Numerical Simulation of Water Quenching of Large Size Steel Forgings: Effects of Macrosegregation and Grain Size on Phase Distribution
Abstract
:1. Introduction
2. Mathematical Models for Finite Element Analysis
2.1. Prediction of Phase Fraction
2.2. Transformation Kinetics
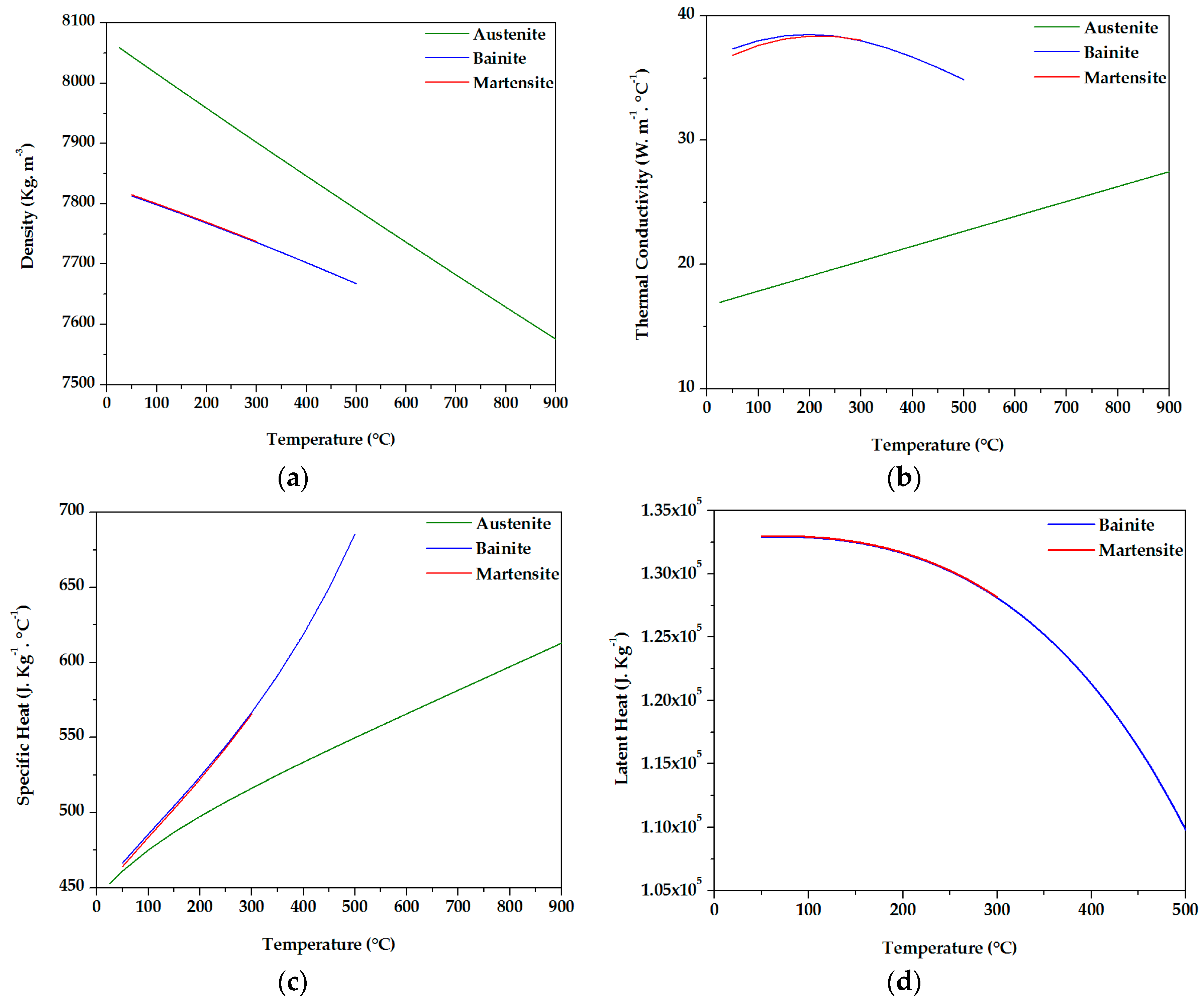
2.3. Model Buildup and Input Data
3. Results and Discussion
3.1. Effect of Carbon Contetnt
3.2. Effect of Variable Grain Size
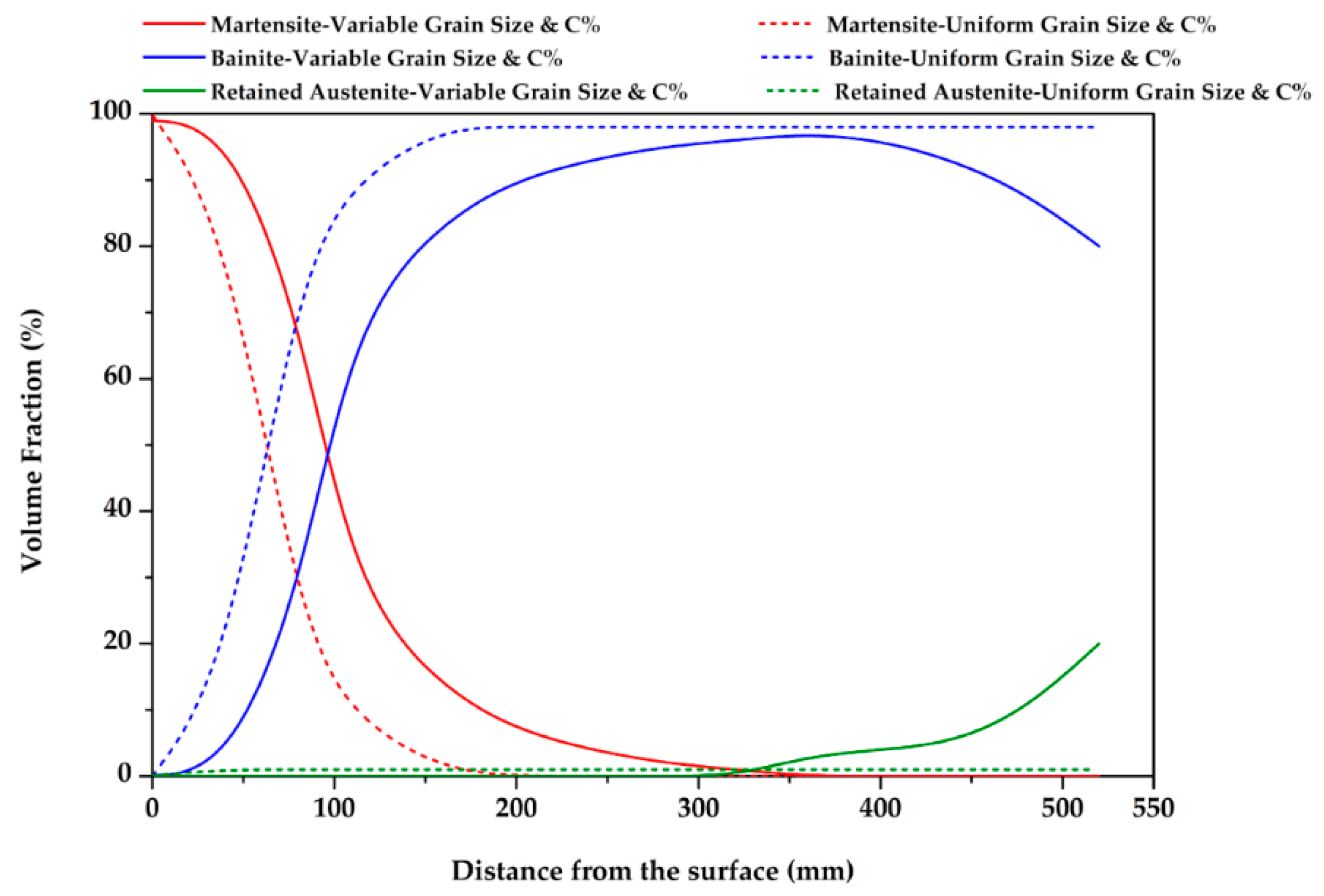
3.3. Effect of Variable Grain Size and Carbon Concentration
3.4. Experimental Validation
4. Conclusions
- The fastest variation in martensite volume fraction was observed for uniform grain size and carbon concentration while an increase in the carbon concentration in austenite led to a decrease in the bainite start temperature Bs.
- The significant dependence of the microstructure and volume fraction of phases on grain size was demonstrated.
- Within the limits considered in the numerical solutions, the grain size had a stronger influence than carbon concentration on the amount of retained austenite being existed in the large-size forged block at the end of the quench process.
- The variation of the carbon content and the austenitic grain size influence the values of the volume fraction of retained austenite, bainite and martensite which were obtained after quenching.
- The simulation results were confirmed by experimental validation demonstrating the applicability of the multiple materials method to the analysis of the quenching process of large-size forged blocks.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Firrao, D.; Matteis, P.; Scavino, G.; Ubertalli, G.; Ienco, M.G.; Pinasco, M.R.; Stagno, E.; Gerosa, R.; Rivolta, B.; Silvestri, A. Relationships between tensile and fracture mechanics properties and fatigue properties of large plastic mould steel blocks. Mater. Sci. Eng. A 2007, 468, 193–200. [Google Scholar] [CrossRef]
- Wegman, D.D.; Wanner, E.A.; Rehrer, W.P.; Widge, S. Heat, Corrosion, and Wear Resistant Steel Alloy and Article. U.S. Patent 4,929,419, 29 May 1990. [Google Scholar]
- Mordike, B.L.; Ebert, T. Magnesium: Properties—Applications—Potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Sinha, A.K.; Division, B.P. Defects and Distortion in Heat-Treated Parts. In ASM Handbook; ASM International: Almere, The Netherlands, 1991; pp. 601–619. [Google Scholar]
- Loucif, A.; Shahriari, D.; Zhang, C.; Jahazi, M.; Lapierre-Boire, L.P.; Tremblay, R. Macrosegregation of Alloying Elements in Hot Top of Large Size High Strength Steel Ingot. Mater. Sci. Forum 2016, 879, 1176–1181. [Google Scholar] [CrossRef]
- Lait, J.E.; Samarasekera, I.V. Novel solidification studies. In Proceedings of the Weinberg International Symposium on Solidification Processing, Hamilton, ON, Canada, 27–29 August 1990; pp. 171–252. [Google Scholar]
- Agrawal, K.; Brimacombe, J.K. Mathematical model of heat flow and austenite-pearlite transformation in eutectoid carbon steel rods for wire. Metall. Trans. B 1981, 12, 121–133. [Google Scholar] [CrossRef]
- Biswas, S.J.; Chen, S.J.; Satyanarayana, A. Optimal temperature tracking for accelerated cooling processes in hot rolling of steel. Dyn. Control 1997, 7, 327–340. [Google Scholar] [CrossRef]
- Woodard, P.R.; Chandrasekar, S.; Yang, H.T.Y. Analysis of temperature and microstructure in the quenching of steel cylinders. Metall. Mater. Trans. B 1999, 30, 815–822. [Google Scholar] [CrossRef]
- Chentouf, S.M.; Jahazi, M.; Lapierre-Boire, L.P.; Godin, S. Characteristics of Austenite Transformation during Post Forge Cooling of Large-Size High Strength Steel Ingots. Metallogr. Microstruct. Anal. 2014, 3, 281–297. [Google Scholar] [CrossRef]
- Johnson, W.A.; Mehl, R.F. Reaction kinetics in processes of nucleation and growth. Trans. Metall. Soc. 1939, 135, 135–416. [Google Scholar]
- Koistinen, D.; Marburger, R. A general equation prescribing the extent of the austenite-martensite transformation in pure iron-carbon alloys and plain carbon steels. Acta Mater. 1959, 7, 59–60. [Google Scholar] [CrossRef]
- Denis, S.; Sjöstrom, S.; Simon, A. Coupled temperature, stress, phase transformation calculation model Numerical illustration of internal stresses evolution during cooling of an eutectoid carbon steel cylinder. Metall. Trans. A 1987, 18, 1203–1212. [Google Scholar] [CrossRef]
- Forge NxT 1.1. Available online: www.transvalor.com (accessed on 21 March 2018).
- Guo, L.; Roelofs, H.; Lembke, M.I.; Bhadeshia, H.K.D. Modelling of recalescence effect on austenite decomposition. Mater. Sci. Technol. 2017, 33, 1258–1267. [Google Scholar] [CrossRef]
- JMatPro. Practical Software for Materials Properties. Available online: www.sentesoftware.co.uk (accessed on 21 March 2018).
- Song, D.L.; Gu, J.F.; Pan, J.S.; Hu, M.J. Numerical simulation of quenching of large sized blocks of 718 steel used for plastic dies. Mater. Sci. Technol. 2013, 20, 1567–1572. [Google Scholar] [CrossRef]
- Denis, S.; Archambault, P.; Aubry, C.; Mey, A.; Louin, J.C.; Simon, A. Modelling of phase transformation kinetics in steels and coupling with heat treatment residual stress predictions. J. Phys. 1999, 9, 323–332. [Google Scholar] [CrossRef]
- Kang, S.H.; Im, Y.T. Three-dimensional thermo-elastic–plastic finite element modeling of quenching process of plain-carbon steel in couple with phase transformation. Int. J. Mech. Sci. 2007, 49, 423–439. [Google Scholar] [CrossRef]
- ASTM E3-11. Standard Guide for Preparation of Metallographic Specimens; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Rizzi, N.; Tschofen, J. Simulation Numérique de la Mise en Forme Multi-Matériaux, Application au Filage de γ-TiAl Sous Gaine; Matériaux: Tours, France, 2002. [Google Scholar]
- Cardinaux, D. Etude et Modelisation Numerique 3D par Élèments Finis D’un Procédé de Traitement Thermique de Tôles Embouties Après Chauffage par Induction: Application à un Renfort de Pied Central Automobile. Ph.D. Thesis, Ecole Nationale Supérieure des Mines de Paris, Paris, France, 2008. [Google Scholar]
- Xing, W.; Shi, D.; Sun, J.; Zhu, Z. Emissivity model of steel 430 during the growth of oxide layer at 800–1100 K and 1.5 μm. Infrared Phys. Technol. 2018, 88, 23–31. [Google Scholar] [CrossRef]
- Sadiq, H.; Wong, M.B.; Tashan, J.; Al-Mahaidi, R.; Zhao, R. Determination of steel emissivity for the temperature prediction of structural steel members in fire. J. Mater. Civ. Eng. 2013, 25, 167–173. [Google Scholar] [CrossRef]
- Bocchini, G.F.; Baggioli, A.; Gerosa, R.; Rivolta, B.; Silva, G. Cooling Rates of P/M Steels. Int. J. Powder Metall. 2004, 4, 57–65. [Google Scholar]
- Boniardi, M.; Guagliano, M.; Casaroli, A.; Andreotti, R.; Ballerini, F. Large Forgings: Microstructural Evolution and Residual Stresses Due to Quenching Treatments—A Combined Numerical and Experimental. Mater. Perform. Charact. 2014, 3, 118–136. [Google Scholar] [CrossRef]
- Huiping, L.; Guoqun, Z.; Lianfang, H.; Yue, M. High-speed data acquisition of the cooling curves and evaluation of heat transfer coefficient in quenching process. Measurement 2008, 41, 676–686. [Google Scholar]
- Aaronson, H.I.; Reynolds, W.T.J.; Shifiet, G.J.; Spanos, G. Bainite Viewed Three. Different Ways. Metall. Mater. Trans. A 1990, 21, 1343–1380. [Google Scholar] [CrossRef]
- Chadha, K.; Shahriari, D.; Jahazi, M. Constitutive Modelling of Ingot Breakdown Process of Low Alloy Steels. Int. J. Ital. Assoc. Metall. 2016, 4, 5–12. [Google Scholar]
- Talebi, S.H.; Ghasemi-Nanesa, H.; Jahazi, M.; Melkonyan, H. In Situ Study of Phase Transformations during Non-Isothermal Tempering of Bainitic and Martensitic Microstructures. Metals 2017, 7, 346. [Google Scholar] [CrossRef]
- Hong, S.Y.; Bhadeshia, H.K.D. Austenite Grain Size and the Martensite-Start Temperature. Scr. Mater. 2009, 60, 493–495. [Google Scholar]
- Umemoto, M.; Komatsubara, N.; Tamura, I. Effect of austenite grain size on the hardenability of eutectoid steel. Tetsu-to-Hagané 1980, 66, 400–409. [Google Scholar] [CrossRef]
- Seok, J.L.; June, S.P.; Young, K.L. Effect of austenite grain size on the transformation kinetics of upper and lower bainite in a low-alloy steel. Scr. Mater. 2008, 59, 87–90. [Google Scholar]
- Matsuzaki, A.; Bhadeshia, H.K.D.H. Effect of Austenite Grain Size and Bainite Morphology on Overall Kinetics of Bainite Transformation in Steels. J. Mater. Sci. Technol. 1999, 15, 518–522. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite in steels. Encyclopedia of materials. Sci. Technol. 2001, 500, 5203–5206. [Google Scholar]
- Wang, J.; Han, Y.; Hua, H.; Wang, X.; Jiang, C. Grain size effect on the martensitic transformation of Ni50Mn25Ga17Cu8 high-temperature shape memory alloy. Intermetallics 2015, 61, 42–46. [Google Scholar] [CrossRef]
- Hu, F.; Hodgson, P.D.; Wu, K.M. Acceleration of the super bainite transformation through a coarse austenite grain size. Mater. Lett. 2014, 122, 240–243. [Google Scholar] [CrossRef]
- ASTM E975-13. Standard Practice for X-ray Determination of Retained Austenite in Steel with Near Random Crystallographic Orientation; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]

















| C | Mn | Si | Ni | Cr | Mo | Cu |
|---|---|---|---|---|---|---|
| 0.35 | 0.99 | 0.41 | 0.5 | 1.86 | 0.53 | 0.16 |
| Material Condition | Designation |
|---|---|
| Uniform grain size and %C | A |
| Uniform grain size and variable %C | B |
| Variable grain size and uniform %C | C |
| Variable grain size and %C | D |
| Zone | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| A | Grain size | 133 | 133 | 133 | 133 | 133 |
| Carbon (wt. %) | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | |
| B | Grain size | 133 | 133 | 133 | 133 | 133 |
| Carbon (wt. %) | 0.3 | 0.35 | 0.38 | 0.42 | 0.55 | |
| C | Grain size | 133 | 205 | 300 | 330 | 360 |
| Carbon (wt. %) | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | |
| D | Grain size | 133 | 205 | 300 | 330 | 360 |
| Carbon (wt. %) | 0.3 | 0.35 | 0.38 | 0.42 | 0.55 | |
| Cooling Rate (°C/s) | —Simulation (%) | —Experimental (%) |
|---|---|---|
| 0.015 | 20 | 18 |
| 3 | 0.006 | 0.0035 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyassami, M.; Shahriari, D.; Ben Fredj, E.; Morin, J.-B.; Jahazi, M. Numerical Simulation of Water Quenching of Large Size Steel Forgings: Effects of Macrosegregation and Grain Size on Phase Distribution. J. Manuf. Mater. Process. 2018, 2, 34. https://doi.org/10.3390/jmmp2020034
Lyassami M, Shahriari D, Ben Fredj E, Morin J-B, Jahazi M. Numerical Simulation of Water Quenching of Large Size Steel Forgings: Effects of Macrosegregation and Grain Size on Phase Distribution. Journal of Manufacturing and Materials Processing. 2018; 2(2):34. https://doi.org/10.3390/jmmp2020034
Chicago/Turabian StyleLyassami, Mountadar, Davood Shahriari, Emna Ben Fredj, Jean-Benoit Morin, and Mohammad Jahazi. 2018. "Numerical Simulation of Water Quenching of Large Size Steel Forgings: Effects of Macrosegregation and Grain Size on Phase Distribution" Journal of Manufacturing and Materials Processing 2, no. 2: 34. https://doi.org/10.3390/jmmp2020034





