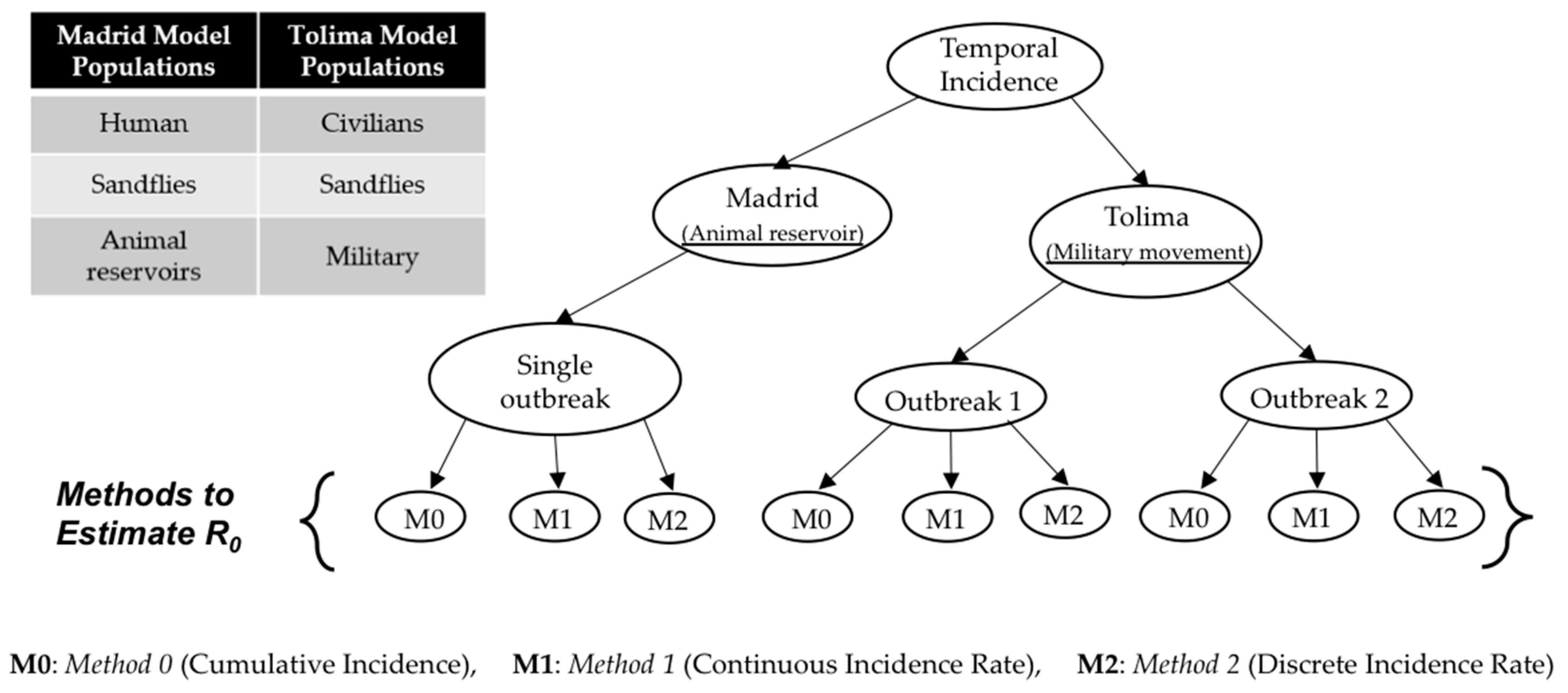
1. Introduction
Background: Leishmaniasis is a disease caused by an intracellular protozoan parasite (genus
Leishmania), which is transmitted by the bite of a female phlebotomine sandfly. The clinical spectrum of leishmaniasis ranges from a self-resolving cutaneous ulcer to a lethal visceral illness. Cutaneous leishmaniasis (CL) is the most common form of leishmaniasis and causes skin lesions on the exposed parts of the body, leaving scars for life. About 95% of CL cases occur in the Americas, the Mediterranean, the Middle East and Central Asia [
1]. More than two-thirds of new cases of CL occur in six countries: Afghanistan, Algeria, Brazil, Colombia, Iran and Syria. An estimated 0.7 million to 1.3 million new cases occur worldwide annually [
1,
2]. Anthroponotic CL (where humans are the major reservoir of the parasite) is predominantly urban and periurban and shows patterns of spatial clustering similar to those of anthroponotic visceral leishmaniasis (VL) in South-East Asia. The epidemiology of CL is complex, with intra- and inter-specific variation in transmission cycles, reservoir hosts, sandfly vectors, clinical manifestations and response to therapy, and there are multiple circulating
Leishmania species in the same geographical area [
1,
2,
3,
4].
Leishmaniasis epidemiology in Spain: Human leishmaniasis in the Mediterranean basin, including Spain, is an endemic zoonotic disease. In Spain, the vector involved in the transmission of the leishmania parasite is a sandfly of the
Phlebotomus genus (primarily
P. perniciosus), which is active between May and October, and dogs are the main reservoir [
5,
6]. During 2000 to 2009, an average of 20 leishmaniasis cases was reported per year in the Madrid autonomous community (with an annual incidence rate of around 0.5 per 100,000 inhabitants) [
5]. However, during the last quarter of 2010, a fivefold increase in the number of cases was detected, compared with the number seen in the previous years. Subsequent research confirmed that an outbreak of leishmaniasis started in July 2009 in the south-west area of the region of Madrid, mainly affecting four geographically close municipalities [
7]. The surveillance system for canine leishmaniasis did not detect any increase in prevalence during the period. Improvements in sanitation and disinfection in affected areas were also carried out as control measures [
5]. Xenodiagnosis studies found that hares may have played a role as active reservoirs for the leishmania parasite [
8]. The discovery of the new reservoir initially posed a challenge for controlling the outbreak. Rabbits were also known to be sources of blood meal for the vector species before this outbreak. Although dogs are the main reservoir host, hares are suspected to be a potential culprit for the surprising increase of cases during this outbreak. This was the largest reported community outbreak of leishmaniasis in Europe, despite Spain being one of the most economically-developed nations in the world, with continued intervention measures to control the disease [
5].
Leishmaniasis epidemiology in Colombia: Colombia is one of two countries in the America region with highest number of leishmaniasis cases [
1,
9]. In 2016, about 10,743 new cases of leishmaniasis were reported from the country [
10]. About 99.3% of all cases that occur in Colombia are CL, and the rest are infected with other forms of leishmaniasis [
11]. The disease prevails in much of the country, moving from sylvatic to domestic cycles; parasites that are in jungle scenarios reach urban areas due to human movement [
1,
12]. CL outbreaks caused by
L. braziliensis,
L. panamensis and
L. guyanensis are associated with intra- and peri-domiciliary transmission, which have been reported since 1984 [
1,
13,
14]. Ramirez et al. [
1] using data collected from 1980 to 2001, confirmed the leishmania species that caused CL in Colombia, and found
L. panamensis (61.3%, 201 of 327 isolates),
L. braziliensis (27.1%, 88/327),
L. infantum chagasi (4%, 12/327),
L. mexicana (2.1%, 8/327), and
L. amazonensis (2.8%, 9/327) to be the primary species. In Colombia, CL is the most common manifestation in army personnel, who are the most vulnerable population, due to the continuous deployment of troops to forested areas of high endemicity and high density of the insect vector [
15].
Health disparities: A central aspect of disparities is to identify and study differences in health status between groups, which negatively impact less advantaged groups. These differences could be because of socioeconomic status, gender and ethnicity disparities, and accessibility to health care and interventions. For example, despite the United States’ economic dominance and status as one of the most developed countries, an estimated 12 million Americans living in poverty suffer from at least one neglected tropical disease (NTD) [
16]. While there are immense challenges to systematically investigating the potential impact of health disparities on an outbreak, overall potential of transmission and size of an infection for distinct populations can be estimated via metrics such as reproduction number, inoculation rate, epidemic size, vectorial capacity, and so forth. In this study, we modeled leishmaniasis outbreaks in the regions of Madrid, Spain and in Tolima, Colombia, as two ‘distinct’ populations to study differences in transmission potential. Spain's healthcare system is regularly rated among the world’s best (with ~90% of patients accessing public healthcare and ~20% accessing some part of private healthcare) and spends about 10% of its gross domestic product (GDP) on healthcare. State healthcare guarantees universal coverage, although one may have to travel far to find, or wait a significant time to access, a public healthcare facility. On the other hand, Colombia ranks 22nd on the WHO’s list of the best healthcare systems, with private healthcare establishments accounting for around 57% of establishments (thus, relatively rapid access to healthcare). Total expenditures on health constitute around 7% of Colombia’s GDP; however, urban and rural areas have significant differences in access to health care (see Table in the
Supplementary Material, Section S.1, for details on potential health disparity between Spain and Colombia). Here, we do not aim to identify (or study) specific factors for health disparity leading to a leishmaniasis epidemic. However, a general discussion of the potential impact of health disparity on the disease outbreak is provided. Significant social and environmental data are needed to truly capture the differences and study the role of health disparity in the transmission dynamics of CL.
Mathematical modeling study of leishmaniasis and reproduction number: Initial mathematical models of CL transmission dynamics were developed by Dye et al. [
17,
18], in which they analyzed simple discrete-time epidemic models to study the mechanism behind observed inter-epidemic periods and the intensity of infection in dog reservoirs. Other studies included models with heterogeneous biting among age-structured dog populations and used serological data for the dog population in Gozo, Malta to estimate the basic reproduction number [
19], a quantity that measures the intensity of an outbreak. Formally, the basic reproduction number (
R0) of an infection can be interpreted as the average number of new cases generated by a typical infectious individual over the course of its infectious period, in an otherwise uninfected population. It is a key parameter, the value of which characterizes the transmission potential of an epidemic and hence, is often used to inform the potential effectiveness of intervention strategies. There are various ways to estimate
R0 [
20,
21]. Here, we derive three novel methods to estimate
R0 for a CL outbreak via a mathematical model with region-dependent features. The methods are tested using data from two ecologically distinct regions—Madrid (a city in a ‘developed’ country, Spain) and Tolima (a city in a ‘developing’ country, Colombia)—as a case study. In the literature, models have especially been used to estimate
R0 [
18] (see
Table 1 for review on
R0 estimates); however, such studies have used data primarily from different unrelated studies to collect point estimates of model parameters instead of applying a rigorous parameter estimation procedure. In the present study, we developed mathematical procedures for the estimation of model parameters via fitting the model to temporal incidence data, using three different techniques.
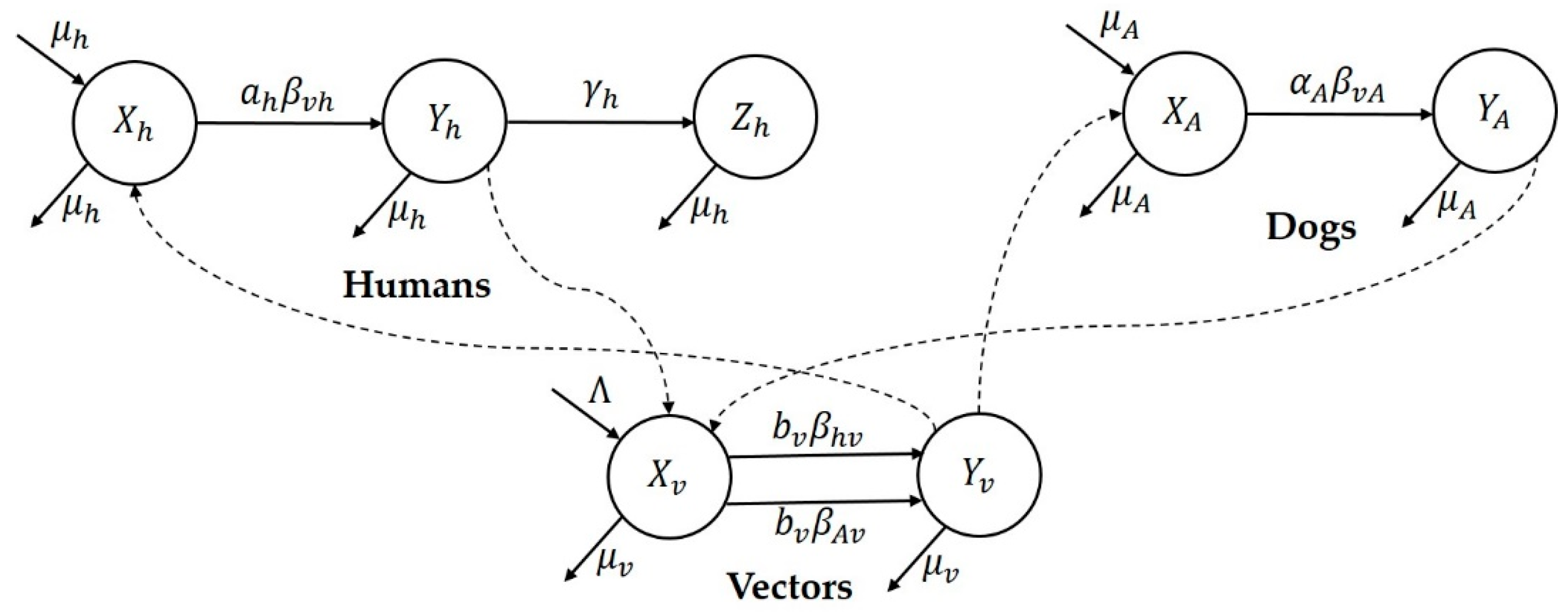
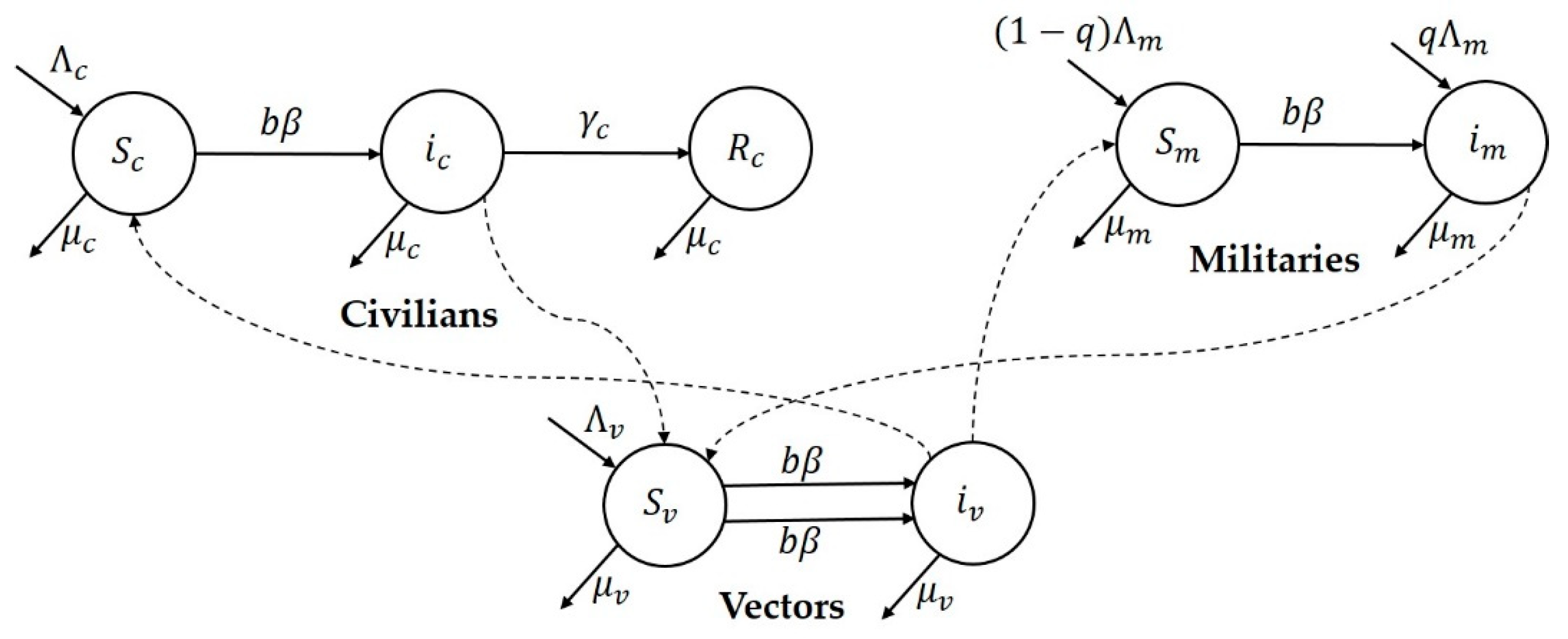
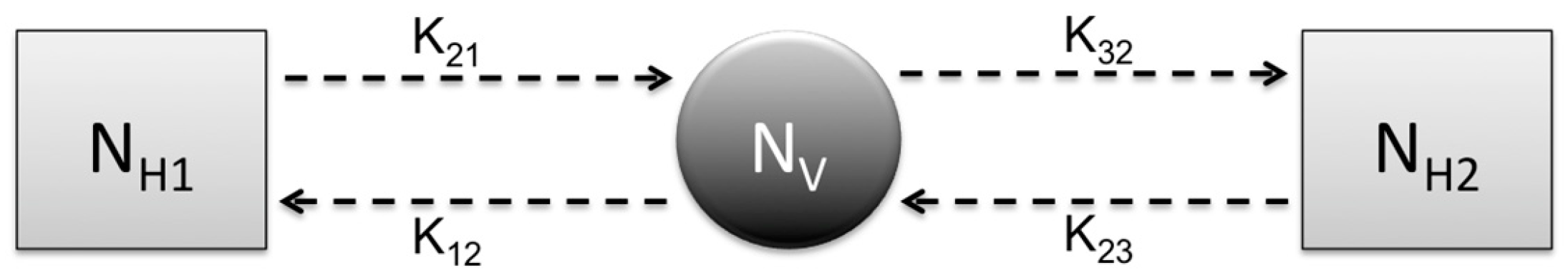
Research focus of the study: This study attempts to understand three major CL outbreaks, the first one in Madrid, Spain from 2009 to 2012 and the second and third outbreaks in Tolima, Colombia—both occurred in 2016. The outbreak in Madrid was started mainly by dogs, which are reservoir hosts of the disease, and the outbreaks in Tolima initiated because of the movement of soldiers, particularly those coming from the jungle to urban areas after staying in forestlands for long periods of time. In this work, we study the dynamics of CL in Spain and Colombia using a simple vector-borne disease model, while incorporating local characteristics and data on the disease. We used the country-specific model to estimate the transmission potential of each of three outbreaks via three novel parameter estimation procedures. These two regions were selected because of their distinct characteristics related to the disease and to highlight the comparison of the transmission potential between the ecologically and economically different regions. The estimated model parameters were then used to estimate the local reproduction number for each outbreak and each region. The difference in the basic reproduction number between regions could shed light on potential differences in health inequality, population immunity and transmissibility of leishmaniasis. This information is also important for designing effective control policies.
5. Discussion
Proper surveillance is crucial for controlling leishmaniasis in endemic countries; however, there is a need to develop methods that can measure disease transmission rates effectively using existing limited data [
29] and can be used to evaluate control programs [
40]. Leishmaniasis-affected regions are primarily resource-constrained and hence face various challenges to gathering regular comprehensive data. In such scenarios, model-driven decisions might be helpful and can provide understanding of region-specific transmission dynamics [
37]. In this study, we provide methodologies to estimate the basic reproduction number,
R0, for CL with regional dependent factors. The estimation methods were tested using case studies from the two economically contrasting regions, Madrid, Spain and Tolima, Colombia. The Madrid model considers dog reservoir hosts (since most cases had contact with infected dogs) whereas the Tolima model takes into account the movement of military personnel on the transmission dynamics of CL. The three estimation procedures were developed for the two models to estimate their parameters using reported incidence data. Unlike the traditionally used estimating process, in which point estimation of model parameters is taken directly from independent studies reported in the literature, the methods in this research provide a simple but consistent way to estimate model parameters. The estimation of model parameters is followed by the estimation of the basic reproduction number,
R0, and the computation of various epidemiologically important quantities, the type reproduction number (RT) vectorial capacity (VC) and entomological inoculation rates (EIR).
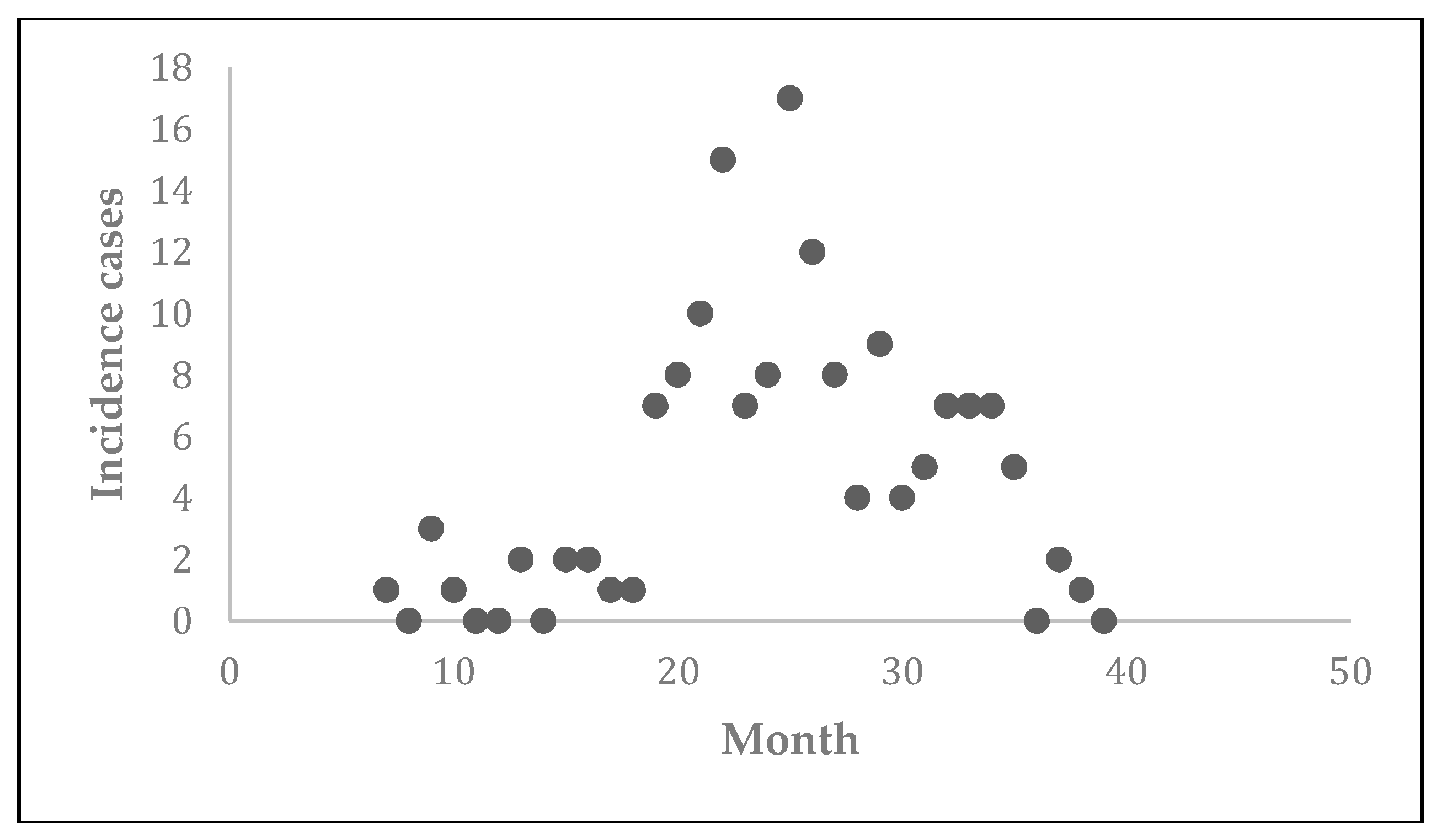
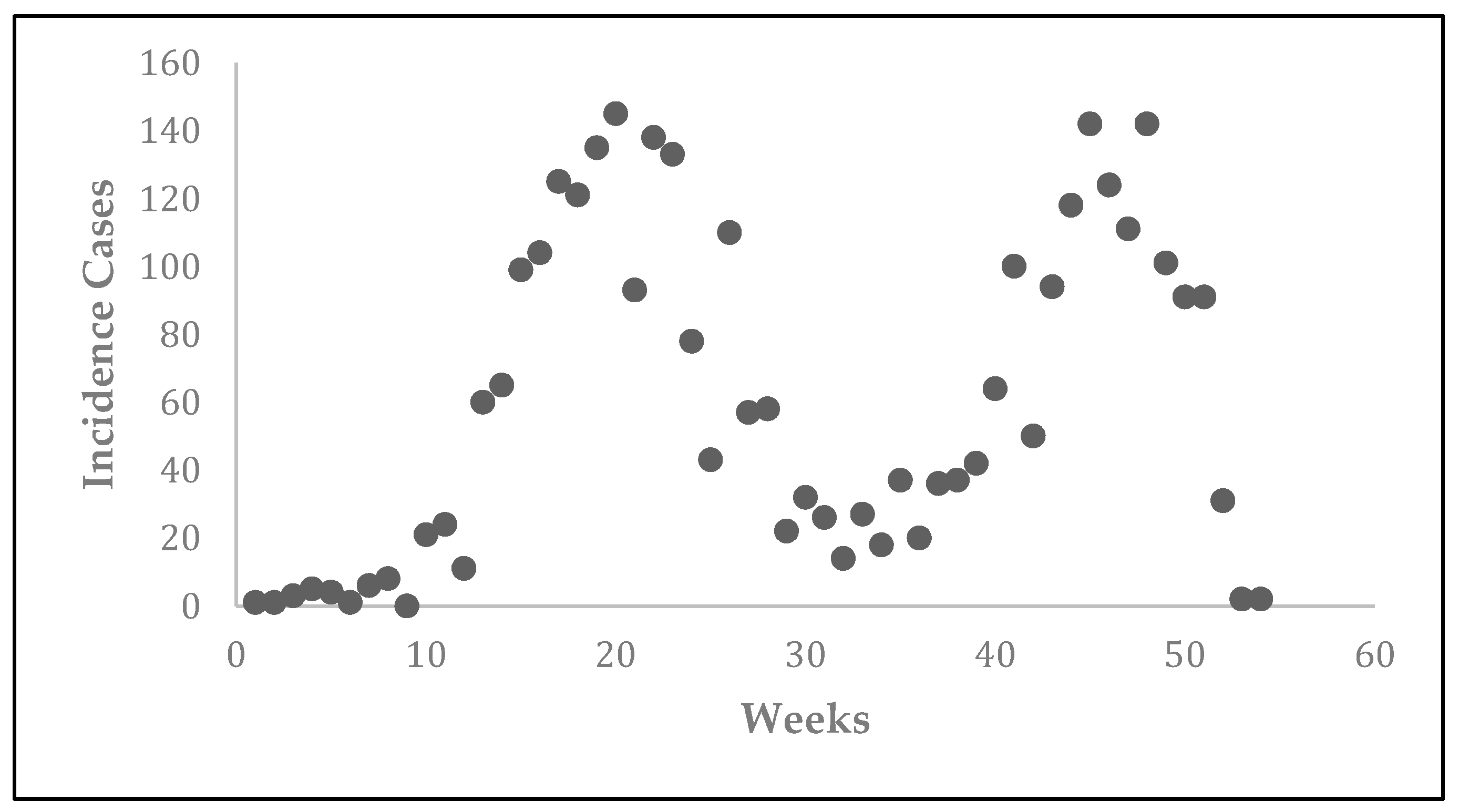
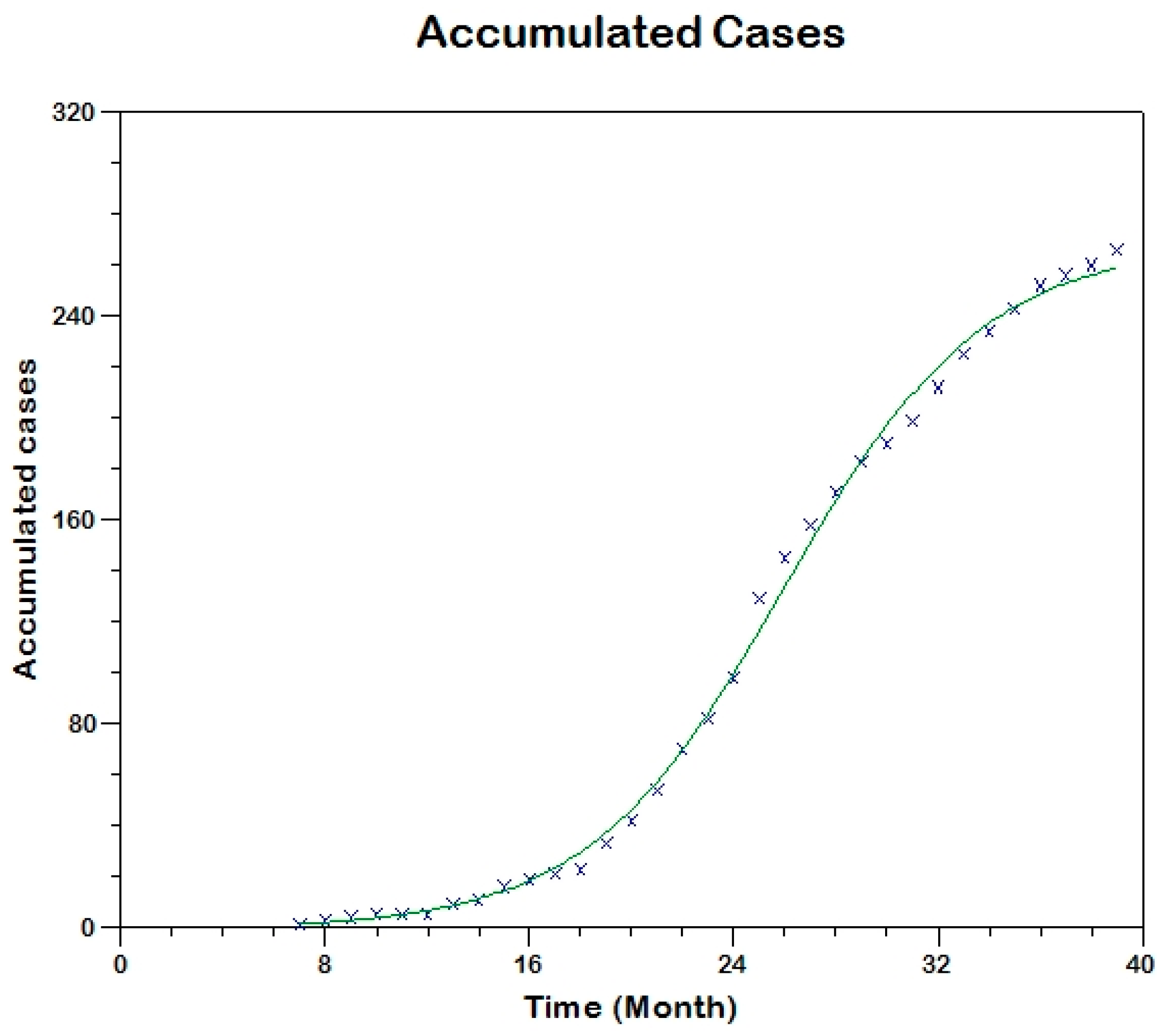
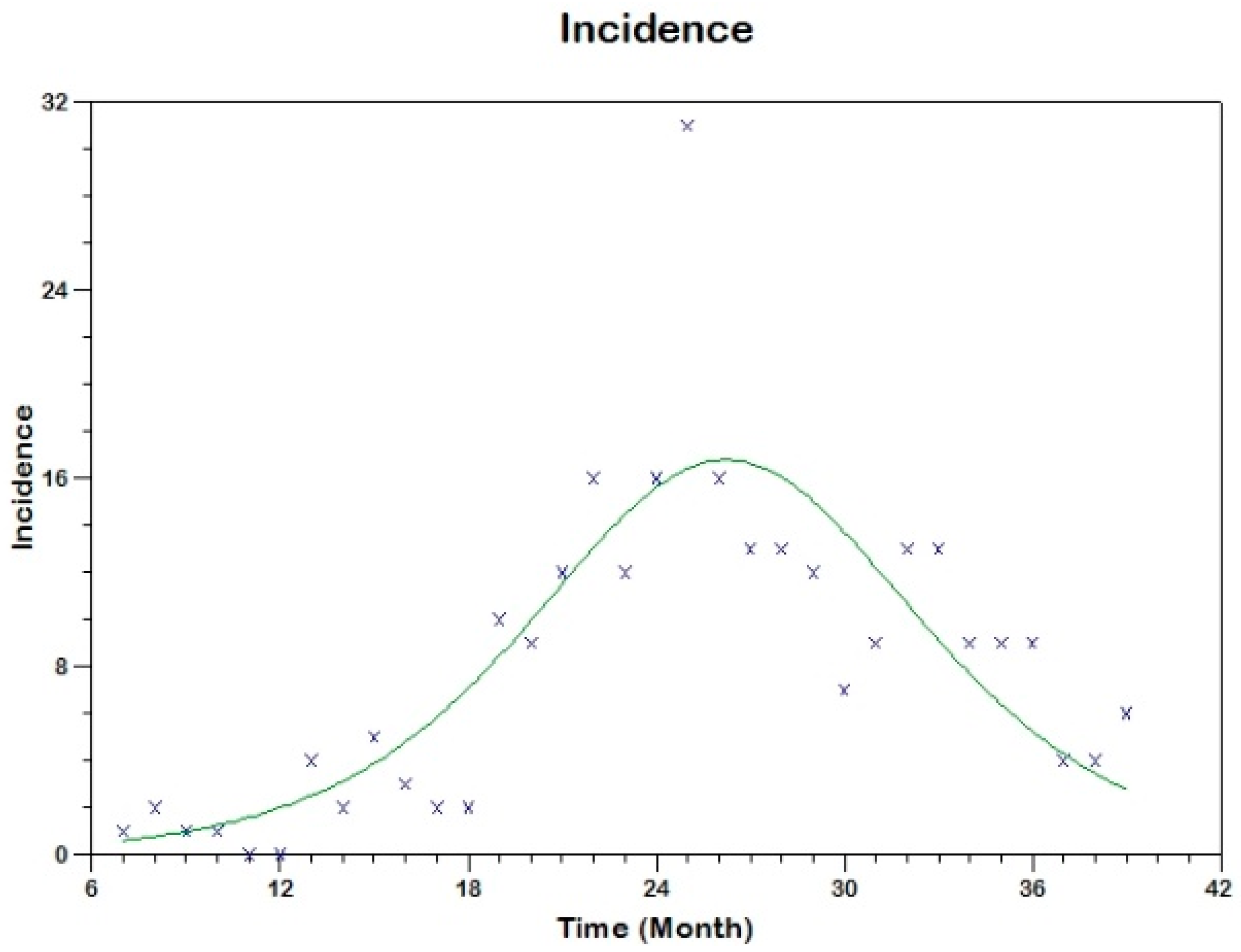
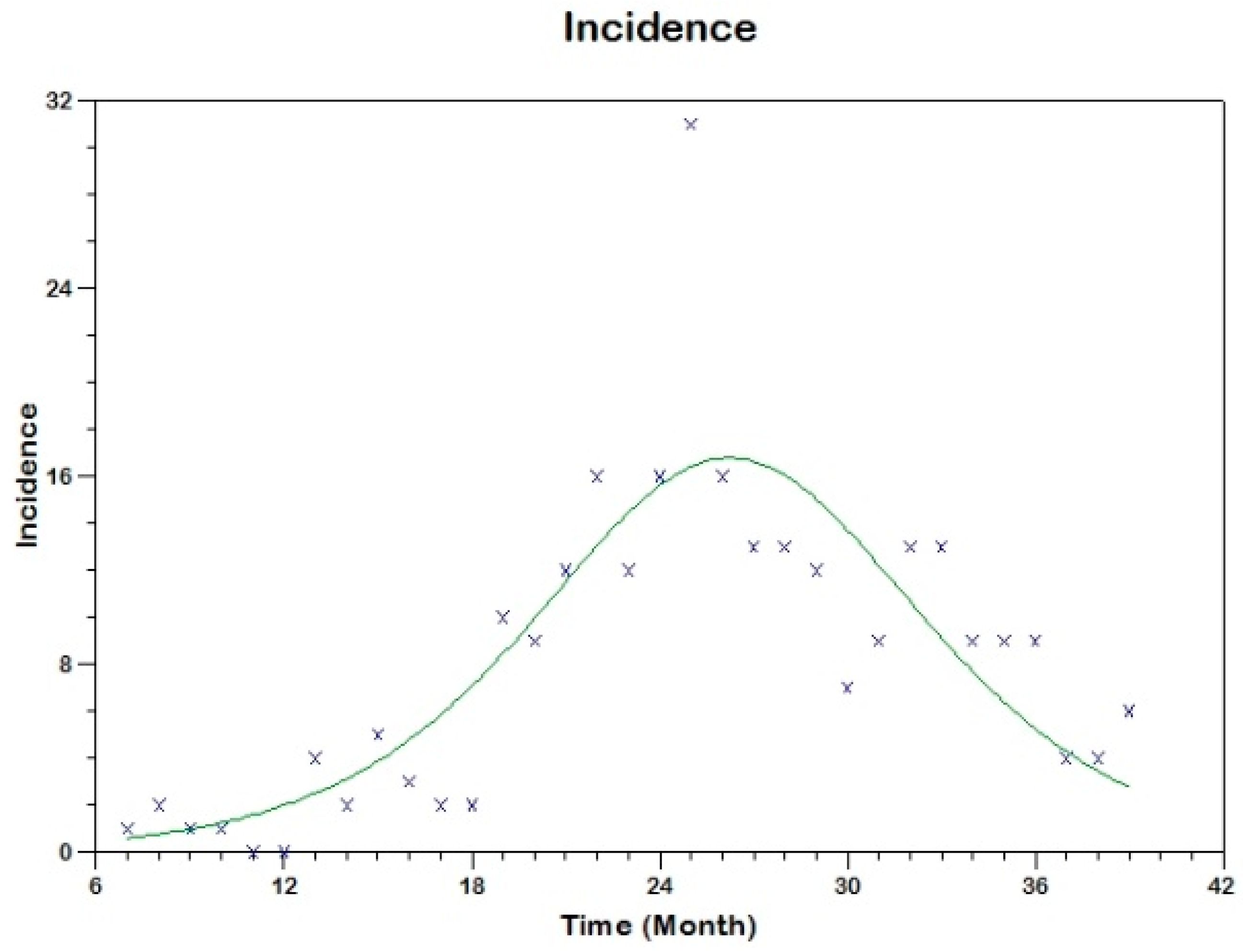
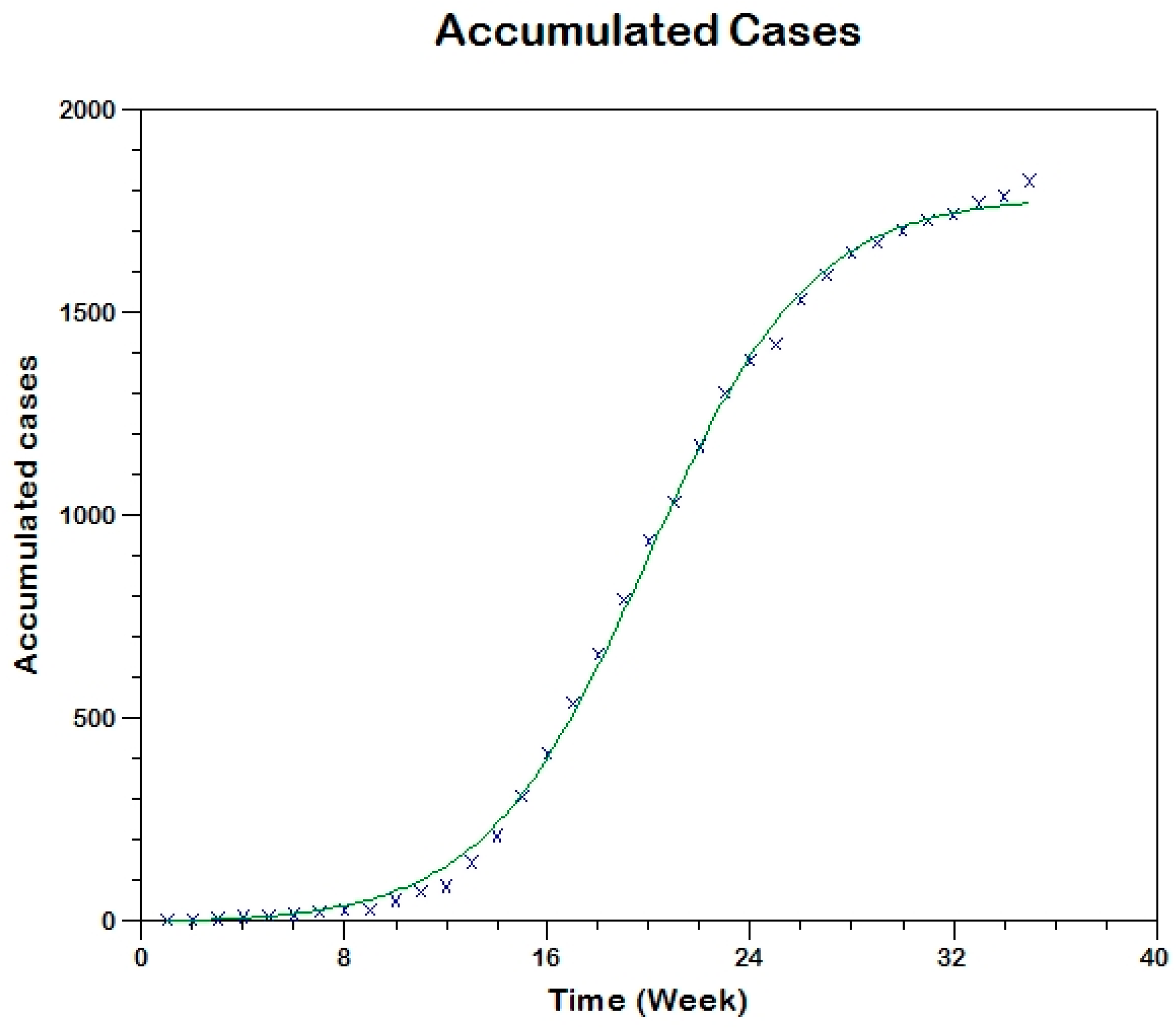
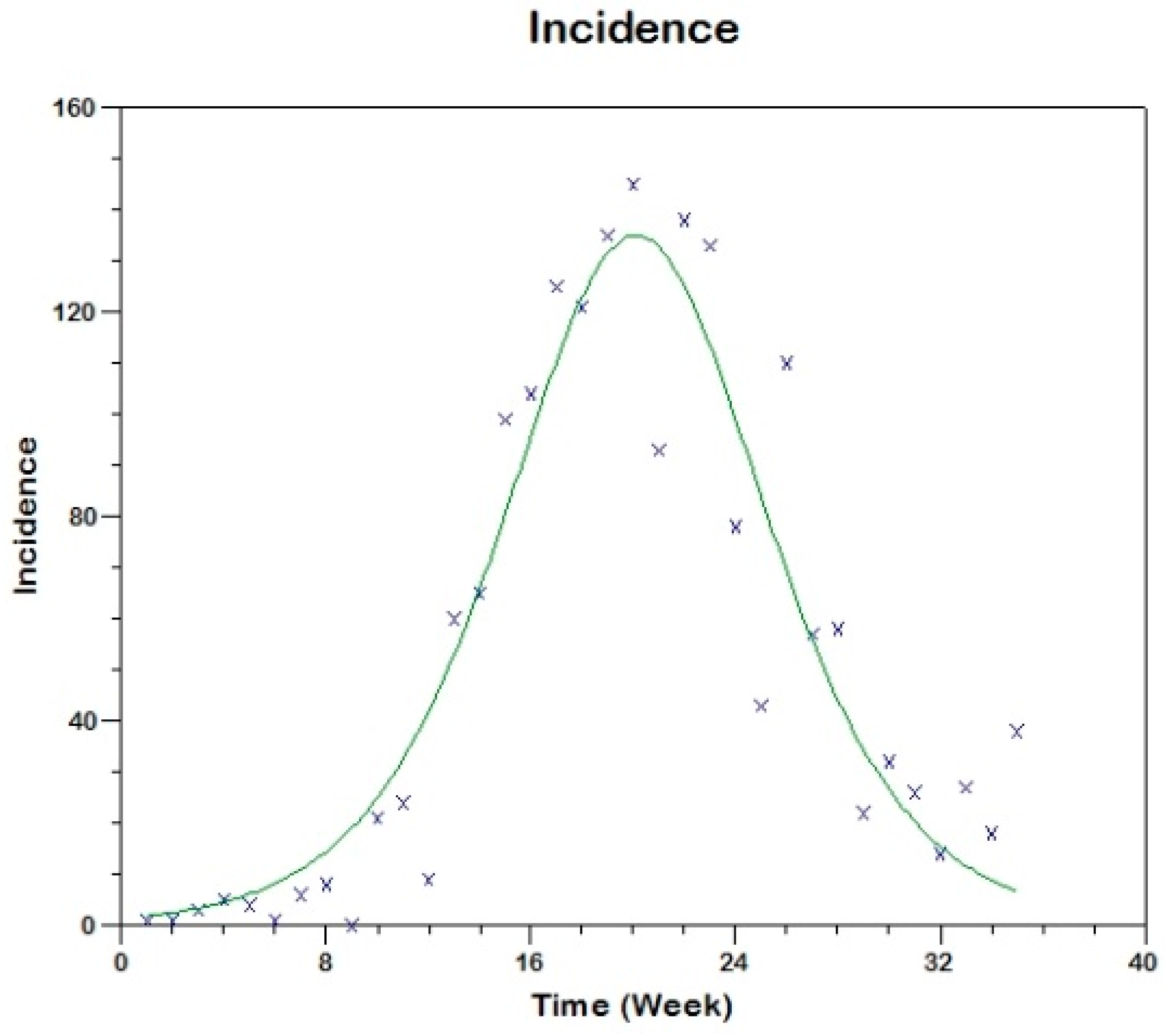
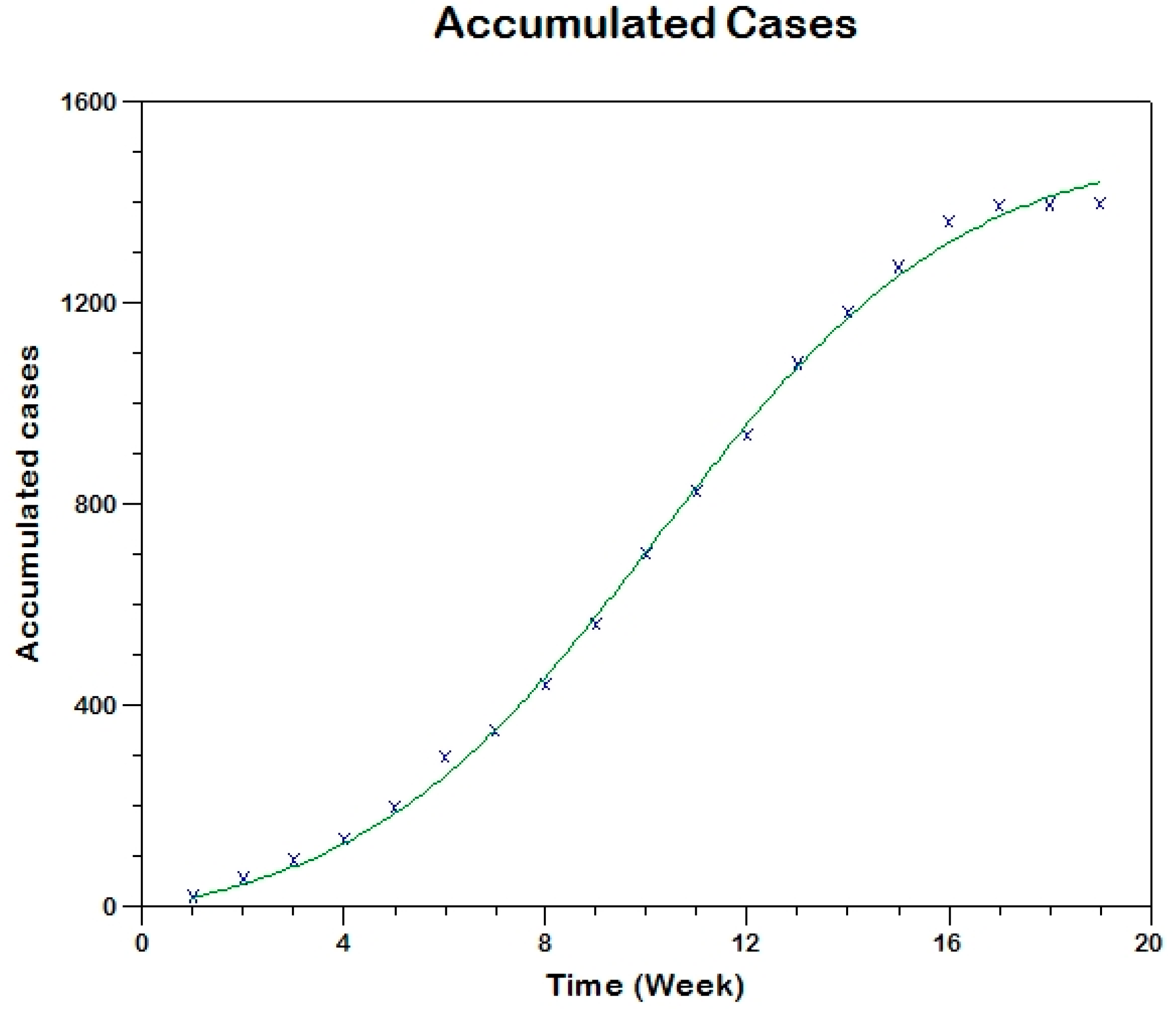
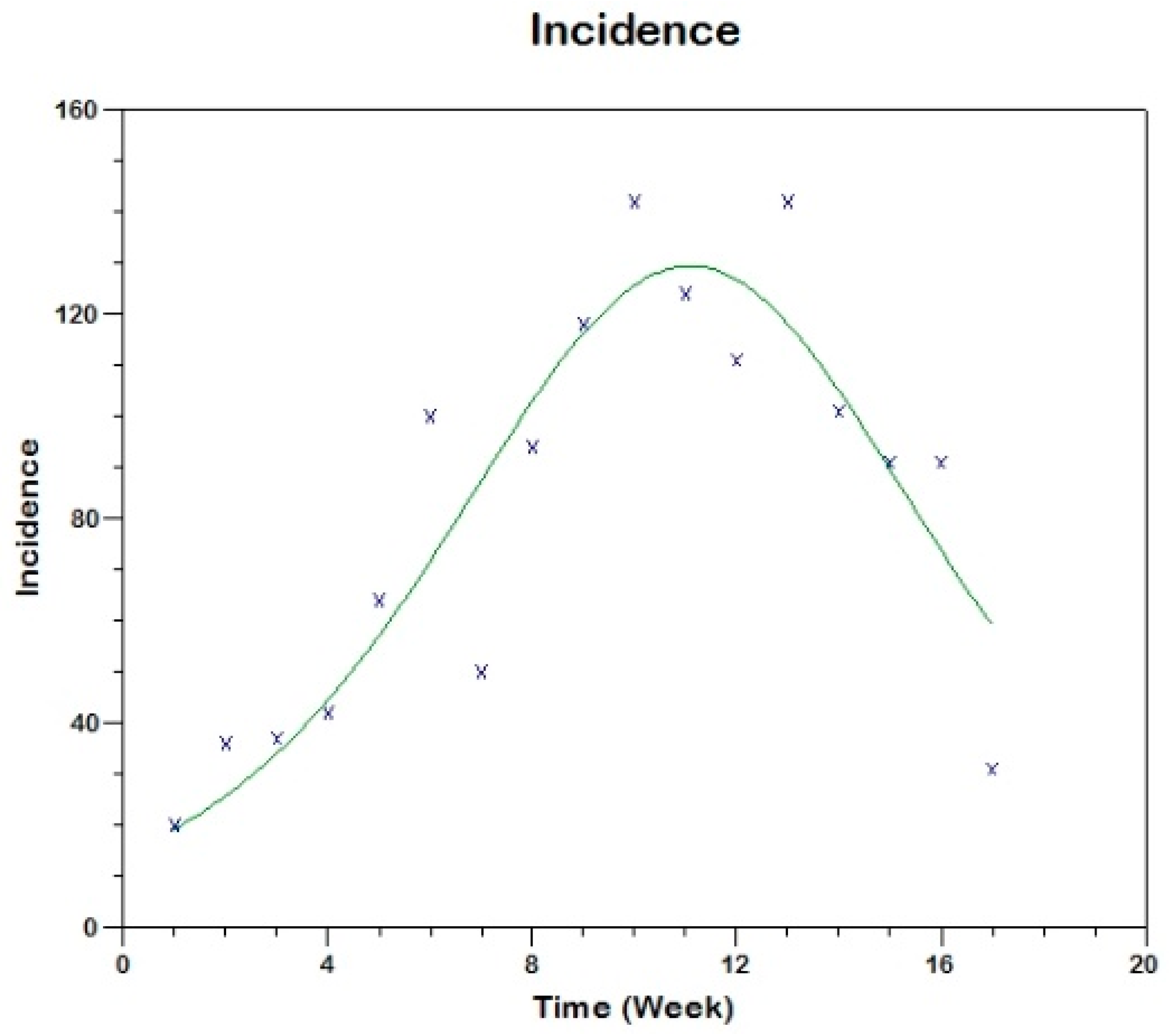
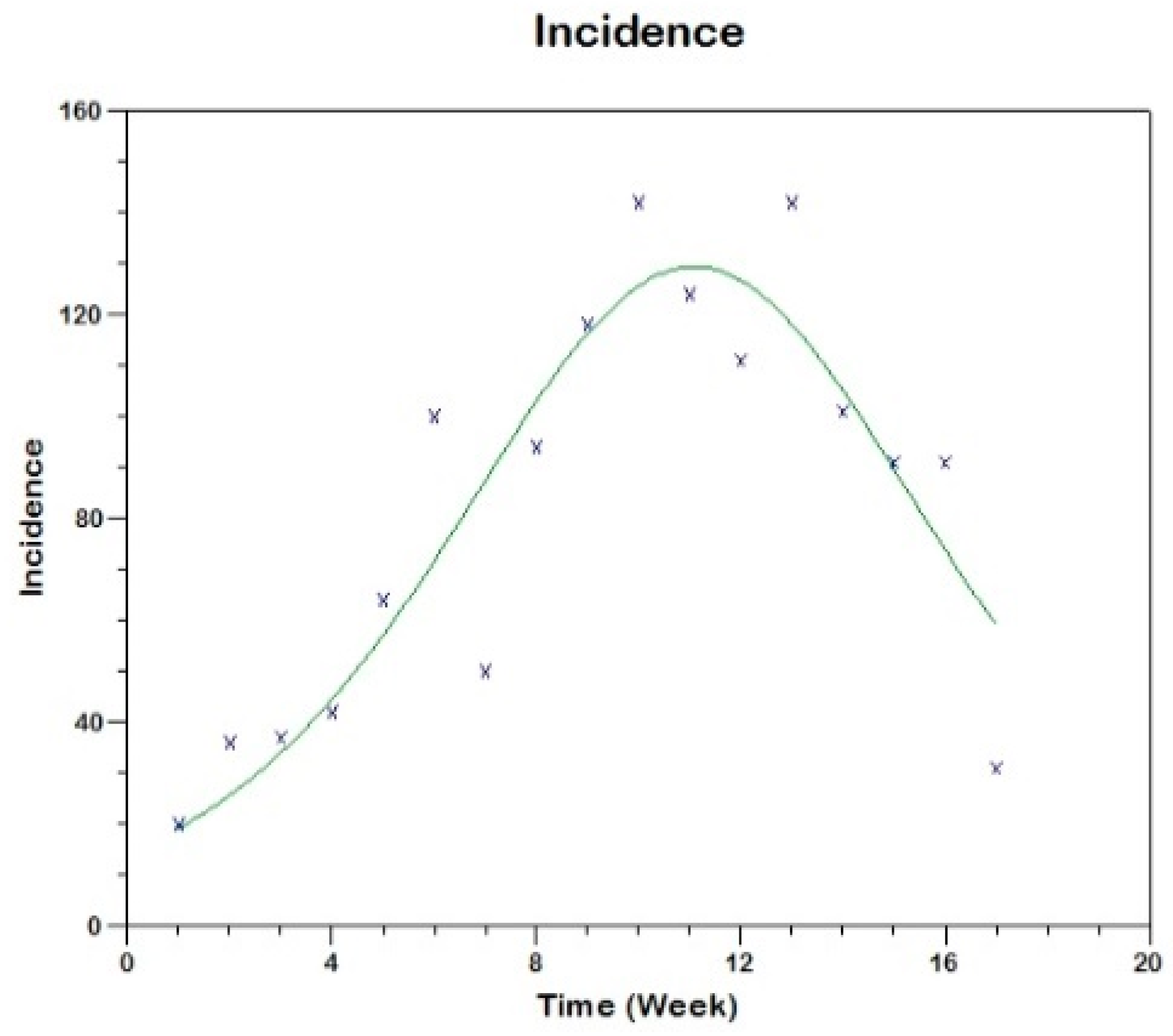
Prior to the estimation of model parameters, outbreak-related CL incidence data from the two ecologically and epidemiologically different regions (Tolima, Colombia and Madrid, Spain) are first analyzed. Various differences are found in the outbreaks: (i) The Madrid incidence data were in months whereas the Tolima data were collected every week (this suggests a difference in the reporting systems of the two countries and potentially different infectious and latent periods between the regions), (ii) The outbreak in Madrid peaked in winters (Dec and Jan) as compared to the outbreak in Tolima, where the highest incidence was observed in Spring (April) and fall (in October), (iii) Each of the Tolima outbreaks was short lived (1/2 year) whereas the outbreak in Madrid lasted for 3 years (the Madrid outbreak was from 2009 to 2012 whereas the two Tolima outbreaks both occurred during 2016), (iv) there were two successive outbreaks in Tolima whereas there was a single outbreak in Madrid (the first outbreak in Tolima was much more lethal than its second outbreak), and (v) Dog reservoirs were important in the Madrid transmission cycle but in the Tolima outbreak, the frequently moving military population played a critical role in its spread.
The key parameter describing the spread of an infection is the basic reproduction numbers,
R0, which is defined as the number of secondary infections generated by an infected index case in otherwise susceptible population. This study uses mathematical models to estimate
R0 for the 2009–2012 CL outbreak in Madrid and the two CL outbreaks in Tolima during 2016. The mean estimates of
R0 are found to be 3.1 for Madrid, 1.2 for the first outbreak of Tolima and 1.01 for the second outbreak of Tolima. The
R0 estimate for Madrid seems to be significantly higher than corresponding estimate for Tolima. This could be a result of differences in the population density (60 persons/km
2 in Tolima vs. 5400 persons/km
2 in Madrid), climatic factors, human mobility, and/or health disparities in sub-communities [
41]. In the Madrid outbreak, dogs were the main reservoir host,
P. perniciosus was the principal vector of leishmania and
L. infantum was the primary parasite species [
5]. Epidemic outbreaks of CL in Tolima were caused by
L. braziliensis,
L. guyanensis and
L. panamensis, with intra- and peri-domiciliary transmission. The military showed the highest incidence of CL due to the continuous deployment of troops to areas of high endemicity.
Risk factors for CL such as urbanization, malnutrition, health seeking behaviors and disparity have been reported in the literature [
42]; however, their impact on the dynamics of CL is less known [
43]. This study attempts to provide a simple framework by which the impact of risk factors can be captured using limited reported data. We made some simplifying assumptions to reduce the dimension of the models and to obtain an explicit analytical formula for estimating
R0. The data used here for fitting the model were obtained through passive case detection and therefore may be prone to high underreporting. Nevertheless, the results in this study suggest a
One Health perspective, for example, if animals are key reservoirs of CL, interventions should not be human focused only, instead control programs should be heterogeneous, focusing on both human and animal hosts [
44]. It suggests identification of disease-affected communities also in
Blue Marble Health countries, wealthy nations with high GDP but also high endemicity of neglected diseases in hidden pockets [
41]. Thus, the current estimation study needs to also include proper cost analysis in order to study transmission dynamics comprehensively [
45]. As more data accumulate in the future, a more thorough analysis will allow for more accurate estimates of
R0, together with less uncertainty around them and greater understanding of impact of socio-economic conditions on its estimates.