Preparation and Performance of Ecofriendly Epoxy/Multilayer Graphene Oxide Composites with Flame-Retardant Functional Groups
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of GO
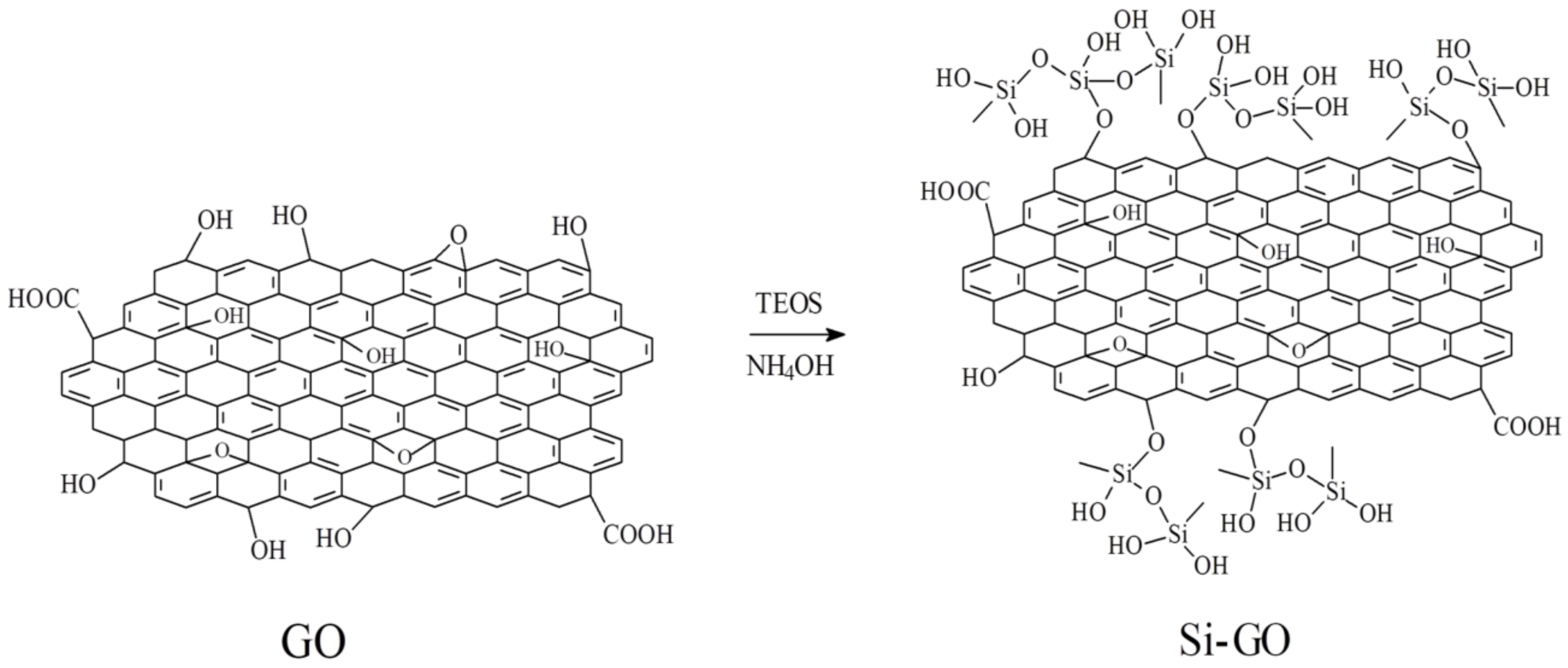
2.3. Preparation of Si-GO
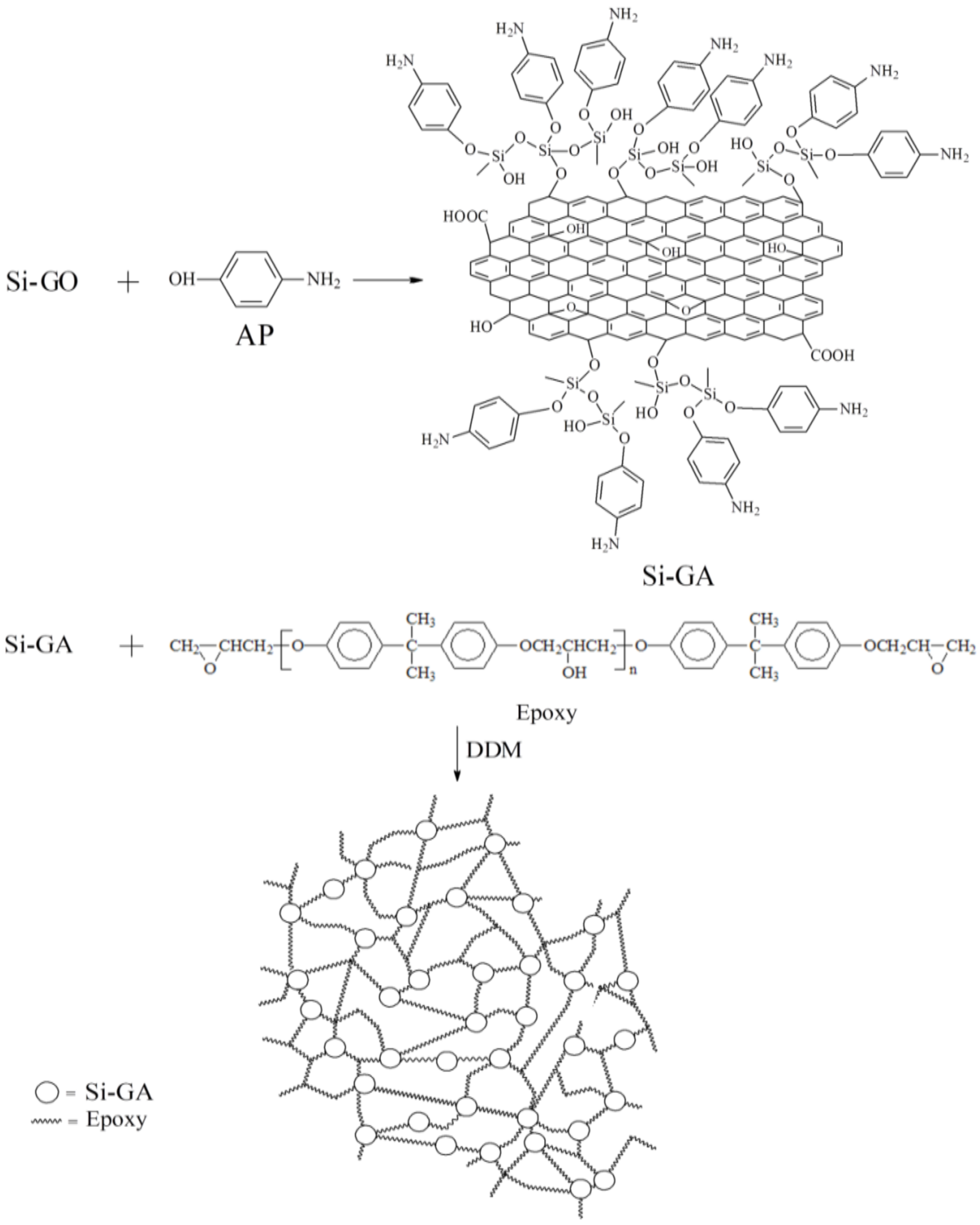
2.4. Preparation of the Epoxy/Si-GA Composite
2.5. Measurements
3. Results and Discussion
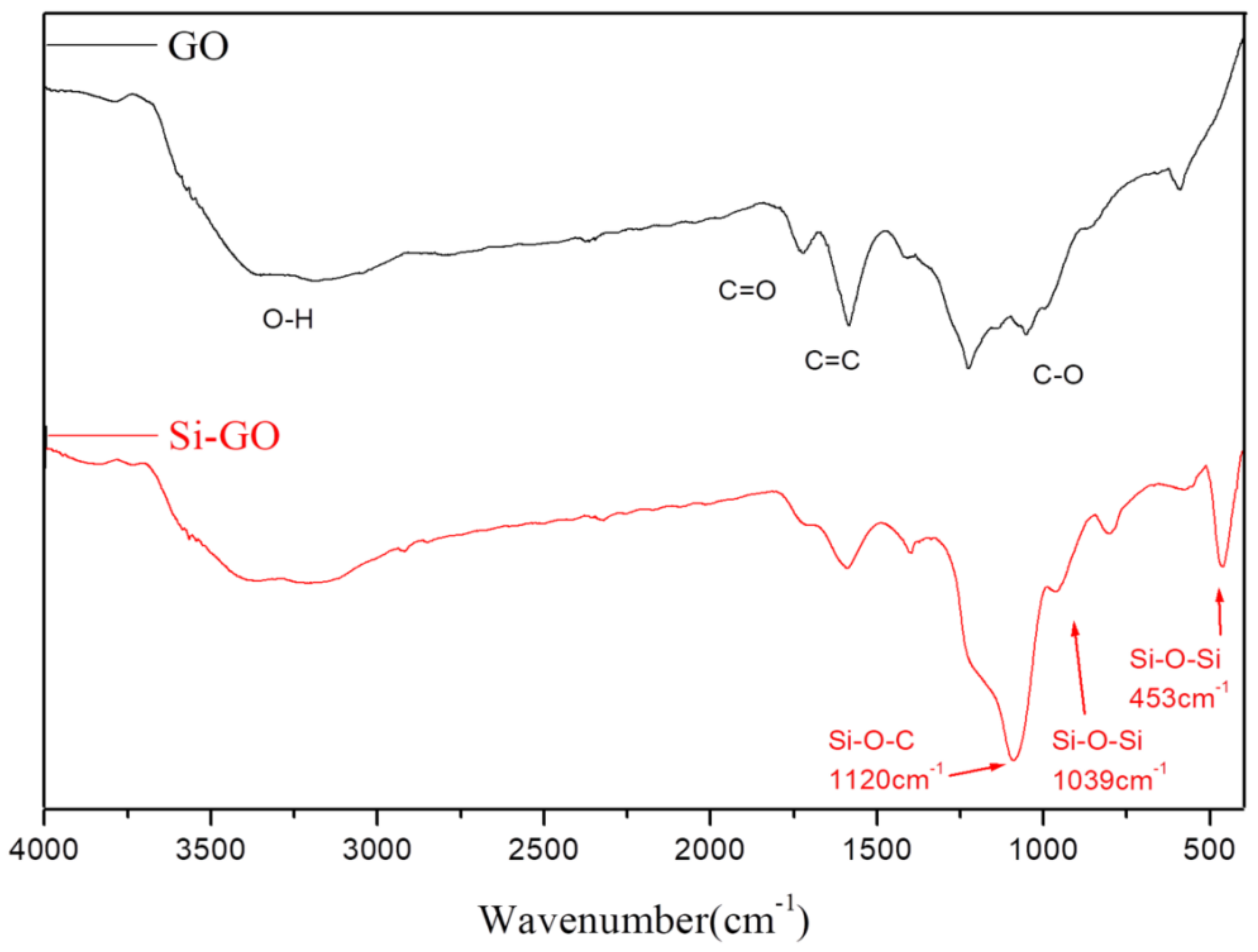
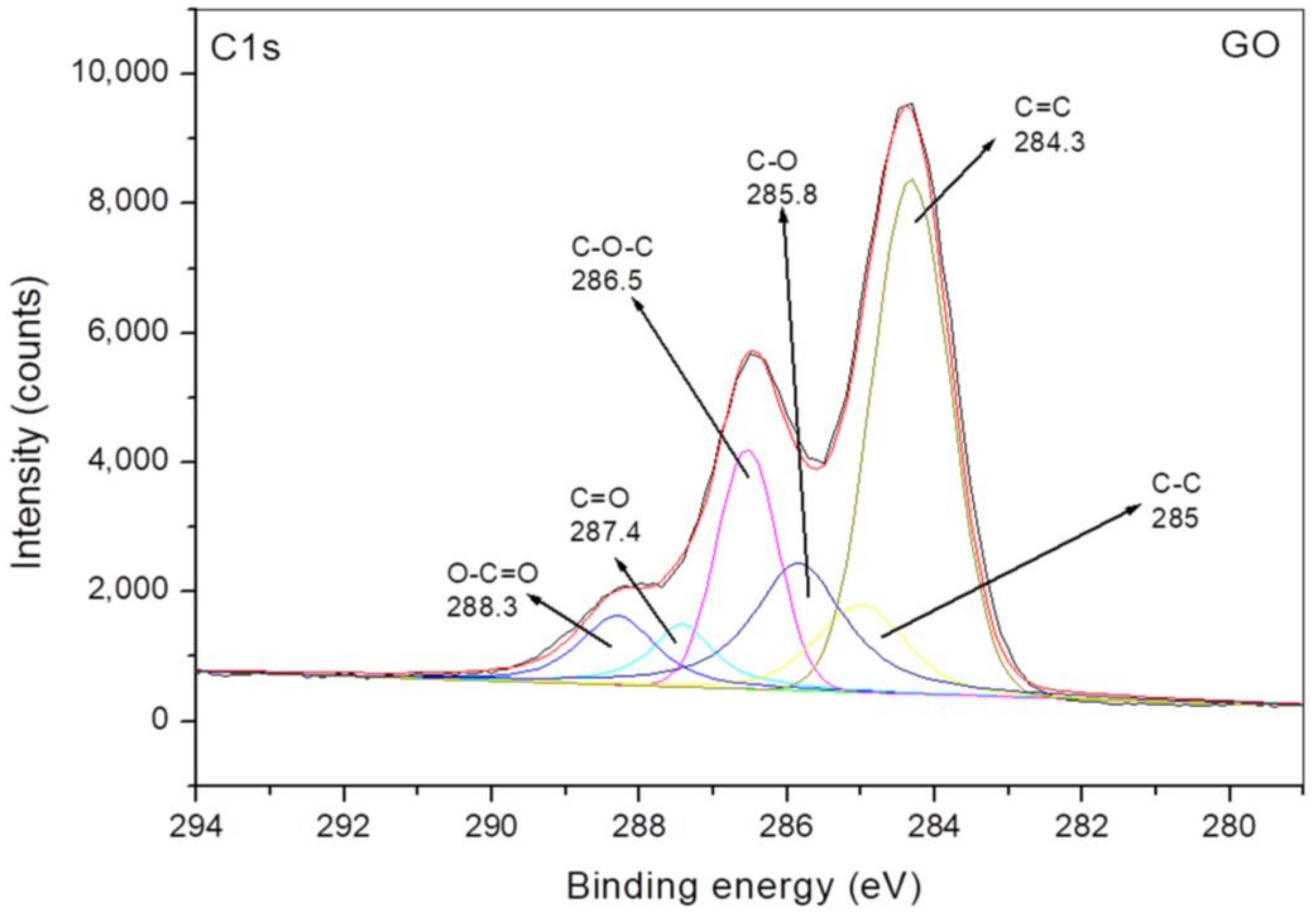
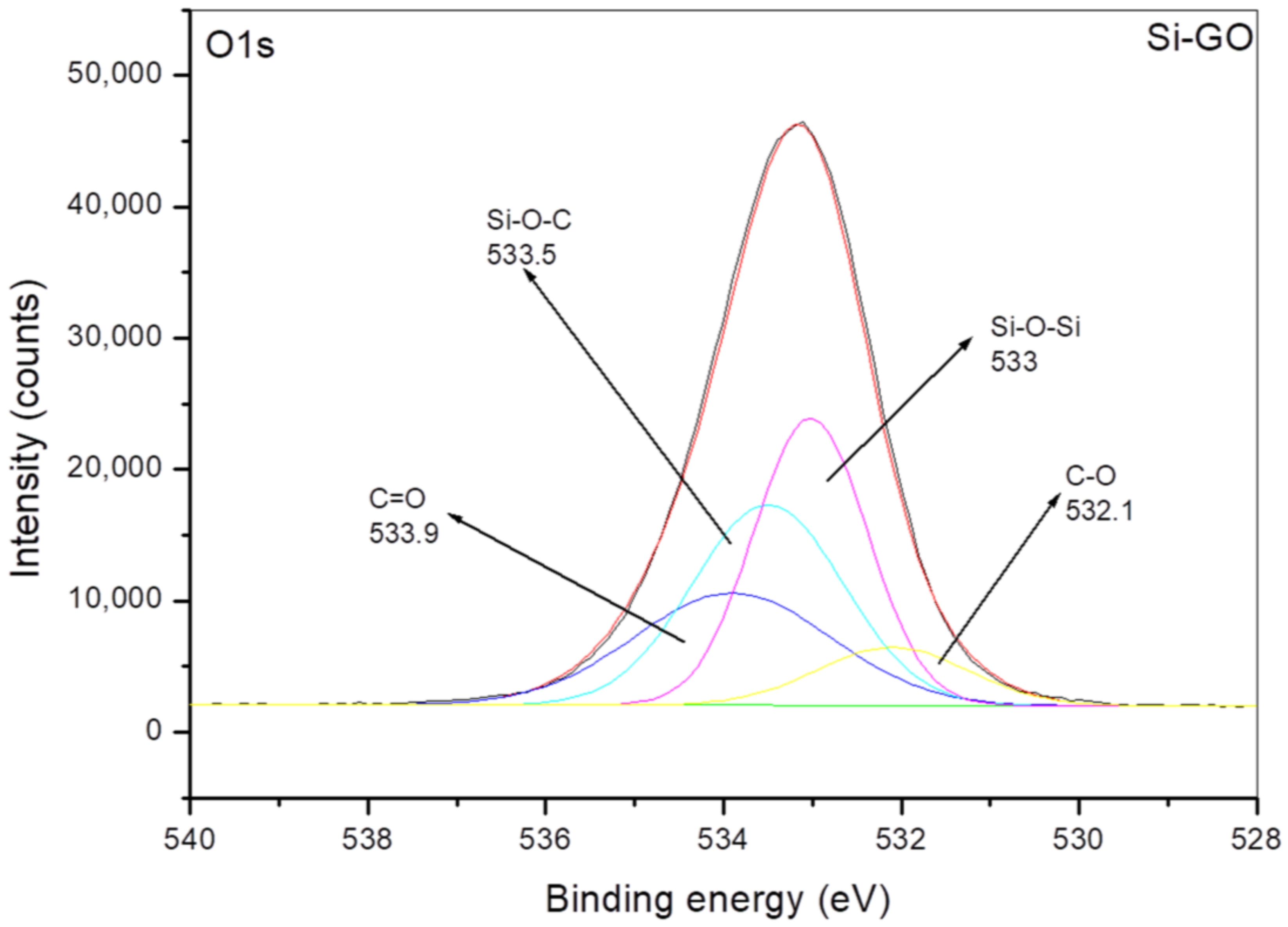
3.1. Characterization of Grafting Reaction
3.2. Thermal Properties of Epoxy/Si-GA Composites
3.3. Interaction between Organic and Inorganic Phases
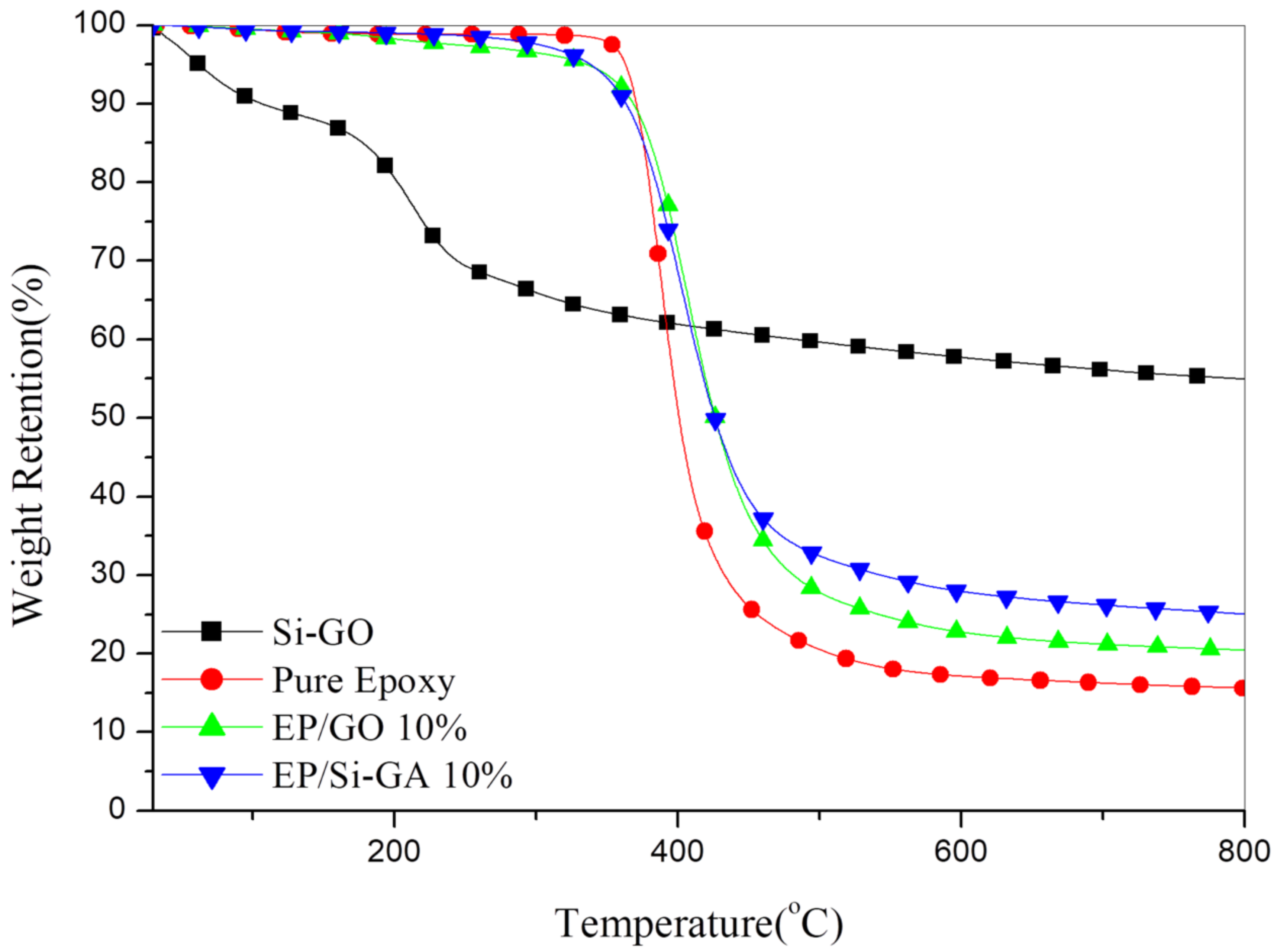
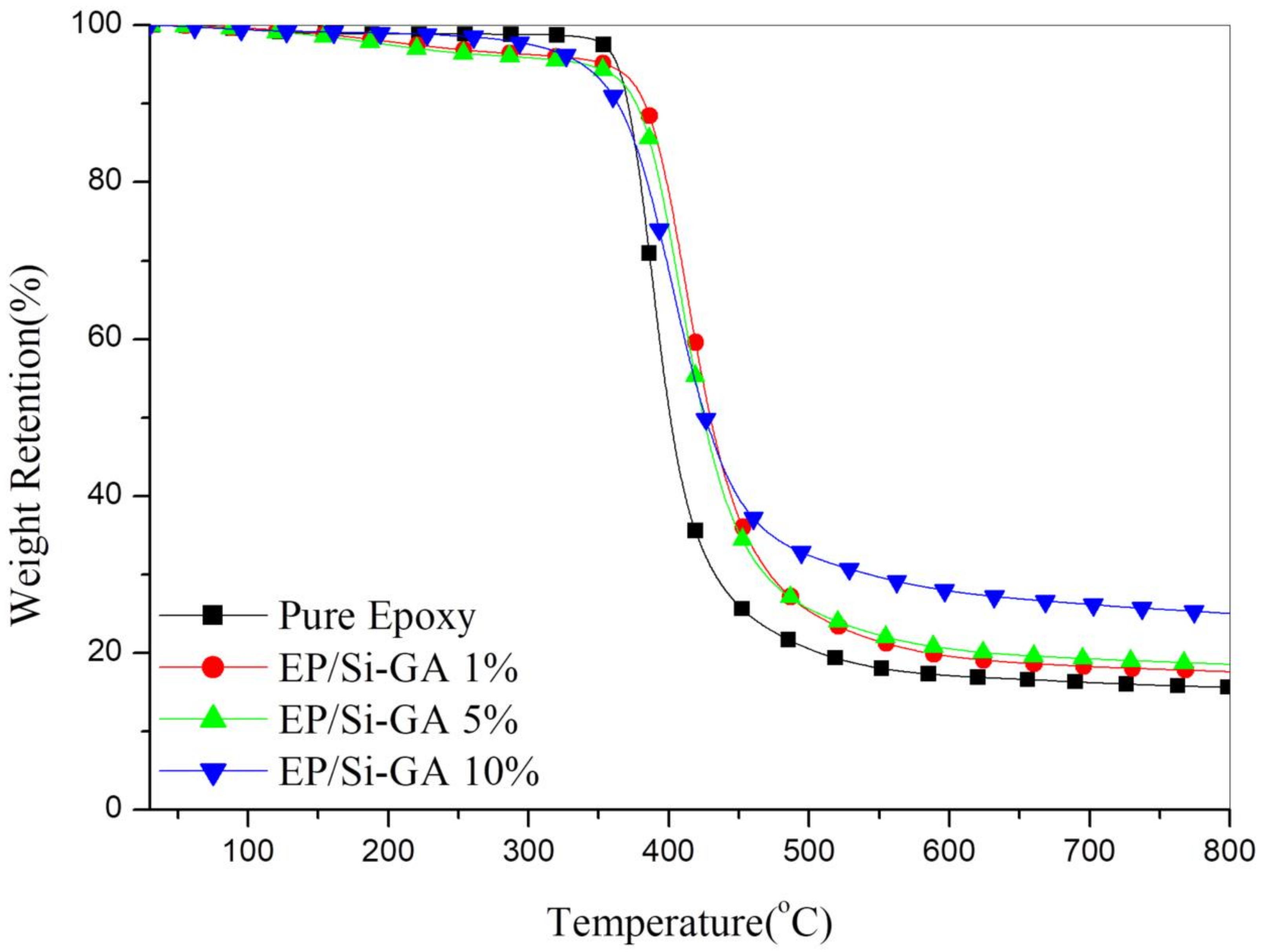
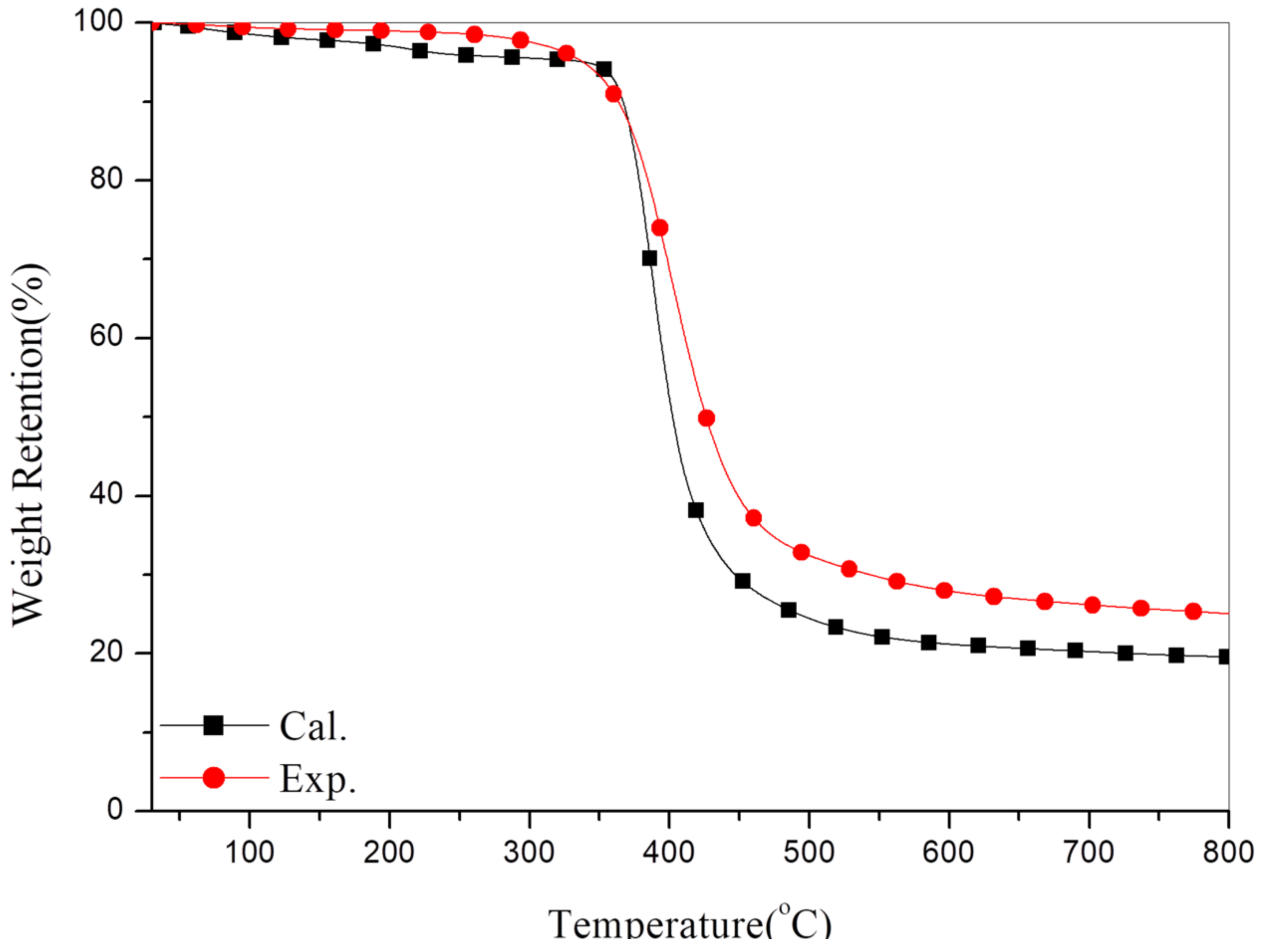
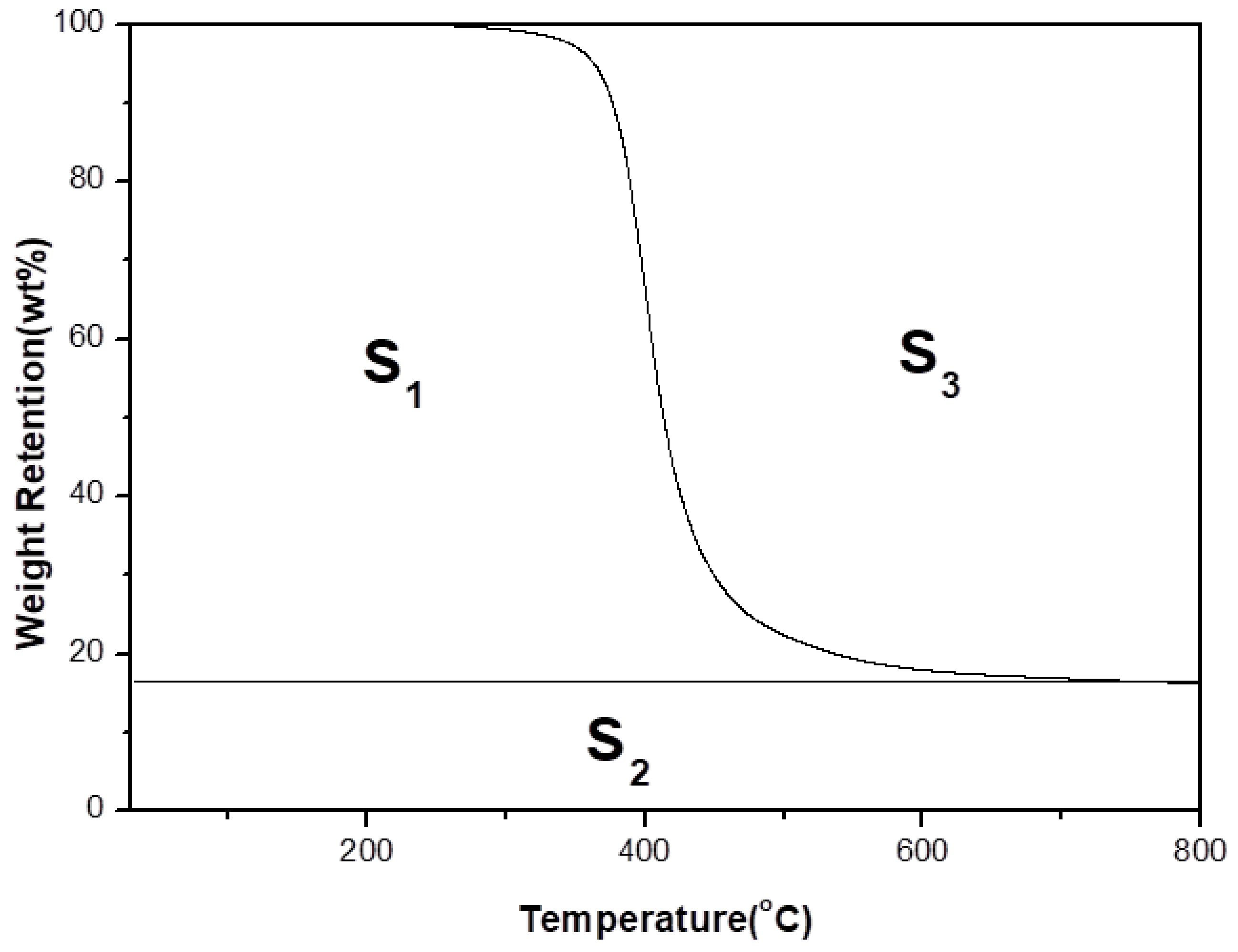
3.4. Thermal Stability of the Composites
- Ti = the initial experimental temperature (100 °C),
- Tf = the final experimental temperature (800 °C),
- A* = (S1 + S2)/(S1 + S2 + S3),
- K* = (S1 + S2)/S1.
3.5. Kinetics of Thermal Degradation
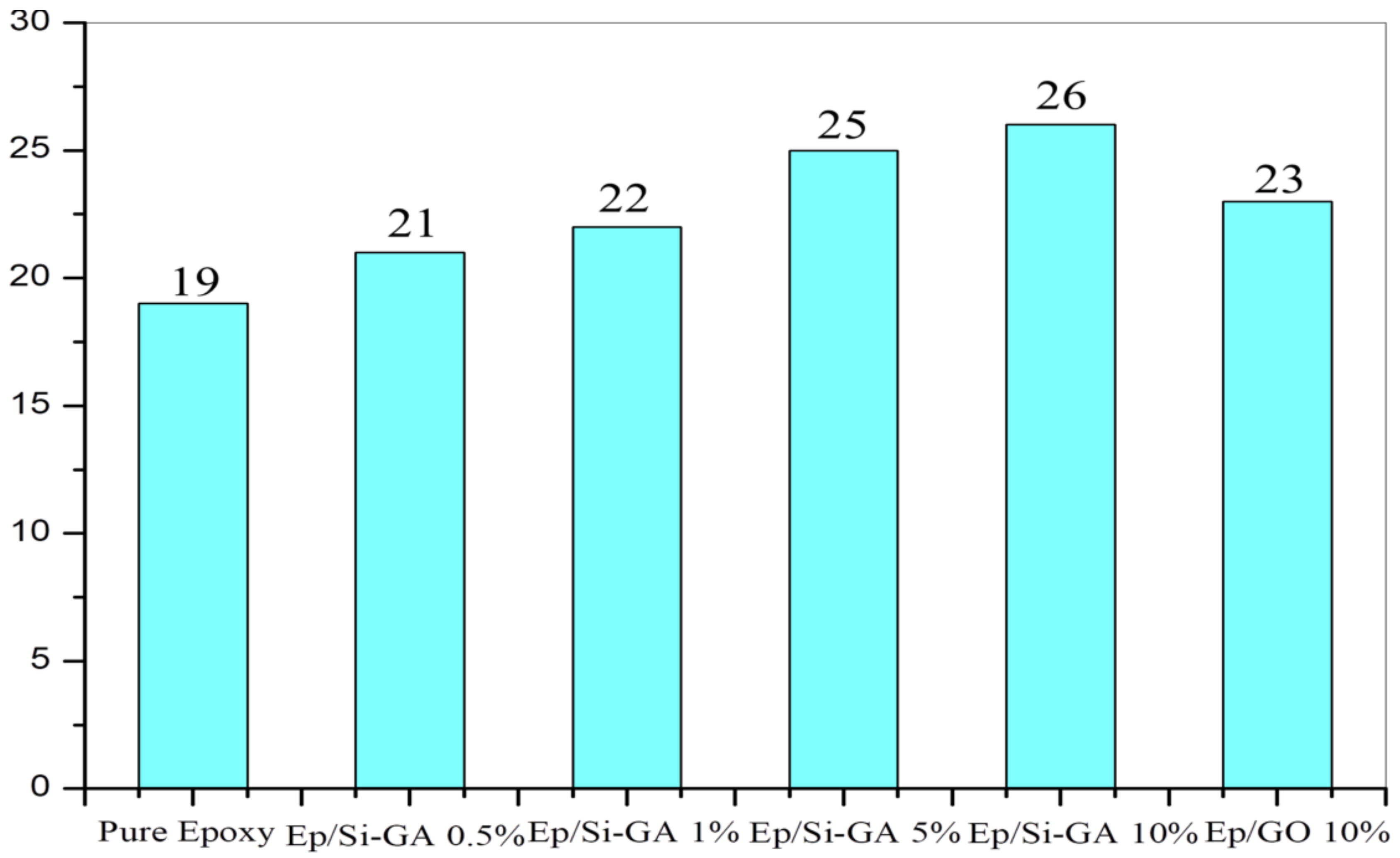
3.6. Flame Retardancy of the Materials
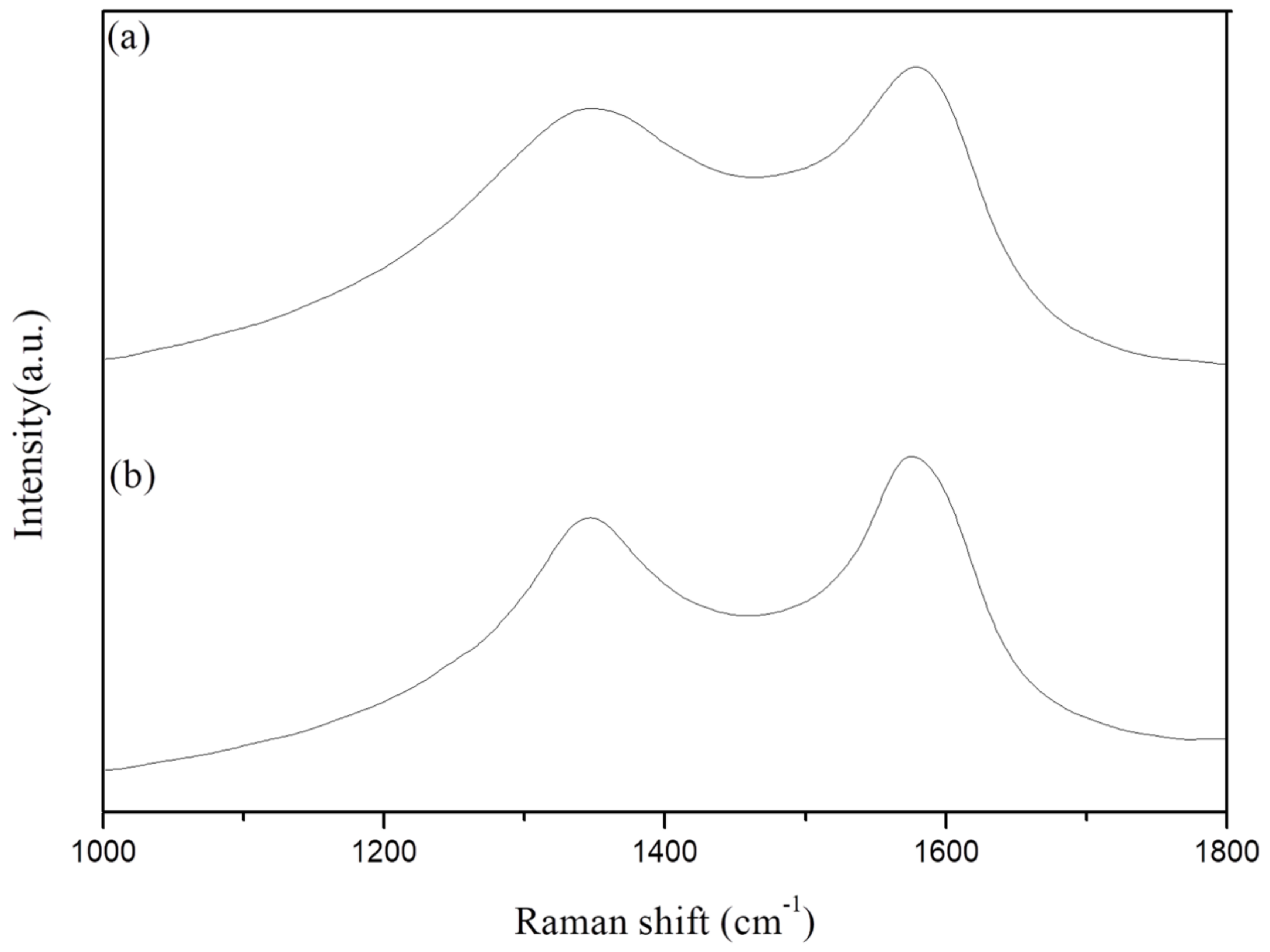
3.7. Analysis of Char
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pan, G.; Du, Z.; Zhang, C.; Li, C.; Yang, X.; Li, H. Synthesis, characterization, and properties of novel novolac epoxy resin containing naphthalene moiety. Polymer 2007, 48, 3686–3693. [Google Scholar] [CrossRef]
- Akatsuka, M.; Takezawa, Y.; Amagi, S. Influences of inorganic fillers on curing reactions of epoxy resins initiated with a boron trifluoride amine complex. Polymer 2001, 42, 3003–3007. [Google Scholar] [CrossRef]
- Perret, B.; Schartel, B.; Stös, K.; Ciesielski, M.; Diederichs, J.; Döring, M.; Krämer, J.; Altstädt, V. Novel DOPO-based flame retardants in high-performance carbon fiber epoxy composites for aviation. Eur. Polym. J. 2011, 47, 1081–1089. [Google Scholar] [CrossRef]
- Wang, X.; Song, L.; Yang, H.; Lu, H.; Hu, Y. Synergistic Effect of Graphene on Antidripping and Fire Resistance of Intumescent Flame Retardant Poly(butylene succinate) Composites. Ind. Eng. Chem. Res. 2011, 50, 5376–5383. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.; Lu, H.; Lv, P.; Jie, G. Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymer 2010, 51, 2435–2445. [Google Scholar] [CrossRef]
- Slonczewski, J.C.; Weiss, P.R. Band structure of graphite. Phys. Rev. 1958, 109, 272. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.P.; Ramesh, P.; Itkis, M.E.; Bekyarova, E.; Haddon, R.C. Graphite nanoplatelet-epoxy composite thermal interface materials. J. Phys. Chem. C 2007, 111, 7565–7569. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Liao, S.H.; Yen, M.Y.; Liu, P.I.; Pu, N.W.; Wang, C.A.; Ma, C.C.M. Preparation of Covalently Functionalized Graphene Using Residual Oxygen-Containing Functional Groups. ACS Appl. Mater. Interfaces 2010, 2, 3092–3099. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tang, B.; Wu, P. Development of novel SiO2–GO nanohybrid/polysulfone membrane with enhanced performance. J. Membr. Sci. 2014, 451, 94–102. [Google Scholar] [CrossRef]
- Wu, G.; Ma, L.; Liu, L.; Chen, L.; Huang, Y. Preparation of SiO2–GO hybrid nanoparticles and the thermal properties of methylphenyl silicone resins/SiO2–GO nanocomposites. Thermochim. Acta 2015, 613, 77–86. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Brito, F.S.; Franceschi, W.; Simonetti, E.A.N.; Cividanes, L.S.; Chipara, M.; Lozano, K. Functionalized graphene oxide as reinforcement in epoxy based Nanocomposites. Surf. Interfaces 2018, 10, 100–109. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Cividanes, L.D.S.; Brito, F.S.; de Menezes, B.R.C.; Franceschi, W.; Simonetti, E.A.N.; Thim, G.P. Functionalization of Carbon Nanotube and Applications, Functionalizing Graphene and Carbon Nanotubes; Springer International Publishing: New York, NY, USA, 2016; pp. 31–61. [Google Scholar]
- Phiri, J.; Gane, P.; Maloney, T.C. General overview of graphene: Production, properties and application in polymer composites. Mater. Sci. Eng. B 2017, 215, 9–28. [Google Scholar] [CrossRef]
- Feng, Y.; He, C.; Wen, Y.; Ye, Y.; Zhou, X.; Xie, X.; Mai, Y.W. Improving thermal and flame retardant properties of epoxy resin by functionalized graphene containing phosphorous, nitrogen and silicon elements. Compos. Part A Appl. Sci. Manuf. 2017, 103, 74–83. [Google Scholar] [CrossRef]
- Yuan, B.; Hu, Y.; Chen, X.; Shi, Y.; Niu, Y.; Zhang, Y.; He, S.; Dai, H. Dual modification of graphene by polymeric flame retardant and Ni(OH)2 nanosheets for improving flame retardancy of polypropylene. Compos. Part A Appl. Sci. Manuf. 2017, 100, 106–117. [Google Scholar] [CrossRef]
- Luo, J.; Yang, S.; Lei, L.; Zhao, J.; Tong, Z. Toughening, synergistic fire retardation and water resistance of polydimethylsiloxane grafted graphene oxide to epoxy nanocomposites with trace phosphorus. Compos. Part A Appl. Sci. Manuf. 2017, 100, 275–284. [Google Scholar] [CrossRef]
- Park, S.J.; Jin, F.L.; Park, J.H.; Kim, K.S. Synthesis of a novel siloxane-containing diamine for increasing flexibility of epoxy resins. Mater. Sci. Eng. A 2005, 399, 377–381. [Google Scholar] [CrossRef]
- Liao, S.H.; Liu, P.L.; Hsiao, M.C.; Teng, C.C.; Wang, C.A.; Ger, M.D.; Chiang, C.L. One-Step Reduction and Functionalization of Graphene Oxide with Phosphorus-Based Compound to Produce Flame-Retardant Epoxy Nanocomposite. Ind. Eng. Chem. Res. 2012, 51, 4573–4581. [Google Scholar] [CrossRef]
- Abdullah, S.I.; Ansari, M.N.M. Mechanical properties of graphene oxide(GO)/epoxy composites. HBRC J. 2015, 11, 151–156. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhou, Y.; Kong, L.; Zhou, T.; Shi, G. A novel composite of SiO2-coated graphene oxide and molecularly imprinted polymers for electrochemical sensing dopamine. Biosens. Bioelectron. 2013, 45, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, X.; Qian, X.; Xing, W.; Yang, H.; Ma, L.; Lin, Y.; Jiang, S.; Song, L.; Hu, Y.; Lo, S. Functionalized graphene oxide/phosphoramide oligomer hybrids flame retardant prepared via in situ polymerization for improving the fire safety of polypropylene. RSC Adv. 2014, 60, 31782–31794. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Zhang, L.; Li, D.W.; Karpuzov, D.; Long, Y.T. Electrocatalytic Oxidation of NADH on Graphene Oxide and Reduced Graphene Oxide Modified Screen-Printed Electrode. Int. J. Electrochem. Sci. 2011, 6, 819–829. [Google Scholar]
- Huang, G.; Chen, S.; Tang, S.; Gao, J. A novel intumescent flame retardant-functionalized graphene: Nanocomposite synthesis, characterization, and flammability properties. Mater. Chem. Phys. 2012, 135, 938–947. [Google Scholar] [CrossRef]
- Hu, W.; Yu, B.; Jiang, S.D.; Song, L.; Hu, Y.; Wang, B. Hyper-branched polymer grafting graphene oxide as an effective flame retardant and smoke suppressant for polystyrene. J. Hazard. Mater. 2015, 300, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Tong, L.; Fang, Z. Effect of a novel phosphorous nitrogen containing intumescent flame retardant on the fire retardancy and the thermal behavior of poly(butylene terephthalate). Polym. Degrad. Stab. 2006, 91, 1295–1299. [Google Scholar] [CrossRef]
- Doyle, C.D. Estimating thermal stability of experimental polymers by empirical thermogravimetric analysis. Anal. Chem. 1961, 33, 77–79. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, S.G.; Kim, S.H. Thermal Decomposition Behavior of Carbon Nanotube Reinforced Thermotropic Liquid Crystalline Polymers. J. Appl. Polym. Sci. 2011, 122, 2060–2070. [Google Scholar] [CrossRef]
- Park, S.J.; Cho, M.S. Thermal stability of carbon-MoSi2-carbon composites by thermogravimetric analysis. J. Mater. Sci. 2000, 35, 3525–3527. [Google Scholar] [CrossRef]
- Ozawa, T. A new Method of Analyzing Thermogravi-metric Data. J. Therm. Anal. 1965, 38, 1881–1886. [Google Scholar]
- Abou-Okeil, A.; El-Sawy, S.M.; Abdel-Mohdy, F.A. Flame retardant cotton fabrics treated with organophosphorus polymer. Carbohydr. Polym. 2013, 92, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- Kiliaris, P.; Papaspyrides, C.D. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Chapkin, W.A.; McNerny, D.Q.; Aldridge, M.F.; He, Y.; Wang, W.; Kieffer, J.; Taub, A.I. Real-time assessment of carbon nanotube alignment in a polymer matrix under an applied electric field via polarized Raman spectroscopy. Polym. Test. 2016, 56, 29–35. [Google Scholar] [CrossRef]
- Silva, K.C.; Corio, P.; Santos, J.J. Characterization of the chemical interaction between single-walled carbon nanotubes and titanium dioxide nanoparticles by thermogravimetric analyses and resonance Raman spectroscopy. Vib. Spectrosc. 2016, 86, 103–108. [Google Scholar] [CrossRef]


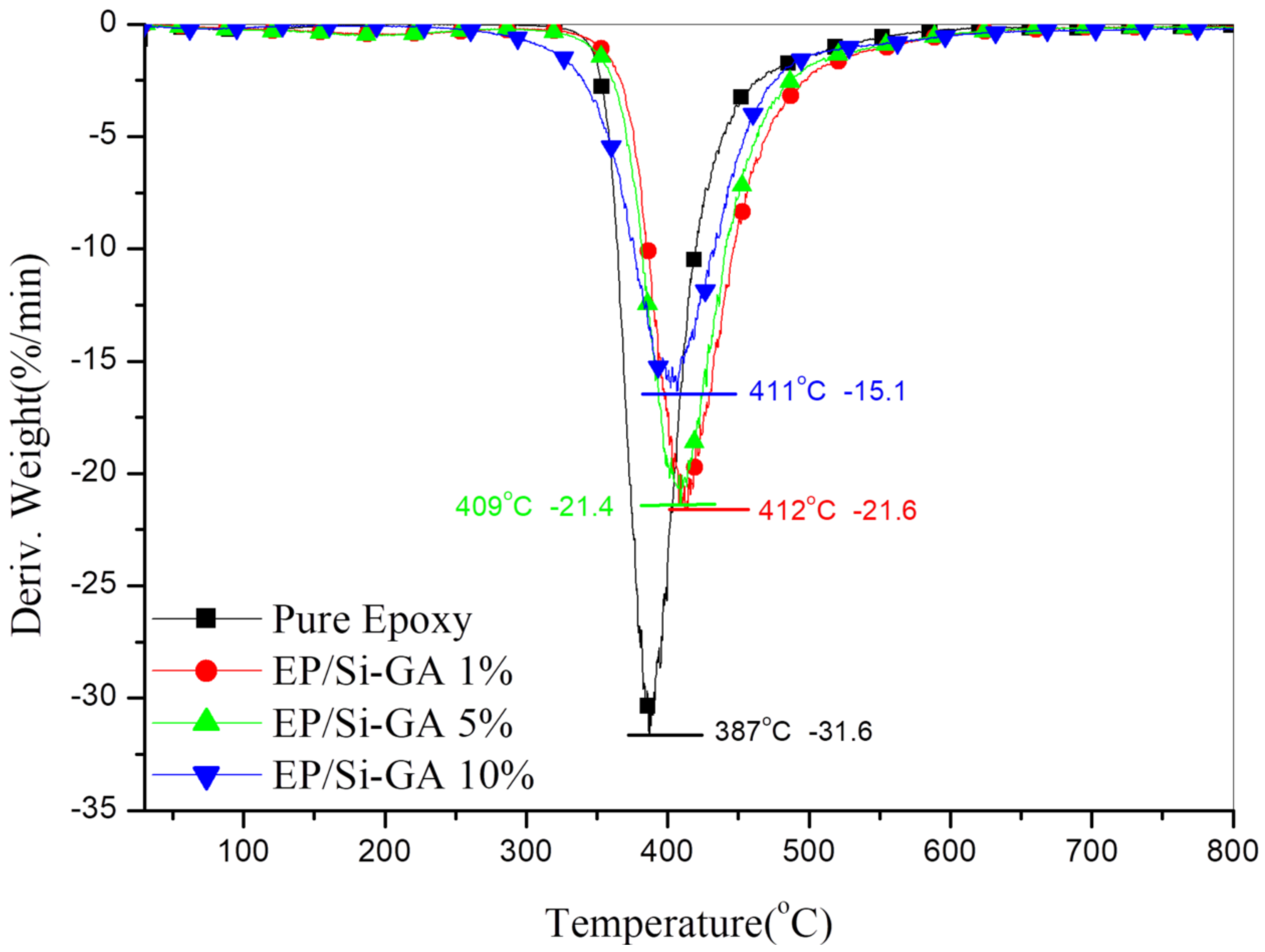
| Sample No. | Tmax (°C) | Rmax (wt %/min) | IPDT1 (°C) | C.Y.2 (wt %) |
|---|---|---|---|---|
| Pure Epoxy | 387 | −31.6 | 629 | 15.6 |
| Epoxy/Si-GA 1% | 409 | −21.6 | 672 | 17.6 |
| Epoxy/Si-GA 5% | 412 | −21.4 | 689 | 18.3 |
| Epoxy/Si-GA 10% | 411 | −15.6 | 825 | 25.0 |
| α | Pure Epoxy | Epoxy/Si-GA 10% | ||
|---|---|---|---|---|
| E(kJ/mole) | R Value | E (kJ/mole) | R Value | |
| 0.2 | 146.8 | 0.88 | 212.8 | 0.99 |
| 0.3 | 138.9 | 0.92 | 194.3 | 0.99 |
| 0.4 | 131.6 | 0.94 | 179.2 | 0.99 |
| 0.5 | 126.8 | 0.95 | 173.6 | 0.99 |
| 0.6 | 122.8 | 0.96 | 169.4 | 0.99 |
| 0.7 | 121.8 | 0.96 | 170.0 | 0.99 |
| 0.8 | 123.5 | 0.94 | 162.0 | 0.98 |
| ΔE(av) | 130.3 | 180.2 | ||
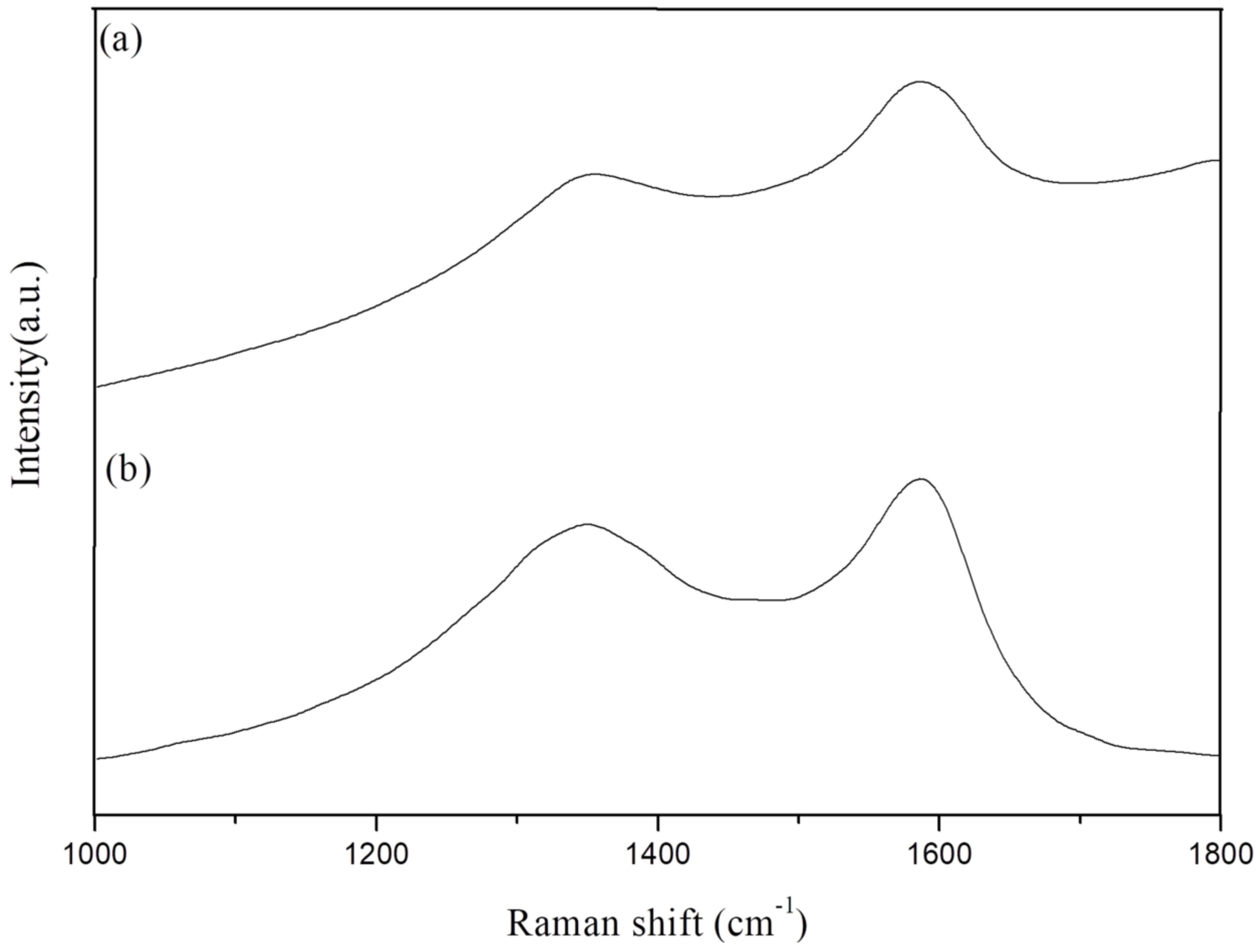
| Sample No. | D-Band | G-Band | D/G | |
|---|---|---|---|---|
| 1356 cm−1 | 1580 cm−1 | |||
| Epoxy/GO 10 | 1 min | 679,856 | 816,332 | 0.83 |
| 5 min | 323,024 | 768,821 | 0.42 | |
| Epoxy/Si-GA 10 | 1 min | 3.97 × 106 | 6.50 × 106 | 0.61 |
| 5 min | 302,028 | 7.60 × 106 | 0.04 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.-H.; Ke, C.-Y.; Chiang, C.-L. Preparation and Performance of Ecofriendly Epoxy/Multilayer Graphene Oxide Composites with Flame-Retardant Functional Groups. J. Compos. Sci. 2018, 2, 18. https://doi.org/10.3390/jcs2020018
Chen M-H, Ke C-Y, Chiang C-L. Preparation and Performance of Ecofriendly Epoxy/Multilayer Graphene Oxide Composites with Flame-Retardant Functional Groups. Journal of Composites Science. 2018; 2(2):18. https://doi.org/10.3390/jcs2020018
Chicago/Turabian StyleChen, Ming-He, Cing-Yu Ke, and Chin-Lung Chiang. 2018. "Preparation and Performance of Ecofriendly Epoxy/Multilayer Graphene Oxide Composites with Flame-Retardant Functional Groups" Journal of Composites Science 2, no. 2: 18. https://doi.org/10.3390/jcs2020018






