Simulation of the Oxygen Permeability of a Composite Container
Abstract
:1. Introduction
2. Experimental
2.1. Composite Material
2.2. Oxygen Permeation
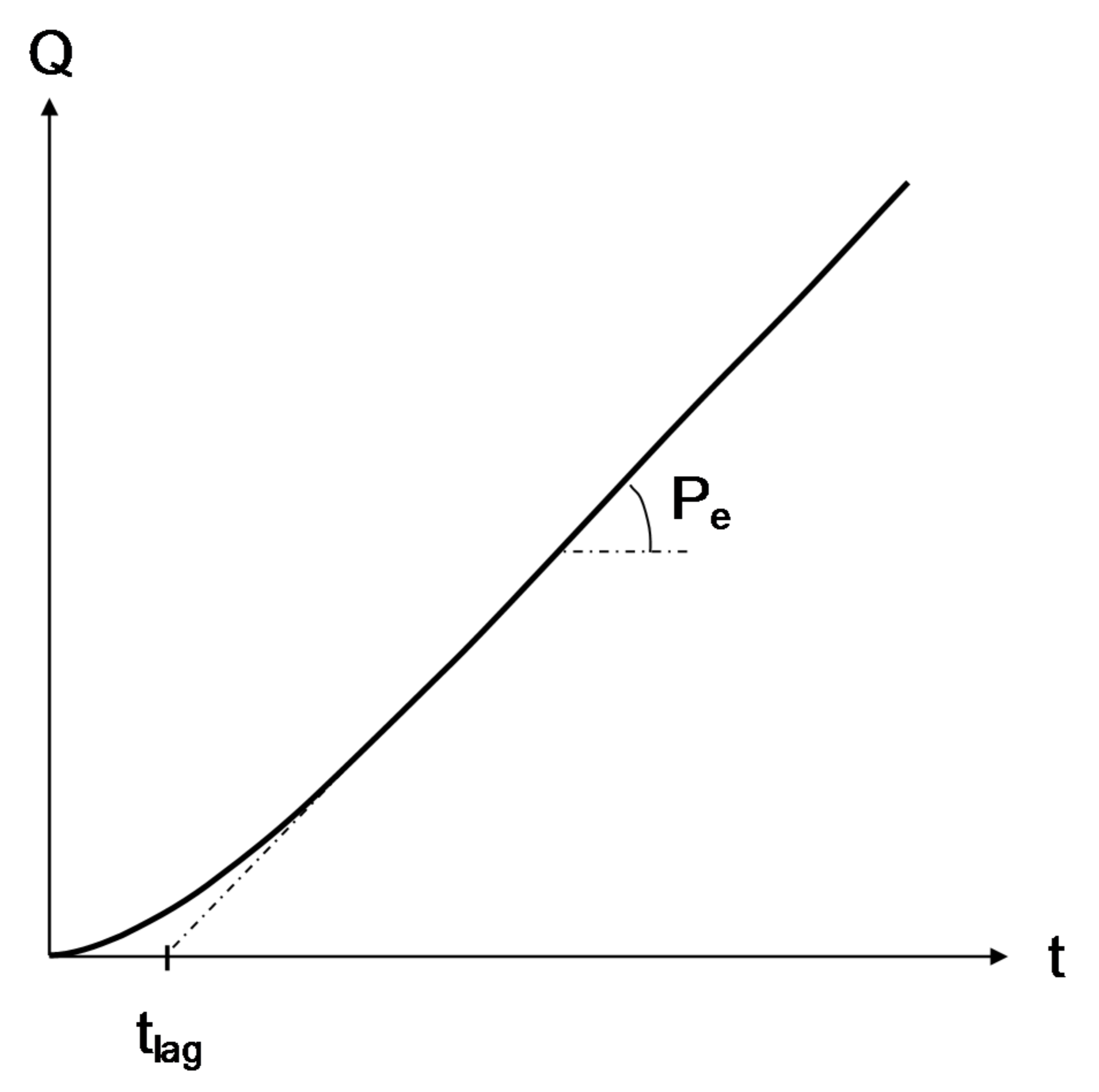
- The time lag (tlag) corresponds to the duration of the transient regime, i.e., the duration for the first oxygen molecules to completely cross the composite disc of thickness E. Thus, tlag is the characteristic time for oxygen diffusion. According to Barrer [7], it is inversely proportional to the coefficient of oxygen diffusion D:
- The permeability (Pe) corresponds to slope of the steady-state regime, i.e., of the linear part of the kinetic curve. Its general mathematical expression is
2.3. Oxygen Transport Properties
3. Kinetic Modeling
3.1. Theory
3.2. Prediction of the Oxygen Permeability
- A reduction of the coefficient of oxygen diffusion across the composite wall thanks to a careful selection of the different constituents (i.e., the fibrous structure and matrix) of the composite material. In particular, the use of a denser fibrous architecture with fibers oriented in several directions (i.e., fabric, braid, or knit) would allow for a significant increase of the fiber fraction and the tortuosity of diffusion paths.
- A reduction of the coefficient of oxygen diffusion thanks to a better control of the processing conditions of the composite material to guarantee minimum ratios of defects and damages (porosity, matrix cracking, fiber/matrix debonding, etc.) and a higher crosslinking density of the epoxy resin in the fiber/matrix interphase.
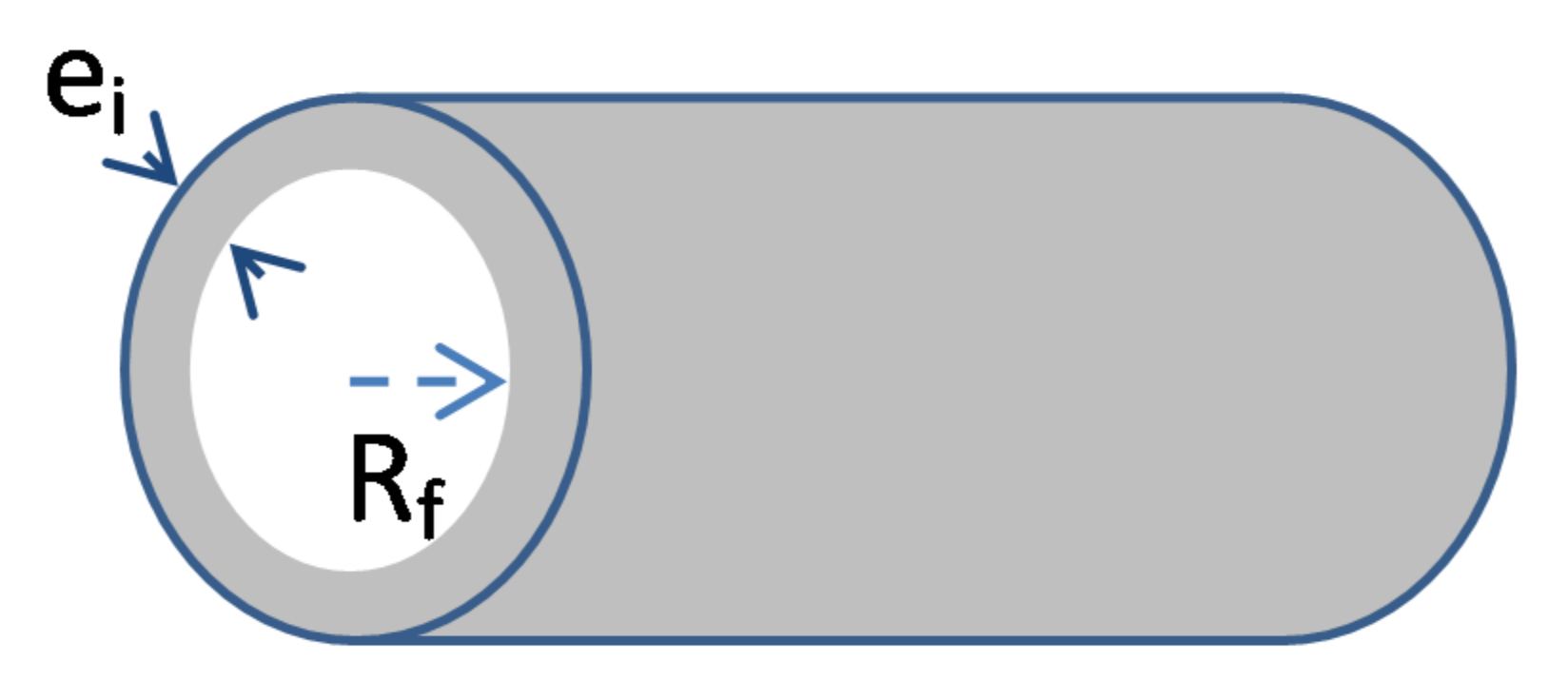
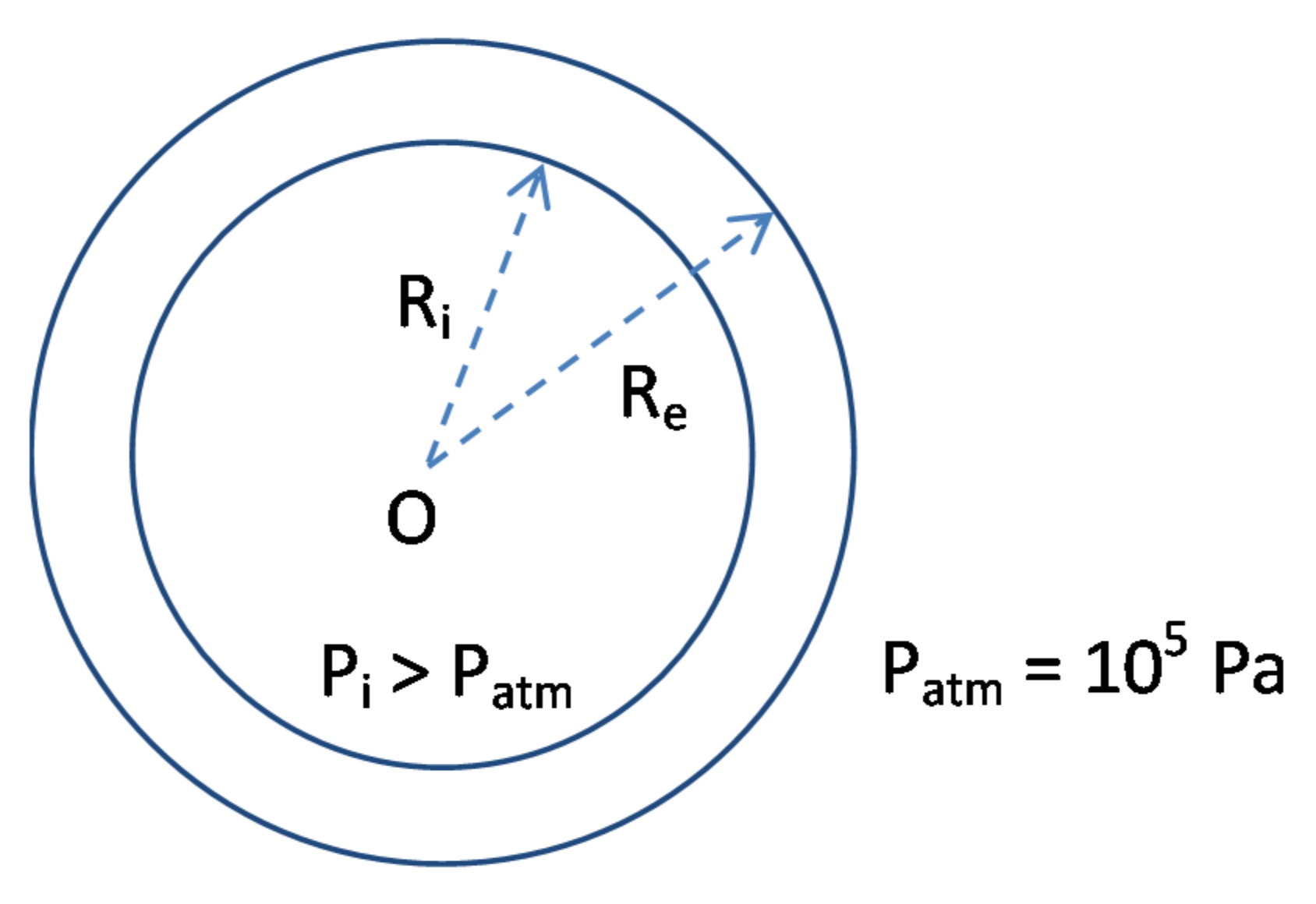
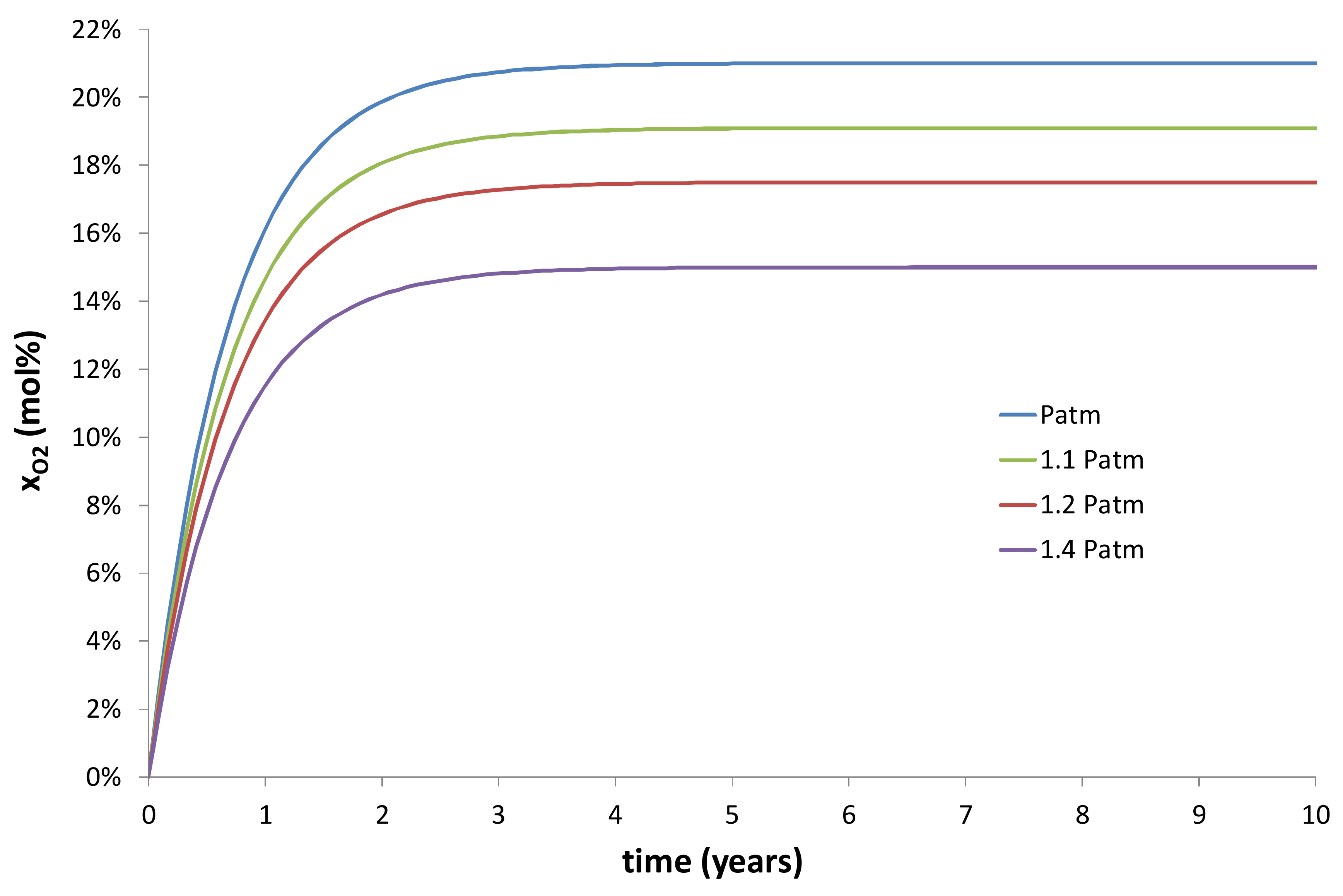
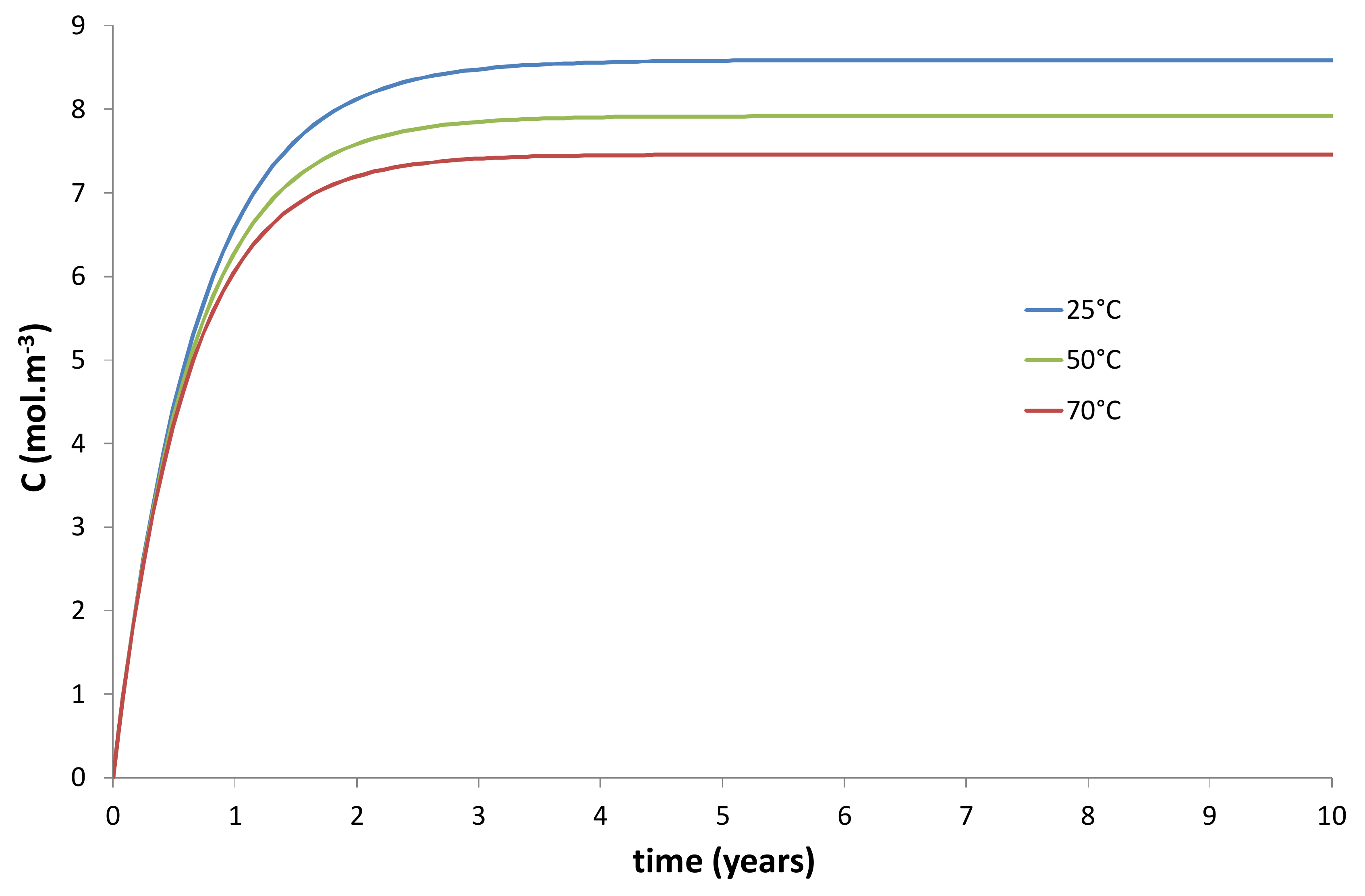
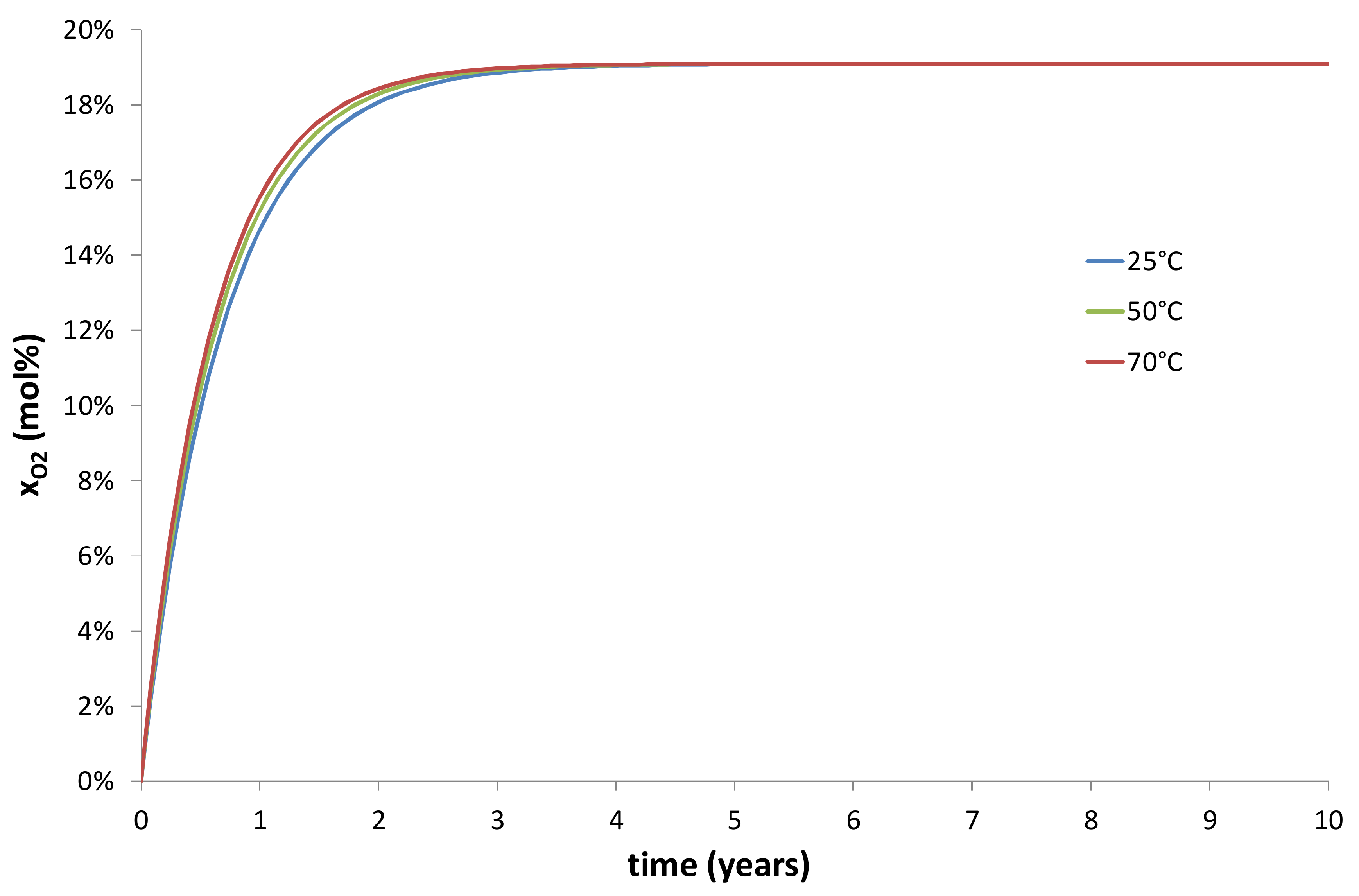
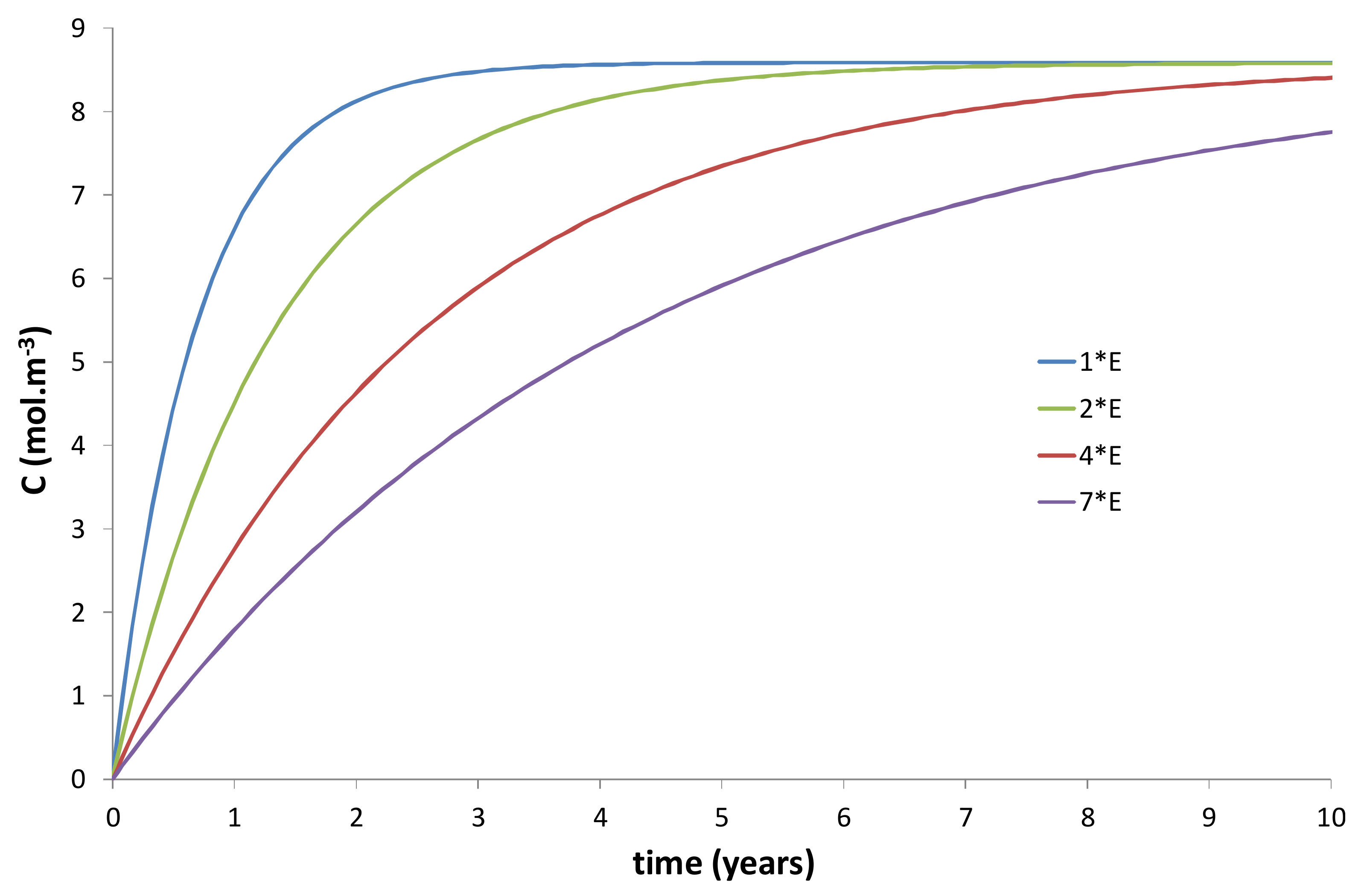
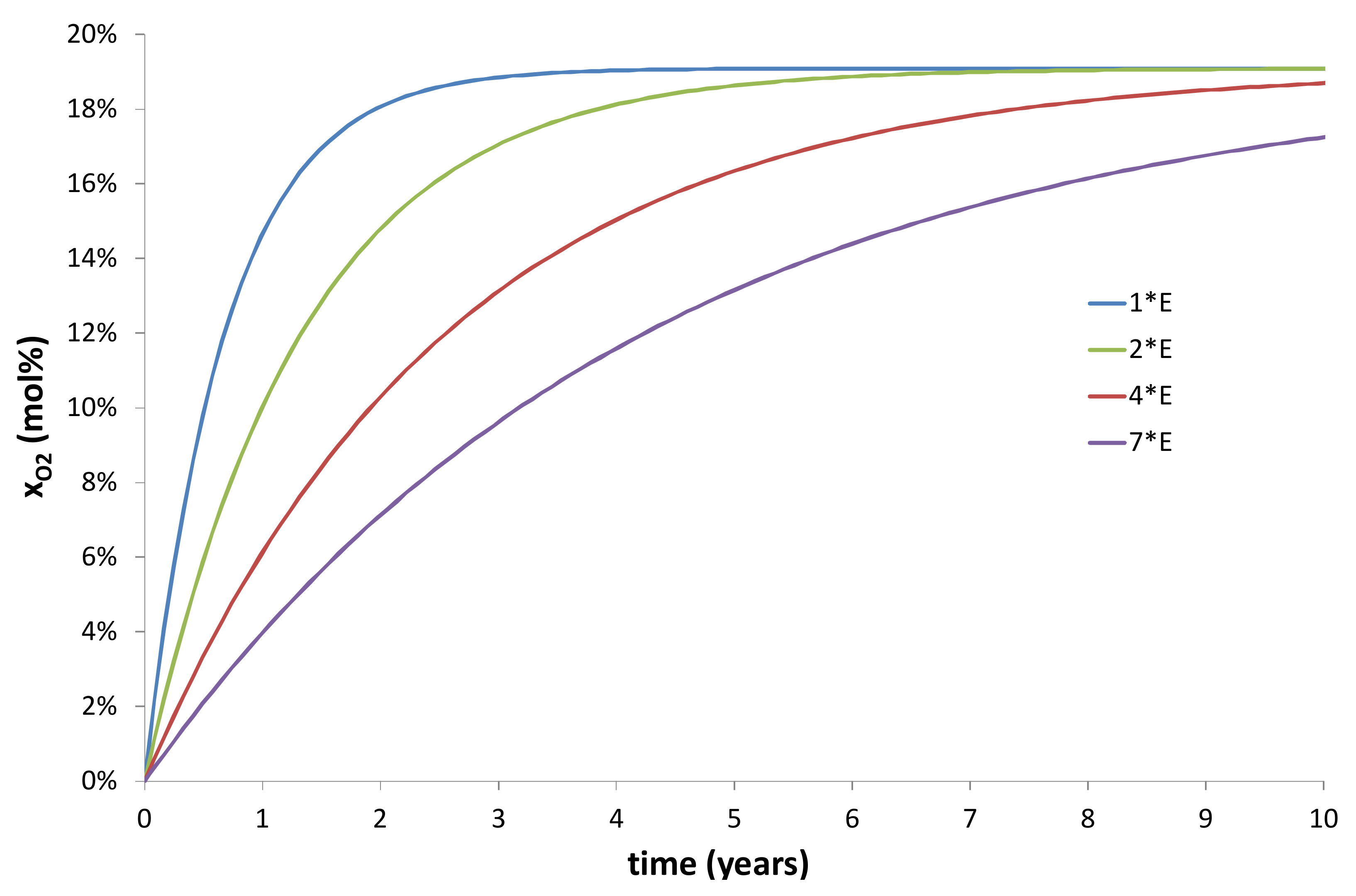
- An increase of the wall thickness of the composite container E = Re − Ri. In the present study, E was approximately fixed at 3 cm. However, this thickness can be progressively increased in order to extend the duration of the transient regime of the kinetic curves, as evidenced in Figure 10 and Figure 11.
- The use of an impermeable coating is certainly another possibility to reach this goal.
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Colin, X.; Fayolle, B.; Audouin, L.; Verdu, J. The classical kinetic model for radical chain oxidation of hydrocarbon substrates initiated by bimolecular hydroperoxide decomposition. Int. J. Chem. Kinent. 2006, 38, 666–676. [Google Scholar] [CrossRef]
- François-Heude, A.; Richaud, E.; Desnoux, E.; Colin, X. A general kinetic model for the photothermal oxidation of polypropylene. J. Photochem. Photobiol. 2015, A296, 48–65. [Google Scholar] [CrossRef] [Green Version]
- Colin, X.; Monchy-Leroy, C.; Audouin, L.; Verdu, J. Lifetime prediction of polyethylene in nuclear plants. Nucl. Instrum. Methods Phys. Res. 2007, B265, 251–255. [Google Scholar] [CrossRef]
- Colin, X.; Audouin, L.; Verdu, J.; Rozental-Evesque, M.; Rabaud, B.; Martin, F.; Bourgine, F. Aging of polyethylene pipes transporting drinking water disinfected by chlorine dioxide. I. Chemical aspects. Polym. Eng. Sci. 2009, 49, 1429–1437. [Google Scholar] [CrossRef]
- Mikdam, A.; Colin, X.; Minard, G.; Billon, N.; Maurin, R. A kinetic model for predicting the oxidative degradation of additive free polyethylene in bleach desinfected water. Polym. Degrad. Stab. 2017, 146, 78–94. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Hoftyser, P.J. Properties of Polymers. Their Estimation and Correlation with Chemical Structure. Their Numerical Estimation and Prediction from Additive Group Contributions, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 656–681. [Google Scholar]
- Barrer, R.M.; Rideal, E.K. Permeation, diffusion and solution of gases in organic polymers. Trans. Faraday Soc. 1939, 35, 628–643. [Google Scholar] [CrossRef]
- Damian, C.; Espuche, E.; Escoubes, M. Influence of three ageing types (thermal oxidation, radiochemical and hydrolytic ageing) on the structure and gas transport properties of epoxy-amine networks. Polym. Degrad. Stab. 2001, 72, 447–458. [Google Scholar] [CrossRef]
- Colin, X.; Marais, C.; Verdu, J. A new method for predicting the thermal oxidation of thermoset matrices: Application to an amine crosslinked epoxy. Polym. Test. 2001, 20, 795–803. [Google Scholar] [CrossRef]
- Colin, X.; Mavel, A.; Marais, C.; Verdu, J. Interaction between cracking and oxidation in organic matrix composites. J. Compos. Mater. 2005, 39, 1371–1389. [Google Scholar] [CrossRef]
- Pochiraju, K.; Tandon, G.P. Interaction of oxidation and damage in high temperature polymeric matrix composites. Compos. Part A 2009, 40, 1931–1940. [Google Scholar] [CrossRef]
- Vu, D.Q. Endommagements Induits Par la Thermo Oxydation Dans les Composites Carbone/Epoxy Unidirectionnels et Stratifies. Ph.D. Thesis, ENSMA, Poitiers, France, 2011. [Google Scholar]
- Kondo, K.; Taki, T. Moisture diffusivity of unidirectional composites. J. Compos. Mater. 1982, 16, 82–93. [Google Scholar] [CrossRef]
- Whitcomb, J.; Xiaodong, T. Micromechanics of moisture diffusion in composites with impermeable fibers. J. Compos. Mater. 2002, 36, 1093–1101. [Google Scholar] [CrossRef]
- Tsai, S.W. Composite Design, 4th ed.; Think Composites: Dayton, OH, USA, 1988. [Google Scholar]
- Roy, S.; Singh, S. Analytical modeling of orthotropic diffusivities in a fiber reinforced composite with discontinuities using homogenization. Compos. Sci. Technol. 2009, 69, 1962–1967. [Google Scholar] [CrossRef]


| Measured Values | Average Value | |||
|---|---|---|---|---|
| T (°C) | 20 °C | 30 °C | 45 °C | From 20 to 45 °C |
| S (mol·m−3·Pa−1) | 2.9 × 10−4 | 1.3 × 10−4 | 1.2 × 10−4 | 1.8 × 10−4 |
| D (m2·s−1) | 6.9 × 10−11 | 8.7 × 10−11 | 7.4 × 10−11 | 7.6 × 10−11 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Defauchy, V.; Le Corre, H.; Colin, X. Simulation of the Oxygen Permeability of a Composite Container. J. Compos. Sci. 2018, 2, 21. https://doi.org/10.3390/jcs2020021
Defauchy V, Le Corre H, Colin X. Simulation of the Oxygen Permeability of a Composite Container. Journal of Composites Science. 2018; 2(2):21. https://doi.org/10.3390/jcs2020021
Chicago/Turabian StyleDefauchy, Virginie, Hélène Le Corre, and Xavier Colin. 2018. "Simulation of the Oxygen Permeability of a Composite Container" Journal of Composites Science 2, no. 2: 21. https://doi.org/10.3390/jcs2020021





