence and risk factors of hookworm infection in a rural community of central Thailand. Am. J. Trop. Med. Hyg. 2011, 84, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Sanguankiat, S.; Yoonuan, T.; Pongvongsa, T.; Keomoungkhoun, M.; Phimmayoi, I.; Boupa, B.; Moji, K.; Waikagul, J. Copro-molecular identification of infections with hookworm eggs in rural Lao PDR. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Scholz, T.; Uhlirova, M.; Ditrich, O. Helminth parasites of cats from the Vientiane province, Laos, as indicators of the occurrence of causative agents of human parasitoses. Parasite 2003, 10, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.S.; Lin, C.K.; Su, K.E.; Liu, C.Y.; Lin, C.C.; Liang, C.C.; Lee, T.H. Diagnosis of Ancylostoma ceylanicum infestation by single-balloon enteroscopy (with video). Gastrointest. Endosc. 2012, 76, 671–672. [Google Scholar] [CrossRef] [PubMed]
- Einsiedel, L.J.; Pham, H.; Woodman, R.J.; Pepperill, C.; Taylor, K.A. The prevalence and clinical associations of HTLV-1 infection in a remote Indigenous community. Med. J. Aust. 2016, 205, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Einsiedel, L.; Woodman, R.J.; Flynn, M.; Wilson, K.; Cassar, O.; Gessain, A. Human T-Lymphotropic Virus type 1 infection in an Indigenous Australian population: Epidemiological insights from a hospital-based cohort study. BMC Public Health 2016, 16, 787. [Google Scholar] [CrossRef] [PubMed]
- Al Maslamani, M.A.; Al Soub, H.A.; Al Khal, A.L.M.; Al Bozom, I.A.; Abu Khattab, M.J.; Chacko, K.C. Strongyloides stercoralis hyperinfection after corticosteroid therapy: A report of two cases. Ann. Saudi Med. 2009, 29, 397–401. [Google Scholar] [PubMed]
- Koticha, A.; Kuyare, S.; Nair, J.; Athvale, A.; Mehta, P. Strongyloides stercoralis hyperinfection syndrome in patients on prolonged steroid treatment: Two case reports. J. Indian Med. Assoc. 2013, 111, 272–274. [Google Scholar] [PubMed]
- Aru, R.G.; Chilcutt, B.M.; Butt, S.; deShazo, R.D. Novel findings in HIV, immune reconstitution disease and Strongyloides stercoralis Infection. Am. J. Med. Sci. 2017, 353, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Llenas-Garcia, J.; Fiorante, S.; Salto, E.; Maseda, D.; Rodriguez, V.; Matarranz, M.; Hernando, A.; Rubio, R.; Pulido, F. Should we look for Strongyloides stercoralis in foreign-born HIV-infected persons? J. Immigr. Minor. Health 2013, 15, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Mobley, C.M.; Dhala, A.; Ghobrial, R.M. Strongyloides stercoralis in solid organ transplantation: Early diagnosis gets the worm. Curr. Opin. Organ Transplant. 2017, 22, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Medicinewise, N. Albendazole (Zentel) listing extended to treat hookworm and strongyloidiasis. Available online: https://www.nps.org.au/radar/articles/albendazole-zentel-listing-extended-to-treat-hookworm-and-strongyloidiasis (accessed on 4 July 2017).
- Refugee health services. Available online: https://www.health.qld.gov.au/public-health/groups/multicultural/refugee-services (accessed on 27 September 2017).
- Refugee and asylum seeker health and wellbeing. Available online: https://www2.health.vic.gov.au/about/populations/refugee-asylum-seeker-health (accessed on 27 September 2017).
- NSW refugee health service. Available online: https://www.swslhd.health.nsw.gov.au/refugee/ (accessed on 27 September 2017).
- Humanitarian entrant health service. Available online: http://ww2.health.wa.gov.au/Articles/F_I/Humanitarian-Entrant-Health-Service (accessed on 27 September 2017).
- Refugee Health Program. Available online: https://www.ntphn.org.au/refugee-health (accessed on 27 September 2017).
- Statistics, A.B.O. Migration, Australia, 2015–2016. Available online: http://www.abs.gov.au/ausstats/[email protected]/mf/3412.0 (accessed on 4 July 2017).
- Bradbury, R.S.; Hii, S.F.; Harrington, H.; Speare, R.; Traub, R. Ancylostoma ceylanicum hookworm in the Solomon Islands. Emerg. Infect. Dis. 2017, 23, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Hanieh, S.; Ryan, N.; Biggs, B. Assessing enteric helminths in refugees, asylum seekers and new migrants. Microbiol. Aust. 2016, 15–19. [Google Scholar] [CrossRef]
- Mayer-Coverdale, J.K.; Crowe, A.; Smith, P.; Baird, R.W. Trends in Strongyloides stercoralis faecal larvae detections in the Northern Territory, Australia: 2002 to 2012. Trop. Med. Infect. Dis. 2017, 2. [Google Scholar] [CrossRef]
- Montes, M.; Sawhney, C.; Barros, N. Strongyloides stercoralis: There but not seen. Curr. Opin. Infect. Dis. 2010, 23, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Knopp, S.; Salim, N.; Schindler, T.; Voules, D.A.K.; Rothen, J.; Lweno, O.; Mohammed, A.S.; Singo, R.; Benninghoff, M.; Nsojo, A.A.; et al. Diagnostic accuracy of Kato-Katz, FLOTAC, Baermann, and PCR methods for the detection of light-intensity hookworm and Strongyloides stercoralis infections in Tanzania. Am. J. Trop. Med. Hyg. 2014, 90, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Knopp, S.; Speich, B.; Hattendorf, J.; Rinaldi, L.; Mohammed, K.A.; Khamis, I.S.; Mohammed, A.S.; Albonico, M.; Rollinson, D.; Marti, H.; et al. Diagnostic accuracy of Kato-Katz and FLOTAC for assessing anthelmintic drug efficacy. PLoS Negl. Trop. Dis. 2011, 5, e1036. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.-D.; Liu, J.-X.; Liu, Y.-M.; Hu, F.; Zhang, Y.-Y.; Xu, J.-M.; Li, J.-Y.; Ji, M.-J.; Bergquist, R.; Wu, G.-L.; et al. Routine Kato-Katz technique underestimates the prevalence of Schistosoma japonicum: A case study in an endemic area of the People’s Republic of China. Parasitol. Int. 2008, 57, 281–286. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.S.; Lustigman, S.; Yang, G.-J.; Barakat, R.M.; García, H.H.; Sripa, B.; Willingham, A.L.; Prichard, R.K.; Basáñez, M.-G. A researcha genda for helminth diseases of humans: Diagnostics for control and elimination programmes. PLoS Negl. Trop. Dis. 2012, 6, e1601. [Google Scholar] [CrossRef] [PubMed]
- Dacombe, R.J.; Crampin, A.C.; Floyd, S.; Randall, A.; Ndhlovu, R.; Bickle, Q.; Fine, P.E.M. Time delays between patient and laboratory selectively affect accuracy of helminth diagnosis. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Cringoli, G. FLOTAC, a novel apparatus for a multivalent faecal egg count technique. Parassitologica 2006, 48, 381–384. [Google Scholar]
- Glinz, D.; Silué, K.D.; Knopp, S.; Lohouringnon, L.K.; Yao, K.P.; Steinmann, P.; Rinaldi, L.; Cringoli, G.; N’Goran, E.K.; Utzinger, J. Comparing diagnostic accuraccy of Kato-Katz, koga agar plate, ether-concentration, and FLOTAC for Schistosoma mansoni and soli-transmitted helminths. PLoS Negl. Trop. Dis. 2010, 4, e754. [Google Scholar] [CrossRef] [PubMed]
- Habtamu, K.; Degarege, A.; Ye-Ebiyo, Y.; Erko, B. Comparison of the Kato-Katz and FLOTAC techniques for the diagnosis of soil-transmitted helminth infections. Parasitol. Int. 2011, 60, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Knopp, S.; Rinaldi, R.; Khamis, I.S.; Stothard, J.R.; Rollinson, D.; Maurelli, M.P.; Steinmann, P.; Marti, H.; Cringoli, G.; Utzinger, J. A single FLOTAC is more sensitive than triplicate Kato-Katz for the diagnosis of low intensity soil-transmitted helminth infections. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Speich, B.; Knopp, S.; Mohammed, A.K.; Khamis, I.S.; Rinaldi, L.; Cringoli, G.; Rollinson, D.; Utzinger, J. Comparative cost assessment of the Kato-Katz and FLOTAC techniques for soil-transmitted helminth diagnosis in epidemiological surveys. Parasit. Vectors 2010, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buonfrate, D.; Formenti, F.; Perandin, F.; Bisoffi, Z. Novel approaches to the diagnosis of Strongyloides stercoralis infection. Clin. Microbiol. Infect. 2015, 21, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Buonfrate, D.; Perandin, F.; Formenti, F.; Bisoffi, Z. A retrospective study comparing agar plate culture, indirect immunofluorescence and real-time PCR for the diagnosis of Strongyloides stercoralis infection. Parasitology 2017, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, H.R.; Koelewijn, R.; Hofwegen, H.; Gilis, H.; Wetsteyn, J.C.; Wismans, P.J.; Sarfati, C.; Vervoort, T.; van Gool, T. Use of enzyme-linked immunosorbent assay and dipstick assay for detection of Strongyloides stercoralis infection in humans. J. Clin. Microbiol. 2007, 45, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.W.; Klein, D.M.; Dornink, S.M.; Jespersen, D.J.; Kubofcik, J.; Nutman, T.B.; Merrigan, S.D.; Couturier, M.R.; Theel, E.S. Comparison of three immunoassays for detection of antibodies to Strongyloides stercoralis. Clin. Vaccine Immunol. CVI 2014, 21, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.A.; Gray, D.J.; Gobert, G.N.; McManus, D.P. DNA amplification approaches for the diagnosis of key parasitic helminth infections of humans. Mol. Cell. Probes 2011, 25, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, T.; Furushima-Shimogawara, R.; Ohmae, H.; Wang, T.P.; Lu, S.; Chen, R.; Wen, L.; Ohta, N. Detection of early and single infections of Schistosoma japonicum in the intermediate host snail, Oncomelania hupensis, by PCR and loop-mediated isothermal amplification (LAMP) assay. Am. J. Trop. Med. Hyg. 2010, 83, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Mugambi, R.M.; Agola, E.L.; Mwangi, I.N.; Kinyua, J.; Shiraho, E.A.; Mkoji, G.M. Development and evaluation of a loop mediated isothermal amplification (LAMP) technique for the detection of hookworm (Necator americanus) infection in fecal samples. Parasit. Vectors 2015, 8, 574. [Google Scholar] [CrossRef] [PubMed]
- Weerakoon, K.G.; Gordon, C.A.; Cai, P.; Gobert, G.N.; Duke, M.; Williams, G.M.; McManus, D.P. A novel duplex ddPCR assay for the diagnosis of schistosomiasis japonica: Proof of concept in an experimental mouse model. Parasitology 2017, 144, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Weerakoon, K.G.; Gordon, C.A.; Gobert, G.N.; Cai, P.; McManus, D.P. Optimisation of a droplet digital PCR assay for the diagnosis of Schistosoma japonicum infection: A duplex approach with DNA binding dye chemistry. J. Microbiol. Methods 2016, 125, 19–27. [Google Scholar] [CrossRef] [PubMed]
- WHO. KITS; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- Pilotte, N.; Papaiakovou, M.; Grant, J.R.; Bierwert, L.A.; Llewellyn, S.; McCarthy, J.S.; Williams, S.A. Improved PCR-based detection of soil transmitted helminth infections using a next-generation sequencing approach to assay design. PLoS Negl. Trop. Dis. 2016, 10, e0004578. [Google Scholar] [CrossRef] [PubMed]
- Belizario, V.Y.; Totanes, F.I.; de Leon, W.U.; Matias, K.M. School-based control of soil-transmitted helminthiasis in western Visayas, Philippines. Southeast Asian J. Trop. Med. Public Health 2014, 45, 556–567. [Google Scholar] [PubMed]
- Insetta, E.R.; Soriano, A.J.; Totanes, F.I.; Macatangay, B.J.; Belizario, V.Y., Jr. Fear of birth defects is a major barrier to soil-transmitted helminth treatment (STH) for pregnant women in the Philippines. PLoS ONE 2014, 9, e85992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, A.; Olveda, R.; Olveda, D.; Harn, D.; Gray, D.J.; McManus, D.P.; Tallo, V.; Chau, T.; Williams, G. Can mass drug administration lead to the sustainable control of schistosomiasis in the Philippines? J. Infect. Dis. 2015, 211, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Sanza, M.; Totanes, F.I.; Chua, P.L.; Belizario, V.Y., Jr. Monitoring the impact of a mebendazole mass drug administration initiative for soil-transmitted helminthiasis (STH) control in the Western Visayas Region of the Philippines from 2007 through 2011. Acta Trop. 2013, 127, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Geerts, S.; Gryseels, B. Drug resistance in human helminths: Current situation and lessons from livestock. Clin. Microbiol. Rev. 2000, 13, 207–222. [Google Scholar] [CrossRef] [PubMed]
- WHO. Monitoring Anthelmintic Efficacy for Soil Transmitted Helminths (STH); WHO: Geneva, Switzerland, 2008. [Google Scholar]
- WHO. Soil-transmitted helminthiases: Number of children treated in 2014. Wkly. Epidemiol. Rec. 2015, 90, 701–712. [Google Scholar]
- Gyorkos, T.W.; MacLean, J.D.; Viens, P.; Chheang, C.; Kokoskin-Nelson, E. Intestinal parasite infection in the Kampuchean refugee population 6 years after resettlement in Canada. J. Infect. Dis. 1992, 166, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Zaha, O.; Hirata, T.; Kinjo, F.; Saito, A.; Fukuhara, H. Efficacy of ivermectin for chronic strongyloidiasis: Two single doses given 2 weeks apart. J. Infect. Chemother. 2002, 8, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Miller, M.M.; Derman, A.I.; Ellis, B.L.; Monnerat, R.G.; Pogliano, J.; Aroian, R.V. Bacillus subtilis strain engineered for treatment of soil-transmitted helminth diseases. Appl. Environ. Microbiol. 2013, 79, 5527–5532. [Google Scholar] [CrossRef] [PubMed]
- Osei-Atweneboana, M.Y.; Awadzi, K.; Attah, S.K.; Boakye, D.A.; Gyapong, J.O.; Prichard, R.K. Phenotypic evidence of emerging ivermectin resistance in Onchocerca volvulus. PLoS Negl. Trop. Dis. 2011, 5, e998. [Google Scholar] [CrossRef] [PubMed]
- Diawara, A.; Schwenkenbecher, J.M.; Kaplan, R.M.; Prichard, R.K. Molecular and biological diagnostic tests for monitoring benzimidazole resistance in human soil-transmitted helminths. Am. J. Trop. Med. Hyg. 2013, 88, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Brockwell, Y.M.; Elliott, T.P.; Anderson, G.R.; Stanton, R.; Spithill, T.W.; Sangster, N.C. Confirmation of Fasciola hepatica resistant to triclabendazole in naturally infected Australian beef and dairy cattle. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Wolstenholme, A.J.; Fairweather, I.; Prichard, R.; von Samson-Himmelstjerna, G.; Sangster, N.C. Drug resistance in veterinary helminths. Trends Parasitol. 2004, 20, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, J.; Albonico, M.; Behnke, J.M.; Kotze, A.C.; Prichard, R.K.; McCarthy, J.S.; Montresor, A.; Levecke, B. Is anthelmintic resistance a concern for the control of human soil-transmitted helminths? Int. J. Parasitol. Drugs Drug Resist. 2011, 1, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Bieri, F.A.M.; Gray, D.J.P.; Williams, G.M.P.; Raso, G.P.; Li, Y.-S.P.; Yuan, L.P.; He, Y.M.P.H.; Li, R.S.B.; Guo, F.-Y.B.A.; Li, S.-M.B.A.; et al. Health-education package to prevent worm infections in Chinese schoolchildren. N. Engl. J. Med. 2013, 368, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Bieri, F.A.; Li, Y.S.; Williams, G.M.; Yuan, L.P.; Henglin, Y.; Du, Z.W.; Clements, A.C.; Steinmann, P.; Raso, G.; et al. Health education and the control of intestinal worm infections in China: A new vision. Parasit. Vectors 2014, 7, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, N.E.; Clements, A.C.; Bryan, S.; McGown, J.; Gray, D.; Nery, S.V. Investigating the differential impact of school and community-based integrated control programmes for soil-transmitted helminths in Timor-Leste: The (S)WASH-D for Worms pilot study protocol. Pilot Feasibility Stud. 2016, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Nery, S.V.; McCarthy, J.S.; Traub, R.; Andrews, R.M.; Black, J.A.; Gray, D.J.; Weking, E.; Atkinson, J.A.; Campbell, S.; Francis, N.; et al. A cluster-randomised controlled trial integrating a community-based water, sanitation and hygiene programme, with mass distribution of albendazole to reduce intestinal parasites in Timor-Leste: The WASH for WORMS research protocol. Br. Med. J. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.; Du, Z.W.; Wu, F.W.; Jiang, J.Y.; Chen, R.; Zhou, X.N.; Hattendorf, J.; Utzinger, J.; Steinmann, P. Rapid re-infection with soil-transmitted helminths after triple-dose albendazole treatment of school-aged children in Yunnan, People’s Republic of China. Am. J. Trop. Med. Hyg. 2013, 89, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Mascarini-Serra, L. Prevention of soil-transmitted helminth infection. J. Glob. Infect. Dis. 2011, 3, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Bundy, D.A.P.; Beegle, K.; Brooker, S.; Drake, L.; de Silva, N.; Montresor, A.; Engels, D.; Jukes, M.; Chitsulo, L.; et al. Helminth infections: soil-transmitted helminth infections and schistosomiasis. In Disease Control Priorities in Developing Countries; Jamison, D.T., Breman, J.G., Measham, A.R., Alleyne, G., Claeson, M., Evans, D.B., Jha, P., Mills, A., Musgrove, P., Eds.; World Bank, The International Bank for Reconstruction and Development/The World Bank Group: Washington, DC, USA, 2006. [Google Scholar]
- Conlan, J.V.; Sripa, B.; Attwood, S.; Newton, P.N. A review of parasitic zoonoses in a changing Southeast Asia. Vet. Parasitol. 2011, 182, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Overgaauw, P.A.M.; van Zutphen, L.; Hoek, D.; Yaya, F.O.; Roelfsema, J.; Pinelli, E.; van Knapen, F.; Kortbeek, L.M. Zoonotic parasites in fecal samples and fur from dogs and cats in The Netherlands. Vet. Parasitol. 2009, 163, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Overgaauw, P.A.M.; van Knapen, F. Veterinary and public health aspects of Toxocara spp. Vet. Parasitol. 2013, 193, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Simonato, G.; Frangipane di Regalbono, A.; Cassini, R.; Traversa, D.; Beraldo, P.; Tessarin, C.; Pietrobelli, M. Copromicroscopic and molecular investigations on intestinal parasites in kenneled dogs. Parasitol. Res. 2015, 114, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, B.; Traversa, D.; Iorio, R.; De Berardinis, A.; Bartolini, R.; Salini, R.; Di Cesare, A. Zoonotic parasites in feces and fur of stray and private dogs from Italy. Parasitol. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Roddie, G.; Stafford, P.; Holland, C.; Wolfe, A. Contamination of dog hair with eggs of Toxocara canis. Vet. Parasitol. 2008, 152, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Heyworth, J.S.; Cutt, H.; Glonek, G. Does dog or cat ownership lead to increased gastroenteritis in young children in South Australia? Epidemiol. Infect. 2006, 134, 926–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, N.I.; Croese, J.; Clouston, A.D.; Parry, M.; Loukas, A.; Prociv, P. Eosinophilic enteritis in northeastern Australia. Pathology, association with Ancylostoma caninum, and implications. Am. J. Surg. Pathol. 1995, 19, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Croese, J.; Loukas, A.; Opdebeeck, J.; Prociv, P. Occult enteric infection by Ancylostoma caninum: A previously unrecognized zoonosis. Gastroenterology 1994, 106, 3–12. [Google Scholar] [CrossRef]
- Loukas, A.; Croese, J.; Opdebeeck, J.; Prociv, P. Detection of antibodies to secretions of Ancylostoma caninum in human eosinophilic enteritis. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 650–653. [Google Scholar] [CrossRef]
- Loukas, A.; Opdebeeck, J.; Croese, J.; Prociv, P. Immunologic incrimination of Ancylostoma caninum as a human enteric pathogen. Am. J. Trop. Med. Hyg. 1994, 50, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Prociv, P.; Croese, J. Human enteric infection with Ancylostoma caninum: Hookworms reappraised in the light of a ‘new’ zoonosis. Acta Trop. 1996, 62, 23–44. [Google Scholar] [CrossRef]
- Prociv, P.; Croese, J. Human eosinophilic enteritis caused by dog hookworm Ancylostoma caninum. Lancet 1990, 335, 1299–1302. [Google Scholar] [CrossRef]
- Croese, J.; Fairley, S.; Loukas, A.; Hack, J.; Stronach, P. A distinctive aphthous ileitis linked to Ancylostoma caninum. J. Gastroenterol. Hepatol. 1996, 11, 524–531. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, S.L.; Leder, K. Global burden of toxocariasis: A common neglected infection of poverty. Curr. Trop. Med. Rep. 2014, 1, 52–61. [Google Scholar] [CrossRef]
- Le, T.H.; Anh, N.T.; Nguyen, K.T.; Nguyen, N.T.; Thuy do, T.T.; Gasser, R.B. Toxocara malaysiensis infection in domestic cats in Vietnam—An emerging zoonotic issue? Infect. Genet. Evol. 2016, 37, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Li, M.W.; Lin, R.Q.; Song, H.Q.; Wu, X.Y.; Zhu, X.Q. The complete mitochondrial genomes for three Toxocara species of human and animal health significance. BMC Genom. 2008, 9, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohd Zain, S.N.; Rahman, R.; Lewis, J.W. Stray animal and human defecation as sources of soil-transmitted helminth eggs in playgrounds of Peninsular Malaysia. J. Helminthol. 2015, 89, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, J.F.; Espinoza, J.V.; Arevalo, F.A. Ocular toxocariasis. J. Pediatr. Ophthalmol. Strabismus 2013, 50, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Blake, R.T.; Overend, D.J. The prevalence of Dirofilaria immitis and other parasites in urban pound dogs in north-eastern Victoria. Aust. Vet. J. 1982, 58, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Dunsmore, J.D.; Thompson, R.C.A.; Bates, I.A. Prevalence and survival of Toxocara canis eggs in the urban environment of Perth, Australia. Vet. Parasitol. 1984, 16, 303–311. [Google Scholar] [CrossRef]
- Carden, S.M.; Meusemann, R.; Walker, J.; Stawell, R.J.; MacKinnon, J.R.; Smith, D.; Stawell, A.M.; Hall, A.J. Toxocara canis: Egg presence in Melbourne parks and disease incidence in Victoria. Clin. Exp. Ophthalmol. 2003, 31, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, W.L.; Stewart, A.C.; Walker, J.C. Toxocariasis: A serological survey of blood donors in the Australian Capital Territory together with observations on the risks of infection. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 217–221. [Google Scholar] [CrossRef]
- Nejsum, P.; Bertelsen, M.F.; Betson, M.; Stothard, J.R.; Murrell, K.D. Molecular evidence for sustained transmission of zoonotic Ascaris suum among zoo chimpanzees (Pan Troglodytes). Vet. Parasitol. 2010, 171, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, M.; Yuan, K.; Hu, N.; Peng, W. Phylogeography of Ascaris lumbricoides and A. suum from China. Parasitol. Res. 2011, 109, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, M.; Yuan, K.; Deng, S.; Peng, W. Pig Ascaris: An important source of human ascariasis in China. Infect. Genet. Evol. 2012, 12, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Traub, R.J.; Robertson, I.D.; Irwin, P.J.; Mencke, N.; Thompson, R.C.A.A. Canine gastrointestinal parasitic zoonoses in India. Trends Parasitol. 2005, 21, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Controlling worms in pigs. Available online: https://www.daf.qld.gov.au/animal-industries/pigs/pig-health-and-diseases/disease-prevention/controlling-worms-in-pigs (accessed on 11 October 2017).
- Graves, S. Human infection with ‘Ascaris suum’ in Tasmania? Ann. ACTM 2005, 6, 16. [Google Scholar]
- Steinmann, P.; Yap, P.; Utzinger, J.; Du, Z.W.; Jiang, J.Y.; Chen, R.; Wu, F.W.; Chen, J.X.; Zhou, H.; Zhou, X.N. Control of soil-transmitted helminthiasis in Yunnan province, People’s Republic of China: Experiences and lessons from a 5-year multi-intervention trial. Acta Trop. 2015, 141, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Luo, N.J. A cross-sectional study of intestinal parasitic infections in a rural district of west China. Can. J. Infect. Dis. 2003, 14, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-J.; Liu, L.; Zhu, H.-R.; Griffiths, S.M.; Tanner, M.; Bergquist, R.; Utzinger, J.; Zhou, X.-N. China’s sustained drive to eliminate neglected tropical diseases. Lancet Infect. Dis. 2014, 14, 881–892. [Google Scholar] [CrossRef]
- Dunn, J.J.; Columbus, S.T.; Aldeen, W.E.; Davis, M.; Carroll, K.C. Trichuris vulpis recovered from a patient with chronic diarrhea and five dogs. J. Clin. Microbiol. 2002, 40, 2703–2704. [Google Scholar] [CrossRef] [PubMed]
- Ravasi, D.F.; O’Riain, M.J.; Davids, F.; Illing, N. Phylogenetic evidence that two distinct Trichuris genotypes infect both humans and non-human primates. PLoS ONE 2012, 7, e44187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawash, M.B.F.; Andersen, L.O.; Gasser, R.B.; Stensvold, C.R.; Nejsum, P. Mitochondrial genome analyses suggest multiple Trichuris species in humans, baboons, and pigs from different geographical regions. PLoS Negl. Trop. Dis. 2015, 9, e0004059. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.H.; Zhou, W.; Nisbet, A.J.; Xu, M.J.; Zhou, D.H.; Zhao, G.H.; Wang, S.K.; Song, H.Q.; Lin, R.Q.; Zhu, X.Q. Characterization of Trichuris trichiura from humans and T. suis from pigs in China using internal transcribed spacers of nuclear ribosomal DNA. J. Helminthol. 2014, 88, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-H.; Gasser, R.B.; Su, A.; Nejsum, P.; Peng, L.; Lin, R.-Q.; Li, M.-W.; Xu, M.-J.; Zhu, X.-Q. Clear genetic distinctiveness between human- and pig-derived Trichuris based on analyses of mitochondrial datasets. PLoS Negl. Trop. Dis. 2012, 6, e1539. [Google Scholar] [CrossRef] [PubMed]
- Bager, P.; Kapel, C.; Roepstorff, A.; Thamsborg, S.; Arnved, J.; Rønborg, S.; Kristensen, B.; Poulsen, L.K.; Wohlfahrt, J.; Melbye, M. Symptoms after ingestion of pig whipworm Trichuris suis eggs in a randomized placebo-controlled double-blind clinical trial. PLoS ONE 2011, 6, e22346. [Google Scholar] [CrossRef] [PubMed]
- Helmby, H. Human helminth therapy to treat inflammatory disorders-where do we stand? BMC Immunol. 2015, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.A.; Friberg, I.M.; Little, S.; Bradley, J.E. Review series on helminths, immune modulation and the hygiene hypothesis: Immunity against helminths and immunological phenomena in modern human populations: Coevolutionary legacies? Immunology 2009, 126, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, J.P.; Grainger, J.R.; Maizels, R.M. Helminth immunoregulation: The role of parasite secreted proteins in modulating host immunity. Mol. Biochem. Parasitol. 2009, 167, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Feary, J.R.; Venn, A.J.; Mortimer, K.; Brown, A.P.; Hooi, D.; Falcone, F.H.; Pritchard, D.I.; Britton, J.R. Experimental hookworm infection: A randomized placebo-controlled trial in asthma. Clin. Exp. Allergy 2010, 40, 299–306. [Google Scholar] [CrossRef] [PubMed]
- TOS. Available online: http://wormswell.com/science-research (accessed on 17 July 2017).
- The helminthic therapy. Available online: https://tanawisa.com/ (accessed on 17 July 2017).
- Turner, K.J.; Quinn, E.H.; Anderson, H.R. Regulation of asthma by intestinal parasites. Investigation of possible mechanisms. Immunology 1978, 35, 281–288. [Google Scholar] [PubMed]
- Mpairwe, H.; Ndibazza, J.; Webb, E.L.; Nampijja, M.; Muhangi, L.; Apule, B.; Lule, S.; Akurut, H.; Kizito, D.; Kakande, M.; et al. Maternal hookworm modifies risk factors for childhood eczema: Results from a birth cohort in Uganda. Pediatr. Allergy Immunol. 2014, 25, 481–488. [Google Scholar] [CrossRef] [PubMed]
- McSorley, H.J.; Gaze, S.; Daveson, J.; Jones, D.; Anderson, R.P.; Clouston, A.; Ruyssers, N.E.; Speare, R.; McCarthy, J.S.; Engwerda, C.R.; et al. Suppression of inflammatory immune responses in celiac disease by experimental hookworm infection. PLoS ONE 2011, 6, e24092. [Google Scholar] [CrossRef] [PubMed]
- Daveson, A.J.; Jones, D.M.; Gaze, S.; McSorley, H.; Clouston, A.; Pascoe, A.; Cooke, S.; Speare, R.; Macdonald, G.A.; Anderson, R.; et al. Effect of hookworm infection on wheat challenge in celiac disease—A randomised double-blinded placebo controlled trial. PLoS ONE 2011, 6, e17366. [Google Scholar] [CrossRef] [PubMed]
- Giacomin, P.; Zakrzewski, M.; Jenkins, T.P.; Su, X.; Al-Hallaf, R.; Croese, J.; de Vries, S.; Grant, A.; Mitreva, M.; Loukas, A.; et al. Changes in duodenal tissue-associated microbiota following hookworm infection and consecutive gluten challenges in humans with coeliac disease. Sci. Rep. 2016, 6, 36797. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.; Walker, A.W.; Reyes, J.; Chico, M.; Salter, S.J.; Vaca, M.; Parkhill, J. Patent human infections with the whipworm, Trichuris trichiura, are not associated with alterations in the faecal microbiota. PLoS ONE 2013, 8, e76573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, A.R.; Dige, A.; Rasmussen, T.K.; Hvas, C.L.; Dahlerup, J.F.; Iversen, L.; Stensvold, C.R.; Agnholt, J.; Nejsum, P. Immune responses and parasitological observations induced during probiotic treatment with medicinal Trichuris suis ova in a healthy volunteer. Immunol. Lett. 2017, 188, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Ruyssers, N.E.; De Winter, B.Y.; De Man, J.G.; Loukas, A.; Herman, A.G.; Pelckmans, P.A.; Moreels, T.G. Worms and the treatment of inflammatory bowel disease: Are molecules the answer? Clin. Dev. Immunol. 2008, 2008, 567314. [Google Scholar] [CrossRef] [PubMed]
- Giacomin, P.; Croese, J.; Krause, L.; Loukas, A.; Cantacessi, C. Suppression of inflammation by helminths: A role for the gut microbiota? Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2015, 370. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.; Esterman, A.; McDermott, R. Control of chronic Strongyloides stercoralis infection in an endemic community may be possible by pharmacological means alone: Results of a three-year cohort study. PLoS Negl. Trop. Dis. 2017, 11, e0005825. [Google Scholar] [CrossRef] [PubMed]
- Flannery, G.; White, N. Immunological Parameters in Northeast Arnhem Land Aboriginies: Consequences of Changing Settlement and Lifestyles; Cambridge University Press: New York, NY, USA, 1993; pp. 202–220. [Google Scholar]
| Years Sampled | Reference | Status | Country of Origin | Parasite Species | Prevalence | Diagnostics |
|---|---|---|---|---|---|---|
| 2000, 2002 | [36] | Immigrant | East Africa Cambodia | S. stercoralis T. trichiura S. stercoralis Hookworm spp. | 11% (n = 124) 4% (n = 124) 42% (n = 230) 1.96% (n = 230) | Faecal samples (method unclear) Serology (method unclear) |
| 7–20 years after resettlement | [37] | Immigrant | Laos | S. stercoralis | 24.21% (n = 95) | Faecal microscopy Strongyloides serology |
| 2–52 years after resettlement 1998–2005 | [38] * | Immigrant | Fiji (1), SEA (5), China (1), Sri Lanka (1), India (2), Seychelles (2), Ethiopia (2), Russia (1), Italy (1), Greece (1) | S. stercoralis | 100% (n = 17) * | Faecal microscopy Strongyloides serology |
| 1998–2005 | [38]* | Returned travellers | Papua New Guinea (1), Vanuatu (1), SEA (7), Africa (2) | S. stercoralis | 100% (n = 11) * | Faecal microscopy Strongyloides serology |
| 2004 | [39] | Returned ADF # member | Solomon Islands | A. ceylanicum | 100% (n = 1) | Harada-Mori culture, direct faecal smear |
| Served 1962–1975 2010 | [40] | ADF veterans | Vietnam | S. stercoralis | 11.6% (n = 249) | Faecal microscopy ELISA |
| 2006–2007 | [41] | RAMSI personnel *** | Solomon Islands | S. stercoralis | 100% (n = 14) * | Faecal microscopy, Serology (ELISA) |
| 2002–2012 | [42] | Residents Northern Territory | Australia | T. trichiura | 0.65% (n = 63,668) ** | Wet mount microscopy, Concentration method |
| 2002–2011 | [43] | Residents Northern Territory | Australia | Hookworm | 0.17% (n = 64,691) ** | Wet mount microscopy, Concentration method |
| 2004–2008 | [44] | Immigrants | Burma | S. stercoralis | 26% (n = 156) | Serology |
| 2002 | [45] | Immigrants | Cambodia | S. stercoralis | 36% (n = 234) * | ELISA, faecal microscopy |
| 2010–2011 | [46] | Residents Northern Territory | Australia | S. stercoralis | 16.5% (n = 124) pre-treatment 12% (n = 30) post-treatment | Serology (NIE ELISA, NIE-DBS-ELISA) |
| 2000–2006 | [29] | Residents | Australia | S. stercoralis | 100% (n = 18) * | Faecal microscopy, serology |
| 1994–1996 | [47] | Residents | Australia | T. canis S. stercoralis S. stercoralis | 21% (n = 29) 28% (n = 29) 19% (n = 314) | Serology Serology Formol-ether |
| [48] | Immigrant | Laos | S. stercoralis | Single patient | Larvae in sputum | |
| 2010–2011 | [49] | Immigrants Residents Residents | Australia | N. americanus A. ceylanicum A. duodenale | (n = 5/227) **** (n = 2/227) (n = 4/227) | PCR Sequencing |
| Ref | Year | Country * | Human/Animal | Prevalence % (Total no.) | Species | Diagnostic |
|---|---|---|---|---|---|---|
| [95] | - | Taiwan | Human | Single patient | A. ceylanicum | Morphology |
| [69] | 2009 | Laos | Human | 17.6% (n = 17) 82.4% (n = 17) | A. ceylanicum N. americanus | Nested PCR |
| [96] | 2012 | Cambodia | Human | 51.6% (n = 124) 51.6% (n = 124) 3.2% (n = 124) | A. ceylanicum N. americanus A. duodenale | Microscopy, PCR |
| Dog | 94.4% (n = 90) 8.9% (n = 90) 1.1% (n = 90) | A. ceylanicum A. caninum N. americanus | Microscopy, PCR | |||
| [49] # | 2010–2011 | Australia | Human | 0.88% (n = 227) 1.76% (n = 227) 1.76% (n = 227) | A. ceylanicum A. duodenale N. americanus a | PCR |
| [97] | - | China | Dog Cat Human | 3 (n = 254) 5 (n = 102) 14 (n = 14) | A. ceylanicum | PCR sequencing |
| [98] | - | Malaysia | Dog | 52% (n = 224) 48% (n = 224) | A. ceylanicum A. caninum | FECT, PCR |
| [99] | 2007–2010 | Malaysia | Cat | 29.5% (n = 543) | A. ceylanicum | Microscopy |
| [100] | 2009–2011 | Malaysia | Human | 87.2 (n = 47) 23.4 (n = 47) | N. americanus A. ceylanicum | Microscopy, PCR |
| [101] | 2013 | Malaysia (Chinese) | Human | Single patient | A. ceylanicum | Microscopy |
| [11] | 2009–2011 | Malaysia | Human | 12.8% (n = 634) 76.6% (n = 634) 10.6% (n = 634) | A. ceylanicum N. americanus Both species | Microscopy, PCR |
| Cats and dogs | 52% (n = 105) 46% (n = 105) | A. caninum A. ceylanicum | Microscopy, PCR | |||
| [102] | - | Myanmar | Human | 72.72% (n = 11) 27.27% (n = 11) | N. americanus A. ceylanicum | PCR sequencing |
| [103] | 2004–2005 | Australia | Dog | 6.5% (n = 92) 70.7% (n = 92) 4.3% (n = 92) 2.2% (n = 92) | A. ceylanicum A. caninum A. caninum + A. ceylanicum A. caninum + U. stenocephala | Microscopy, PCR-RFLP |
| Cat | 30% (n = 10) | A. caninum | ||||
| [12] | 2011–2013 | Thailand | Human | 60% (n = 10) 30% (n = 10) 10% (n = 10) | N. americanus A. ceylanicum A. duodenale | PCR sequencing |
| [86] | >2007 | Australia | Wild dog | 100% (n = 26) 11.5% (n = 26) | A. caninum A. ceylanicum + A. caninum | Microscopy, PCR |
| Dog scat | 65.31% (n = 89) 71.43% (n = 89) 38.78% (n = 89) | A. ceylanicum A. caninum A. caninum + A. ceylanicum | ||||
| [39] | 2004 | Australia (Solomon Islands) | Human | Single patient | A. ceylanicum | Microscopy |
| [51] | 2004–2005 | Thailand | Dog | 77% (n = 229) 9% (n = 229) 14% (n = 229) | A. ceylanicum A. caninum Both species | PCR |
| Human | 71.43% (n = 204) 28.57% (n = 204) | N. americanus A. ceylanicum | ||||
| [104] | 2008 | India | Dog | 50.46% (n = 325) 51.92% (n = 104) 33.65% (n = 104) 15.38% (n = 104) | Hookworm spp. A. caninum A. ceylanicum A. caninum + A. ceylanicum | Microscopy, PCR-RFLP |
| [105] | 2000 | India | Dog | 36% (n = 101) 38% (n = 101) | A. caninum A. caninum + A. braziliense | Microscopy, PCR-RFLP + sequencing |
| [85] | 2011 | Australia | Dog | 96.4% (n = 84) 16.67%(n = 84) 14.0% (n = 84) | A. caninum A. ceylanicum A. caninum + A. ceylanicum | Microscopy, PCR |
| [106] | 2013–2014 | Malaysia | Dog | 29.6% (n = 227) 6.6% (n = 227) | A. ceylanicum A. caninum | FECT, PCR |
| Soil samples | 14.3% (n = 126) 2.4% (n = 126) | A. ceylanicum A. caninum | ||||
| Cat | 29.6% (n = 152) 6.6% (n = 152) | A. ceylanicum A. caninum | ||||
| [107] | 2015 | Japan (Lao) | Human | Single patient | A. ceylanicum | Microscopy, PCR |
| [108] | 2013–2015 | India | Human | 100% (n = 143) 16.8% (n = 143) 8.4% (n = 143) | N. americanus A. caninum A. duodenale | |
| Dog | 27.9% 76.4% | A. ceylanicum A. caninum | PCR-RFLP | |||
| Soil samples | 60.2% (n = 78) 29.4% (n = 78) 16.6% (n = 78) 1.4% (n = 78) | A. ceylanicum A. caninum A. duodenale N. americanus | ||||
| [109] | 2014 | Thailand | Dog Cat | 33.0% (n = 197) 58.46% (n = 180) | A. ceylanicum | Microscopy, PCR |
| [110] | 2014 | France (Myanmar) | Human | Single patient | A. ceylanicum | Microscopy, PCR |
| [111] | 2014 | Vietnam | Dog | 54.3% (n = 94) 33% (n = 94) 12.7% (n = 94) | A. ceylanicum A. caninum Both species | PCR-RFLP, PCR (cox1) |
| [112] | 2014 | China | Cat | 40.8% (n = 112) 59.2% (n = 112) 20.4% (n = 112) | A. ceylanicum A. caninum Both species | Microscopy, PCR |
| [113] | - | India | Human | 95% 15% 5% | N. americanus A. duodenale A. ceylanicum | PCR-RFLP |
| [114] | 2005 | Thailand | Human | 92% 4% 2% 2% | N. americanus A. ceylanicum A. duodenale N. americanus + A. ceylanicum | KK, PCR |
| [115] | 2008 | Lao | Human | 5.91% 2.46% 1.48% 0.49% | N. americanus A. duodenale A. caninum A. ceylanicum | KK, PCR |
| [99] | 2007–2010 | Malaysia | Feral cats | 29.5% (n = 251) | A. ceylanicum | Microscopy of adults (staining paracarmine) |
| [116] | - | Lao | Feral cats | 69% (n = 55) | A. ceylanicum | Microscopy of adults (staining Mayers carmine) |
| [117] | - | Taiwan | Human | Single patient | A. ceylanicum | Method unclear. Adult identification. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gordon, C.A.; Kurscheid, J.; Jones, M.K.; Gray, D.J.; McManus, D.P. Soil-Transmitted Helminths in Tropical Australia and Asia. Trop. Med. Infect. Dis. 2017, 2, 56. https://doi.org/10.3390/tropicalmed2040056
Gordon CA, Kurscheid J, Jones MK, Gray DJ, McManus DP. Soil-Transmitted Helminths in Tropical Australia and Asia. Tropical Medicine and Infectious Disease. 2017; 2(4):56. https://doi.org/10.3390/tropicalmed2040056
Chicago/Turabian StyleGordon, Catherine A., Johanna Kurscheid, Malcolm K. Jones, Darren J. Gray, and Donald P. McManus. 2017. "Soil-Transmitted Helminths in Tropical Australia and Asia" Tropical Medicine and Infectious Disease 2, no. 4: 56. https://doi.org/10.3390/tropicalmed2040056





