A Tubing-Free, Microfluidic Platform for the Realization of Physiologically Relevant Dosing Curves on Cellular Models †
Abstract
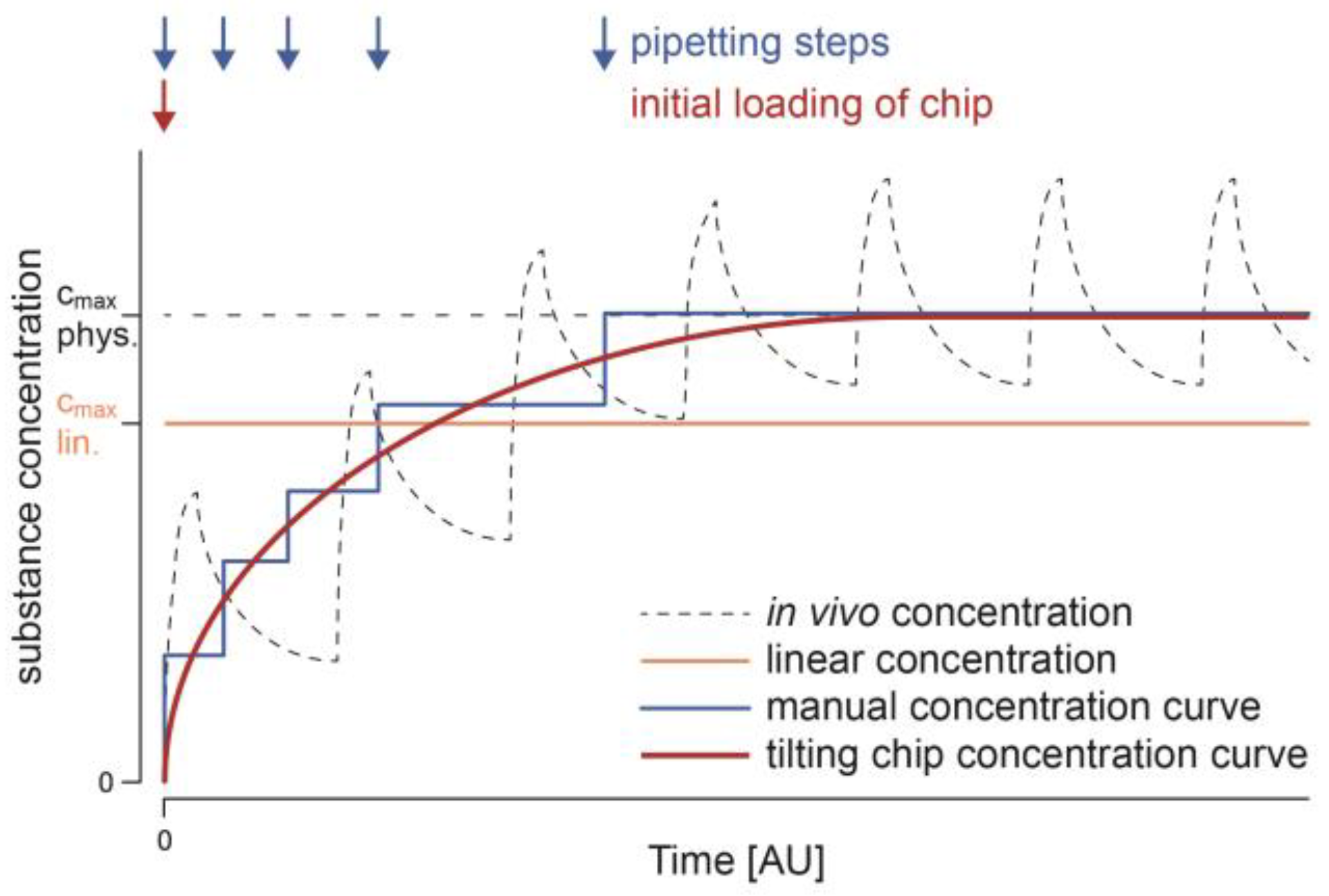
:1. Introduction
2. Methods
2.1. Fabrication
2.2. Device Operation
2.3. Assessment of Concentration Changes
2.4. Microtissue Analyses
3. Results and Discussion
4. Conclusions and Outlook
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Leist, M.; Hartung, T.; Nicotera, P. The dawning of a new age of toxicology. ALTEX 2008, 25, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Tsaioun, K.; Blaauboerand, B.J.; Hartung, T. Evidence-based absorption, distribution, metabolism, excretion (ADME) and its interplay with alternative toxicity methods. ALTEX 2016, 33, 343–358. [Google Scholar] [CrossRef]
- Kim, J.Y.; Fluri, D.A.; Marchan, R.; Boonen, K.; Mohanty, S.; Singh, P.; Hammad, S.; Landuyt, B.; Hengstler, J.G.; Kelm, J.M.; et al. 3D spherical microtissues and microfluidic technology for multi-tissue experiments and analysis. J. Biotechnol. 2015, 205, 24–35. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.; Ajdari, A.; Mezic, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lohasz, C.; Frey, O.; Renggli, K.; Hierlemann, A. A Tubing-Free, Microfluidic Platform for the Realization of Physiologically Relevant Dosing Curves on Cellular Models. Proceedings 2017, 1, 497. https://doi.org/10.3390/proceedings1040497
Lohasz C, Frey O, Renggli K, Hierlemann A. A Tubing-Free, Microfluidic Platform for the Realization of Physiologically Relevant Dosing Curves on Cellular Models. Proceedings. 2017; 1(4):497. https://doi.org/10.3390/proceedings1040497
Chicago/Turabian StyleLohasz, Christian, Olivier Frey, Kasper Renggli, and Andreas Hierlemann. 2017. "A Tubing-Free, Microfluidic Platform for the Realization of Physiologically Relevant Dosing Curves on Cellular Models" Proceedings 1, no. 4: 497. https://doi.org/10.3390/proceedings1040497




