Processing Stability and the Significance of Variation in Extrusion Speeds and Temperatures on SSB® 55 Pharma Grade Shellac for Oral Drug Delivery
Abstract
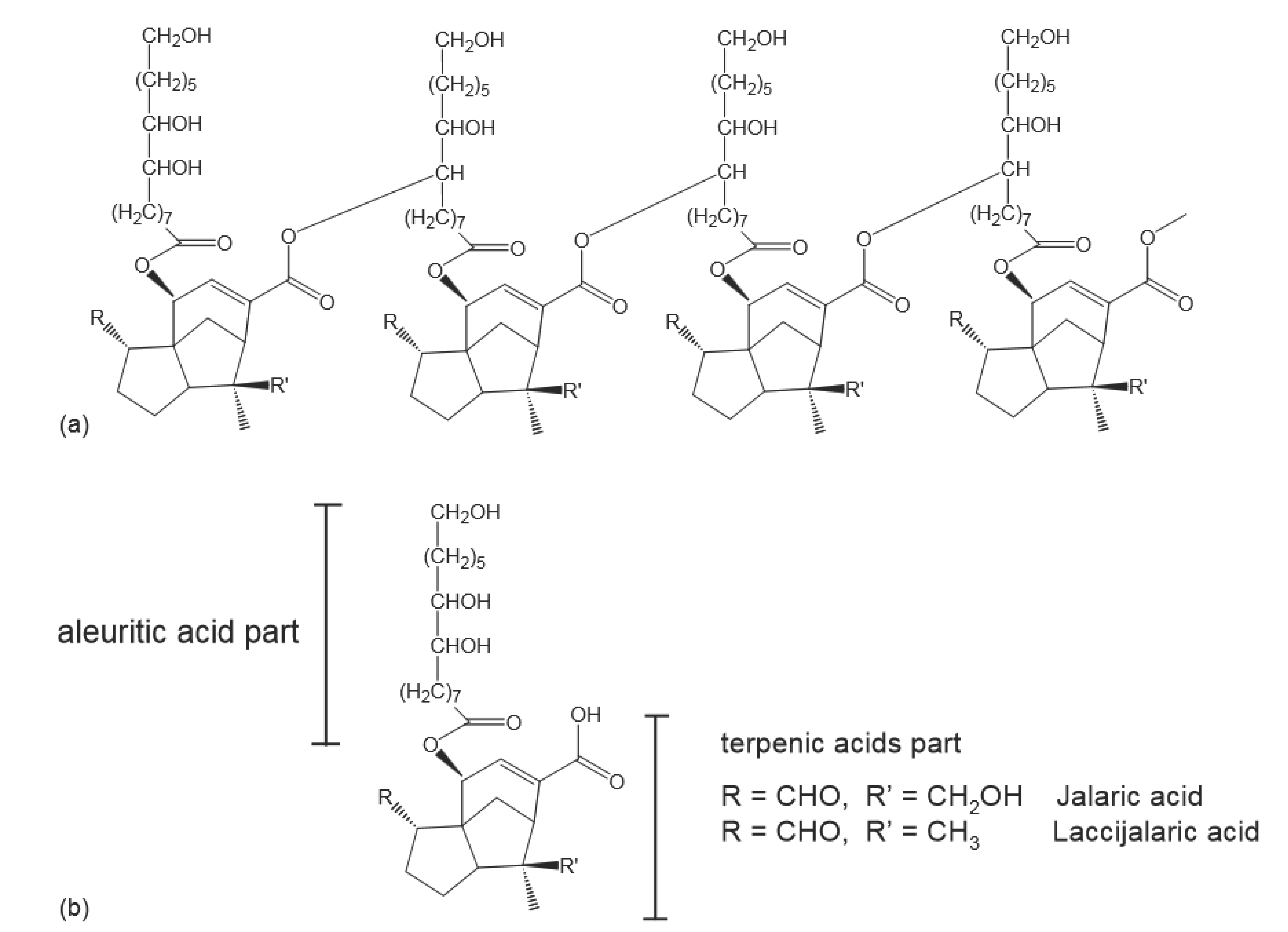
:1. Introduction
2. Materials and Methods
2.1. Hot Melt Extrusion Conditions
2.2. Differential Scanning Calorimetry (DSC)
2.3. Rheological Analysis
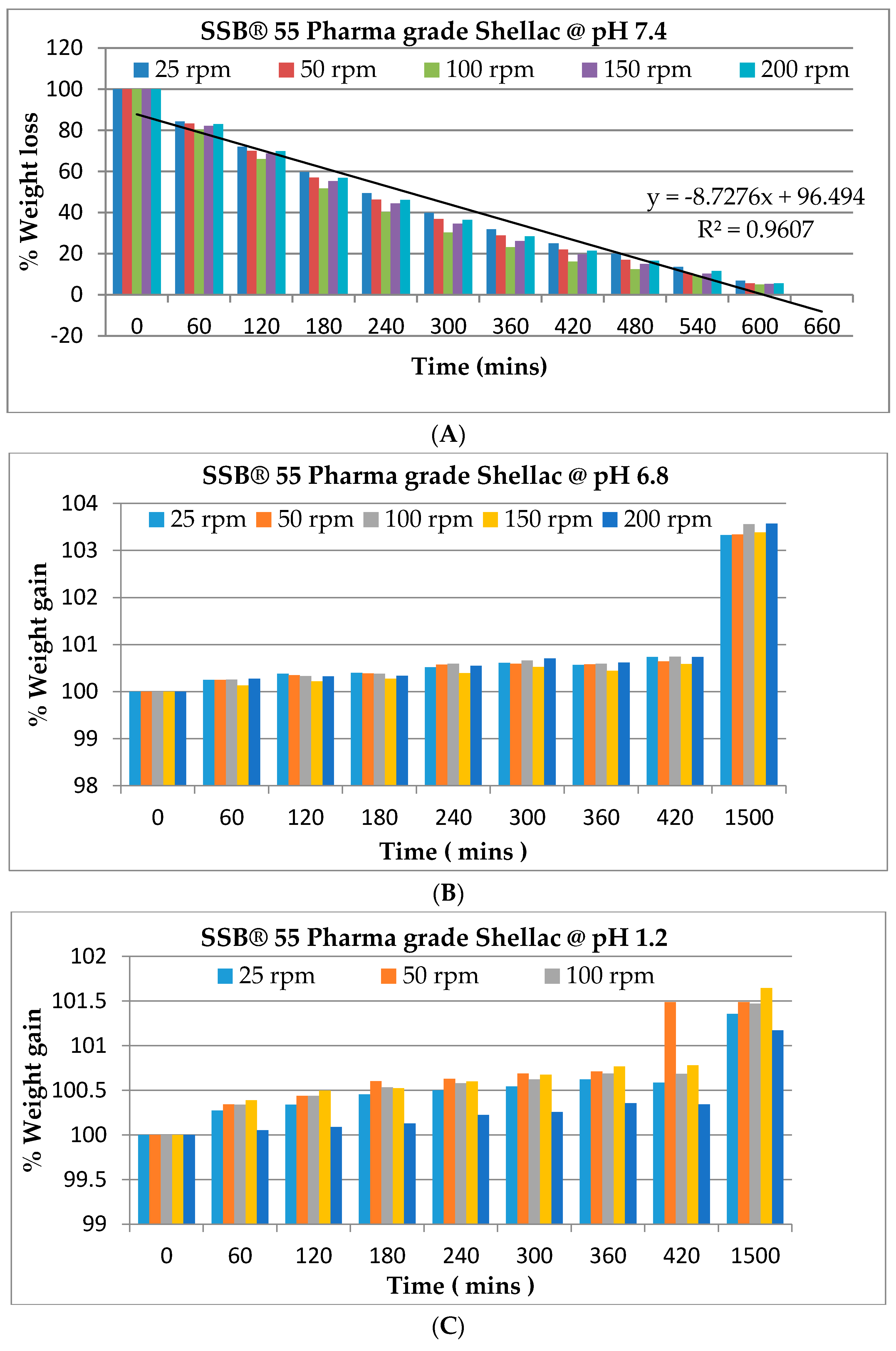
2.4. Swelling/Degradation Studies
3. Results
3.1. Hot Melt Extrusion
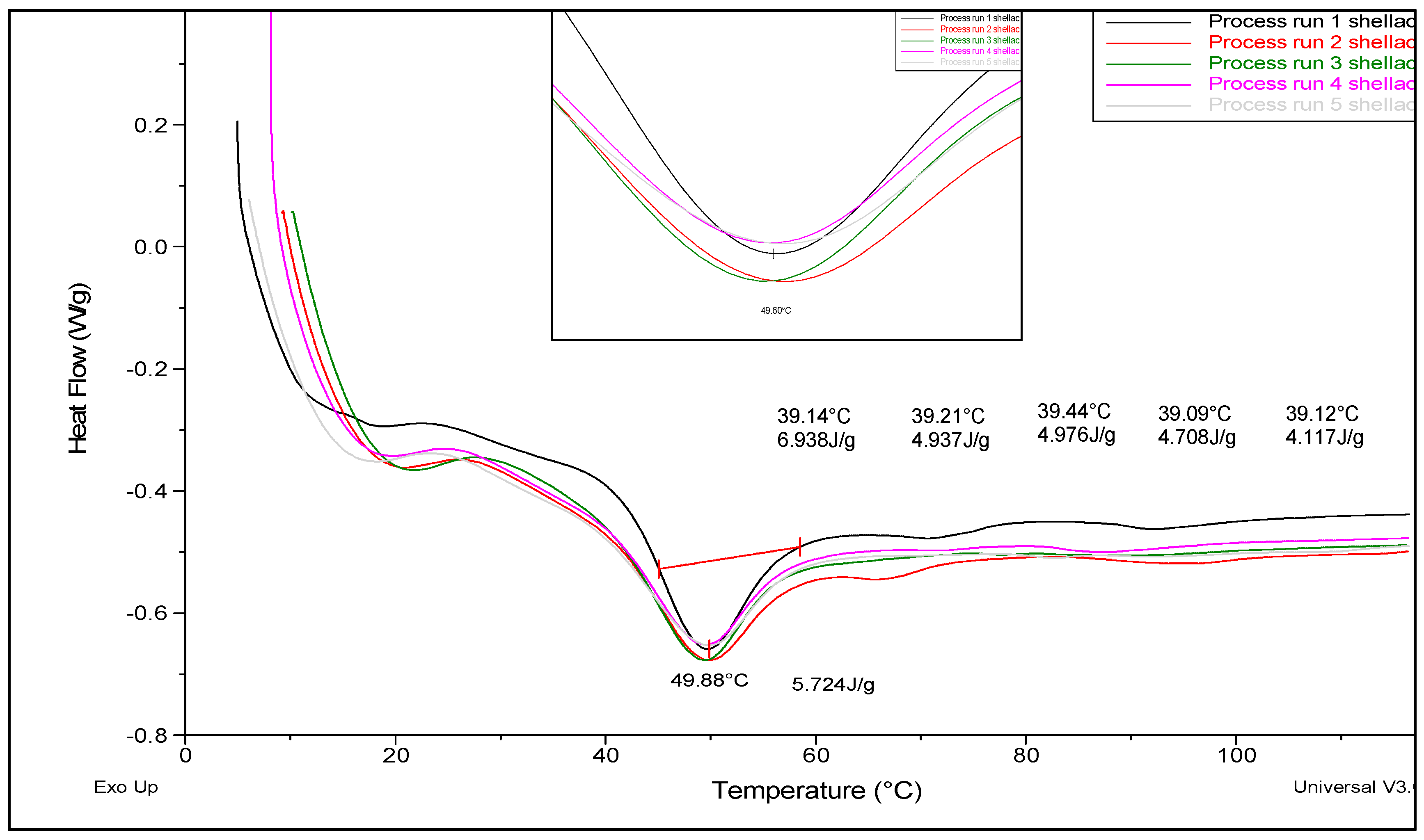
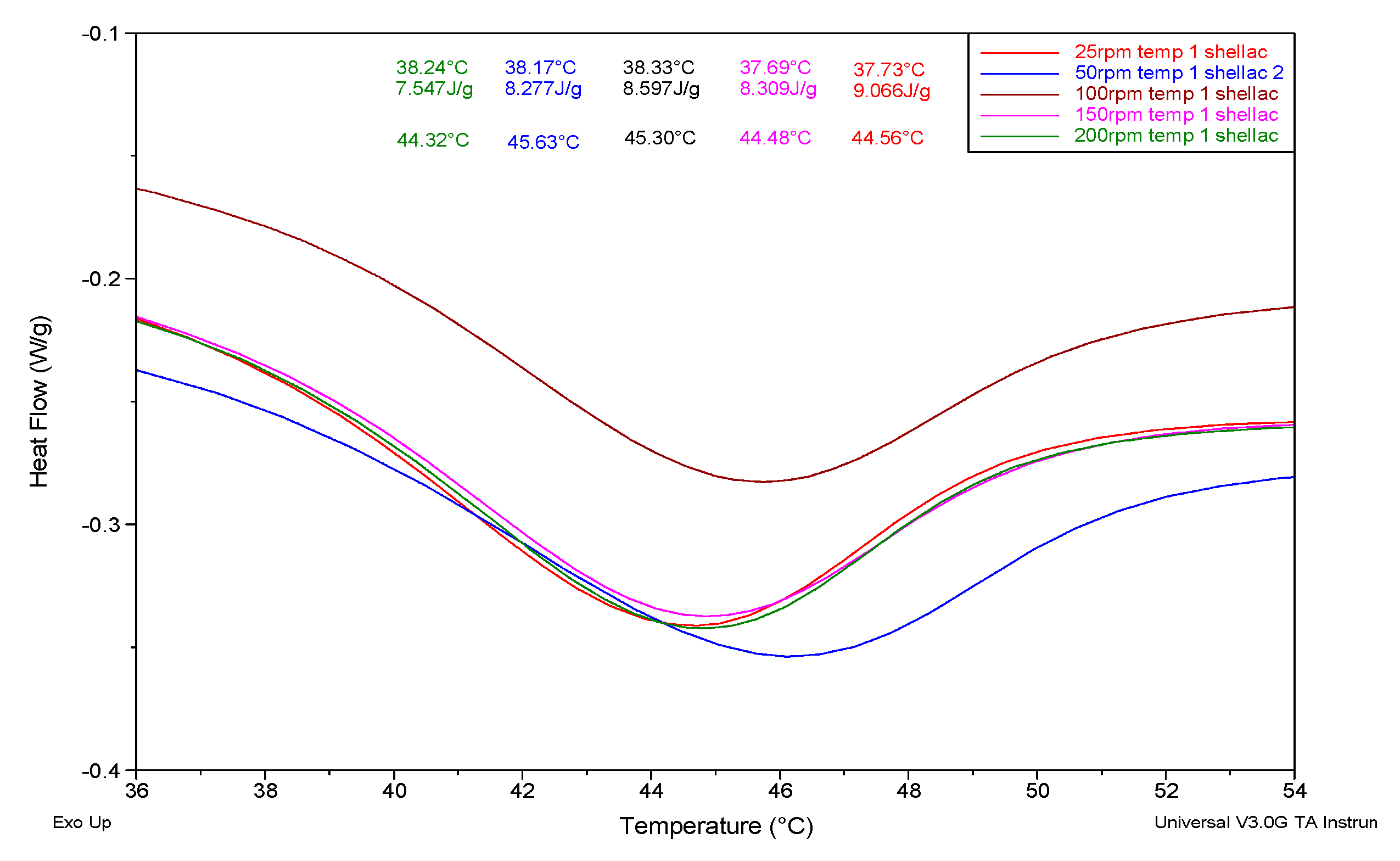
3.2. Differential Scanning Calorimetry
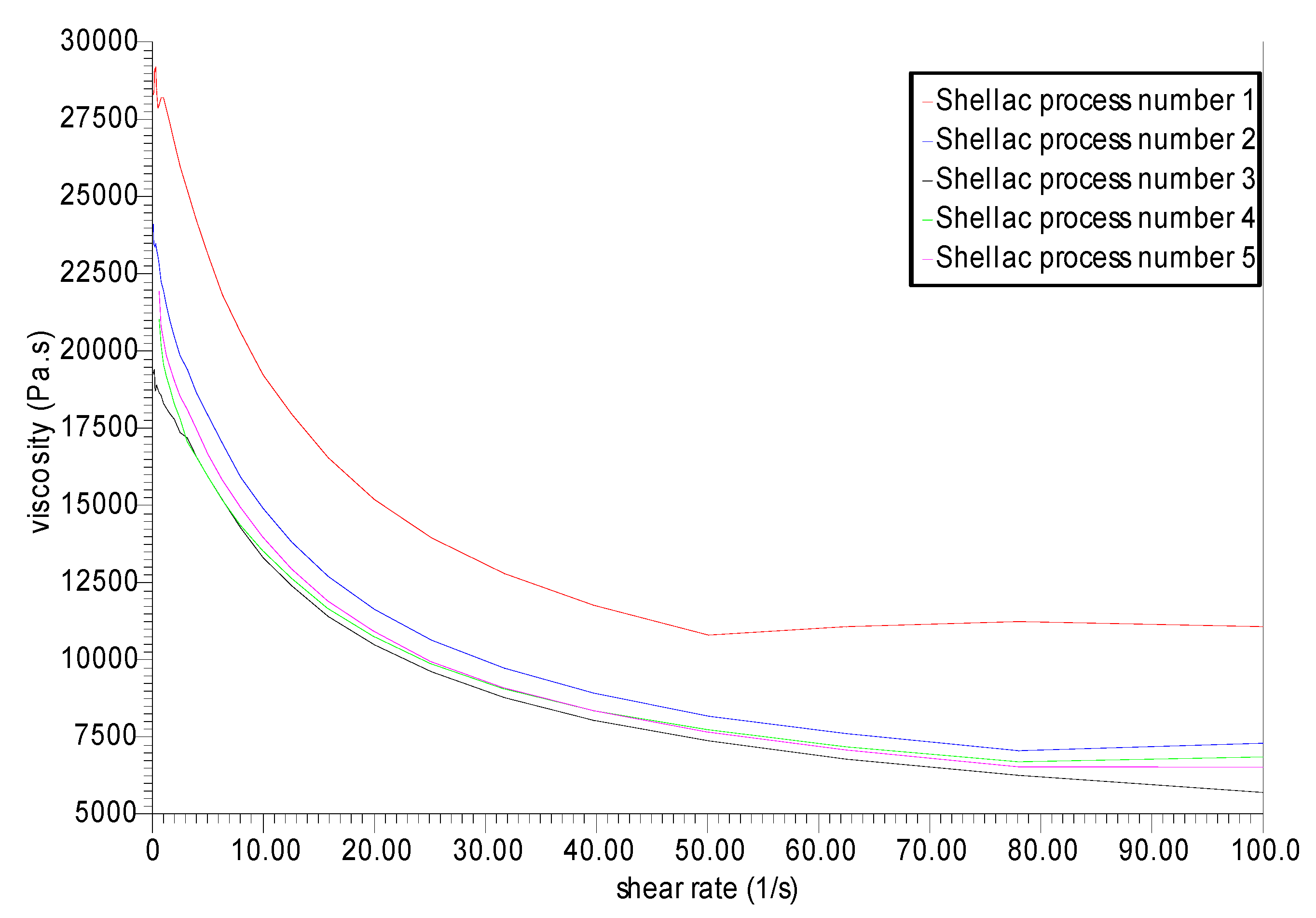
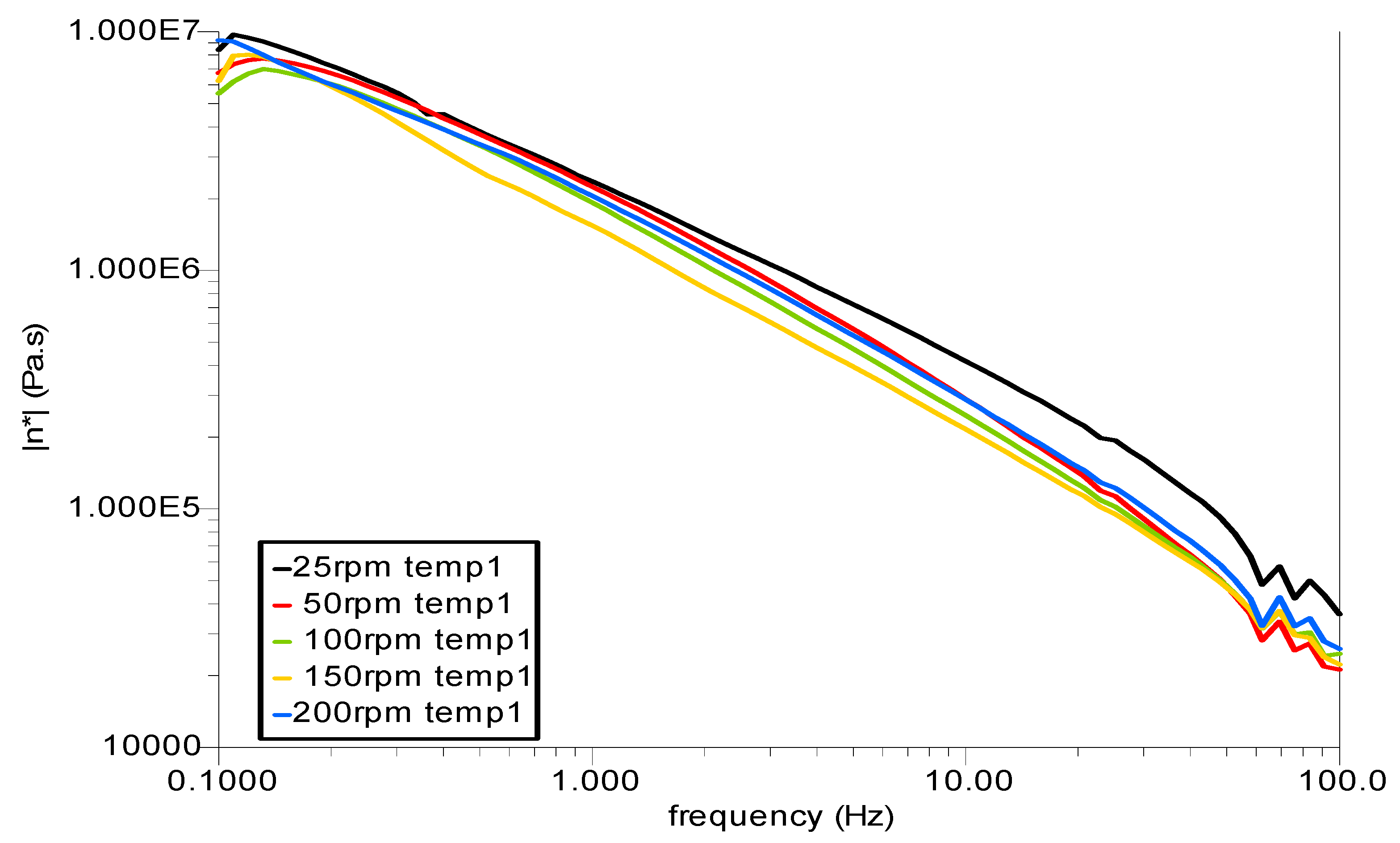
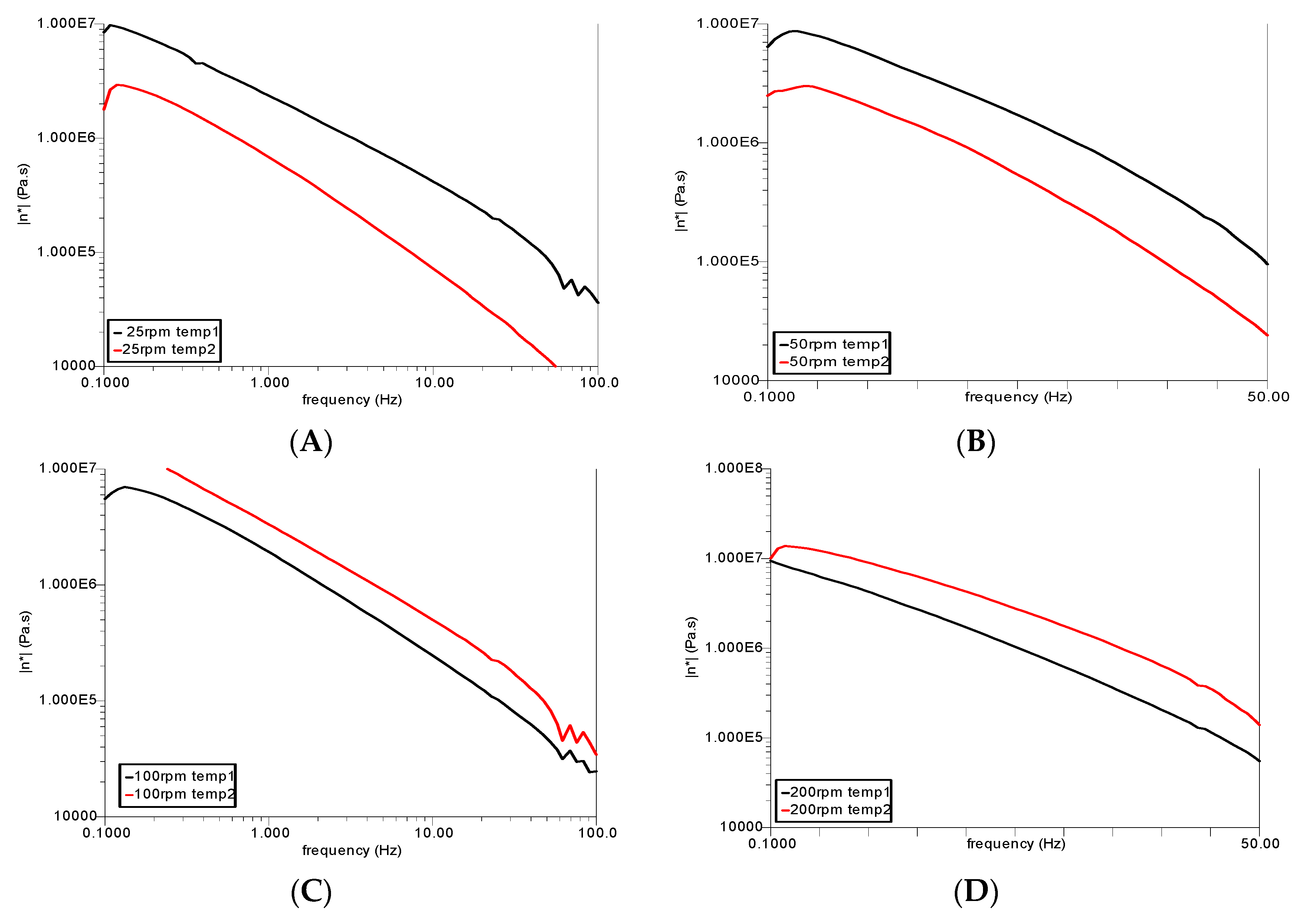
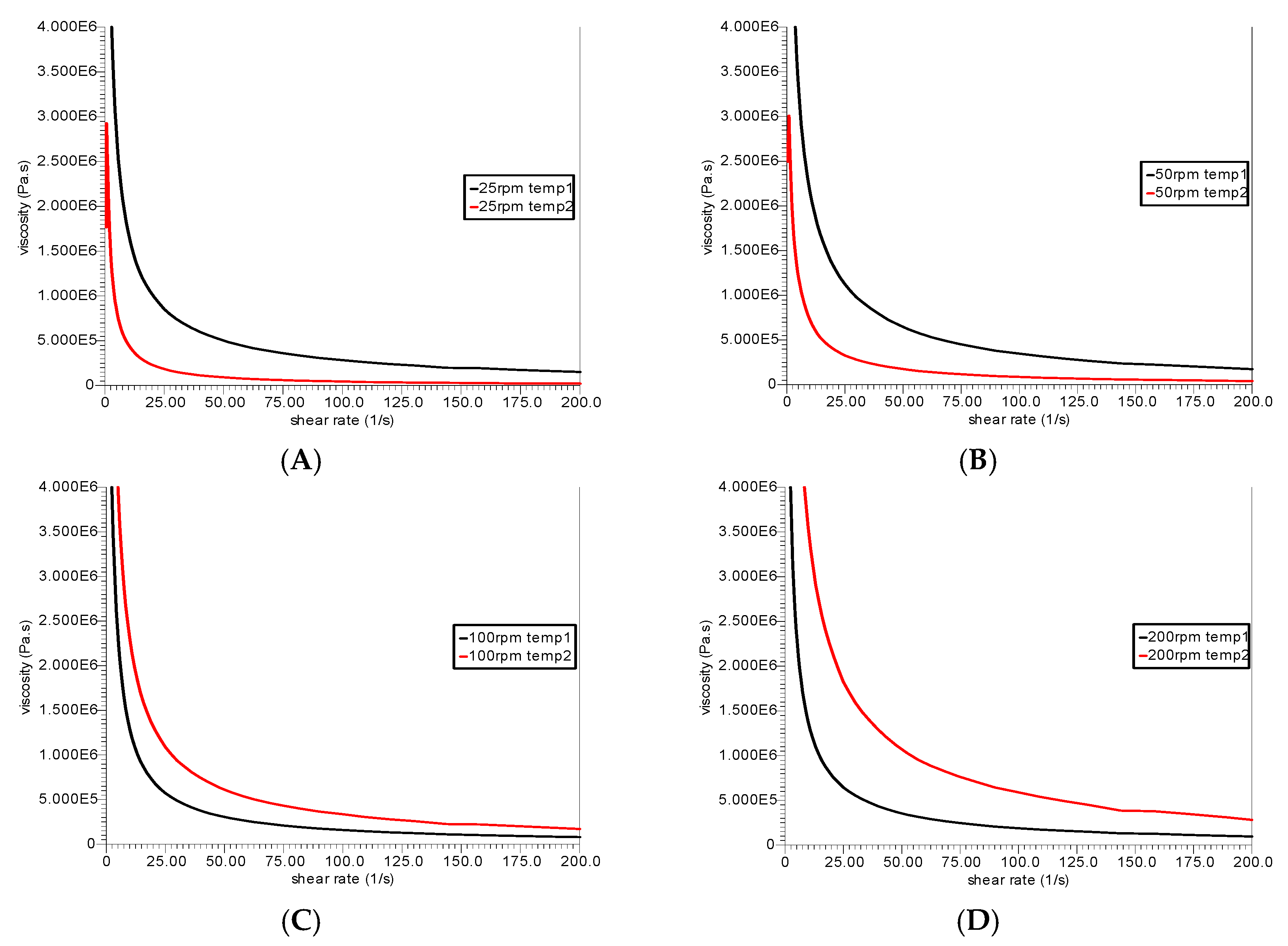
3.3. Rheological Analysis
3.4. Swelling Studies of Variation in Screw Speed and Extrusion Temperature on a Novel SSB® 55 Pharma Grade Shellac
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Farag, Y.; Leopold, C.S. Physicochemical Properties of Various Shellac Types. Dissolut. Technol. 2009, 16, 33–39. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Crowley, M.M.; Zhang, F.; Koleng, J.J.; Mcginity, J.W. Stability of polyethylene oxide in matrix tablets prepared by hot-melt extrusion. Biomaterials 2002, 23, 4241–4248. [Google Scholar] [CrossRef]
- Lyons, J.G.; Blackie, P.; Higginbotham, C.L. The significance of variation in extrusion speeds and temperatures on a PEO/PCL blend based matrix for oral drug delivery. Int. J. Pharm. 2008, 351, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach, J. Melt extrusion: From process to drug delivery technology. Eur. J. Pharm. Biopharm. 2002, 54, 107–117. [Google Scholar] [CrossRef]
- Brydson, J.A. Plastics Materials; Elsevier Science: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Ambrósio, J.D.; Pessan, L.A.; Otaguro, H.; Chinelatto, M.A.; Junior, E.H. The effect of extrusion conditions and the use of a compatibilizer in the crystallization of PBT/ABS blends. Mater. Res. 2013, 16, 1220–1228. [Google Scholar] [CrossRef]
- Goswami, D.N.; Jha, P.C.; Mahato, K. Rheological studies of shellac. Pigment Resin Technol. 2003, 32, 107–112. [Google Scholar] [CrossRef]
- Rosen, S.L. Fundamental Principles of Polymeric Materials; Wiley: Hoboken, NJ, USA, 1971. [Google Scholar]
- Alzahrani, H.; Bedir, Y.; Al-Hayani, A. Efficacy of shellac, a natural product, for the prevention of wet gangrene. J. Int. Med. Res. 2013, 41, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Limmatvapirat, S.; Limmatvapirat, C.; Luangtana-anan, M.; Nunthanid, J.; Oguchi, T.; Tozuka, Y.; Yamamoto, K.; Puttipipatkhachorn, S. Modification of physicochemical and mechanical properties of shellac by partial hydrolysis. Int. J. Pharm. 2004, 278, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Limmatvapirat, S.; Nunthanid, J.; Luangtana-Anan, M.; Puttipipatkhachorn, S. Effect of alkali treatment on properties of native shellac and stability of hydrolyzed shellac. Pharm. Dev. Technol. 2005, 10, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Stummer, S.; Salar-Behzadi, S.; Unger, F.M.; Oelzant, S.; Penning, M.; Viernstein, H. Application of shellac for the development of probiotic formulations. Food Res. Int. 2010, 43, 1312–1320. [Google Scholar] [CrossRef]

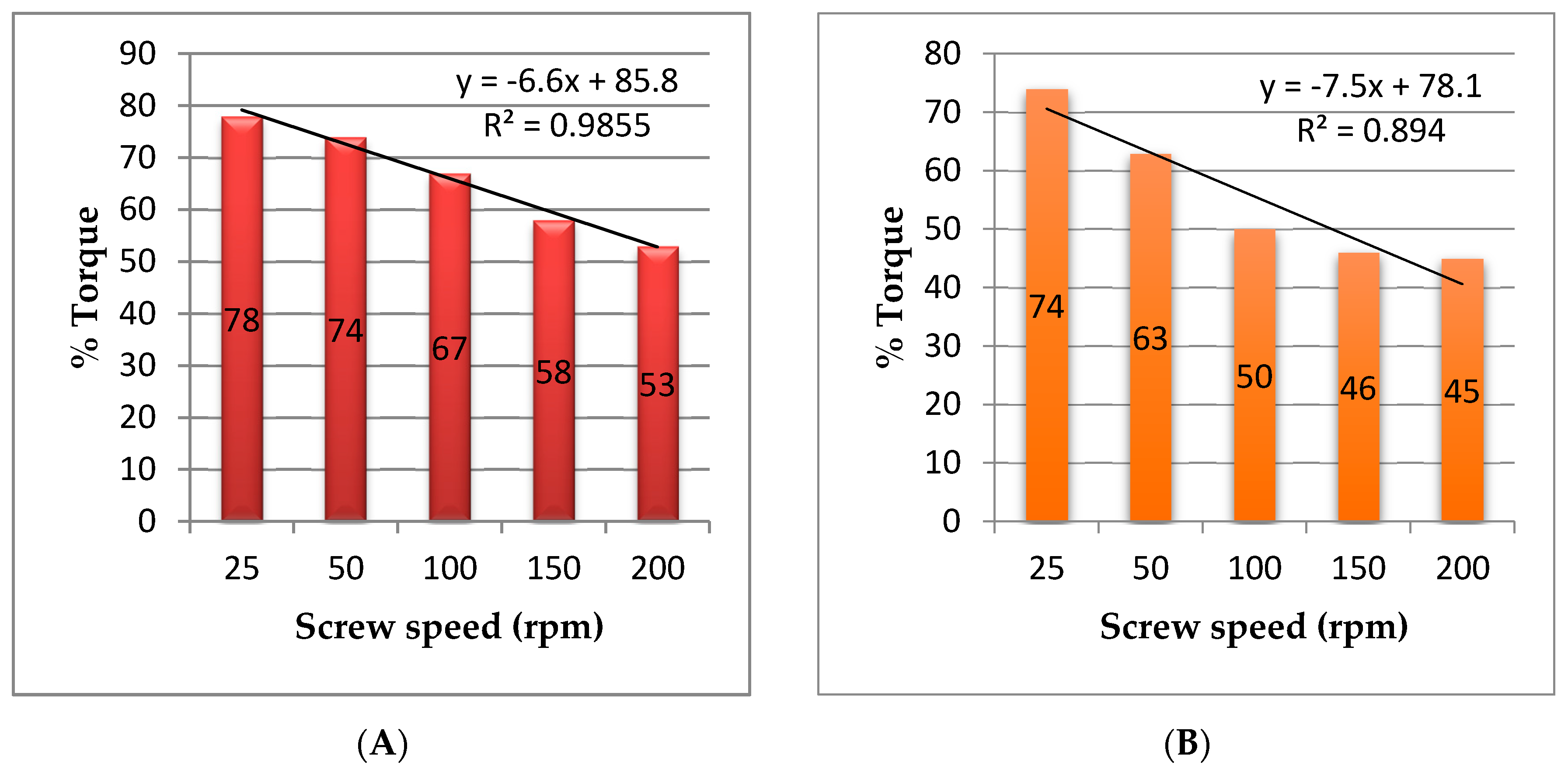


| Screw Speed (RPM) | Temperature (°C) | ||||||
|---|---|---|---|---|---|---|---|
| Zone 1 | Zone 2 | Zone 3 | Zone 4 | Zone 5 | Zone 6 | Die | |
| 100 | 30 | 35 | 40 | 50 | 60 | 65 | 70 |
| Screw Speed (RPM) | Temperature (°C) | |||||||
|---|---|---|---|---|---|---|---|---|
| Zone 1 | Zone 2 | Zone 3 | Zone 4 | Zone 5 | Zone 6 | Die | ||
| 50 | Temp. 1 | 30 | 35 | 40 | 50 | 60 | 65 | 70 |
| 50 | Temp. 2 | 40 | 45 | 50 | 60 | 70 | 80 | 90 |
| Sample | Peak Temperature (°C) | Enthalpy (J/g) | Relative Crystallinity (%) |
|---|---|---|---|
| Base Material | 56.47 | 3.61 | 100.00 |
| 25 rpm | 44.32 | 7.55 | 208.88 |
| 50 rpm | 45.63 | 8.28 | 229.09 |
| 100 rpm | 45.30 | 8.60 | 237.95 |
| 150 rpm | 44.48 | 8.31 | 229.98 |
| 200 rpm | 44.56 | 9.07 | 250.93 |
| Sample | Temp Profile 1 (Tm °C) | Temp Profile 2 (Tm °C) | Temp. Profile 1 Enthalpy (J/g) | Temp. Profile 2 Enthalpy (J/g) |
|---|---|---|---|---|
| 25 rpm | 44.32 | 44.57 | 9.47 | 8.99 |
| 50 rpm | 45.63 | 45.34 | 7.09 | 6.64 |
| 100 rpm | 45.30 | 45.17 | 9.42 | 8.99 |
| 150 rpm | 44.48 | 45.37 | 8.25 | 8.81 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gately, N.M.; Kennedy, J.E. Processing Stability and the Significance of Variation in Extrusion Speeds and Temperatures on SSB® 55 Pharma Grade Shellac for Oral Drug Delivery. J. Manuf. Mater. Process. 2017, 1, 9. https://doi.org/10.3390/jmmp1010009
Gately NM, Kennedy JE. Processing Stability and the Significance of Variation in Extrusion Speeds and Temperatures on SSB® 55 Pharma Grade Shellac for Oral Drug Delivery. Journal of Manufacturing and Materials Processing. 2017; 1(1):9. https://doi.org/10.3390/jmmp1010009
Chicago/Turabian StyleGately, Noel M., and James E. Kennedy. 2017. "Processing Stability and the Significance of Variation in Extrusion Speeds and Temperatures on SSB® 55 Pharma Grade Shellac for Oral Drug Delivery" Journal of Manufacturing and Materials Processing 1, no. 1: 9. https://doi.org/10.3390/jmmp1010009





