The Use of Polymer and Surfactants for the Microencapsulation and Emulsion Stabilization
Abstract
:1. Introduction
2. Emulsion Stabilization by Polymers and Surfactants: Interfacial Tension and Dilational Rheology of PS Mixtures at Water/Oil Interface as Key Properties
3. Encapsulation Processes
3.1. Encapsulation of Active Ingredients Using O/W Emulsion Stabilized by Surfactants and Polymers
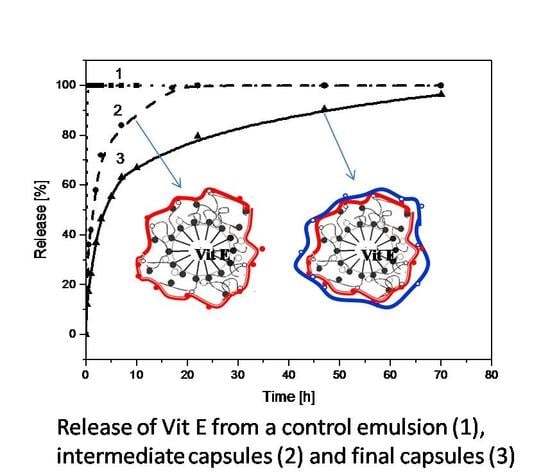
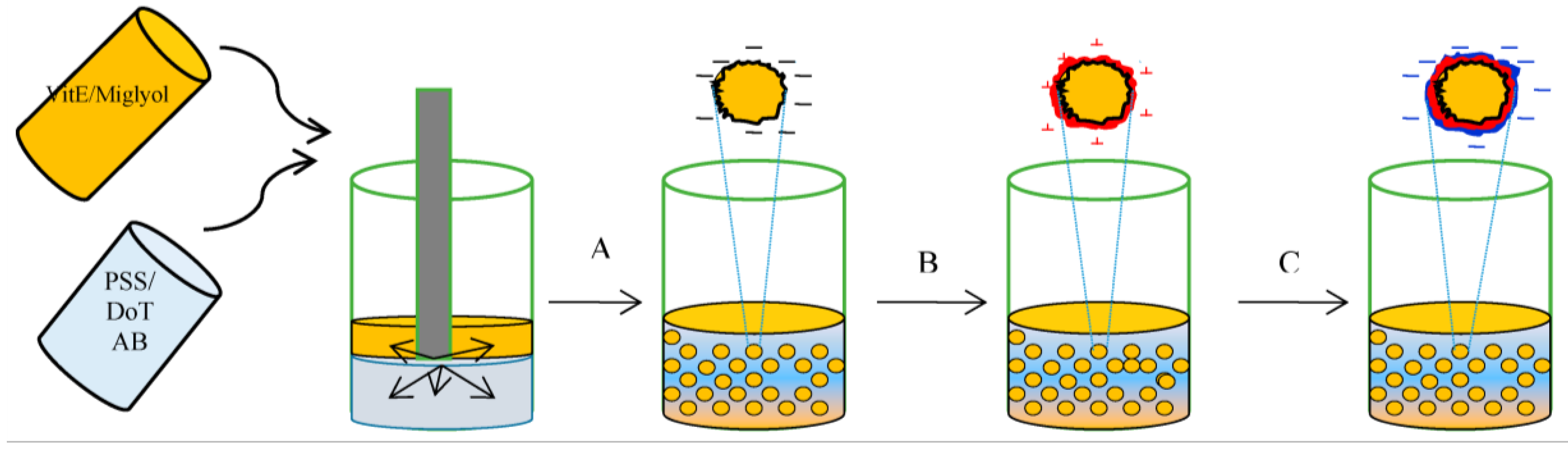
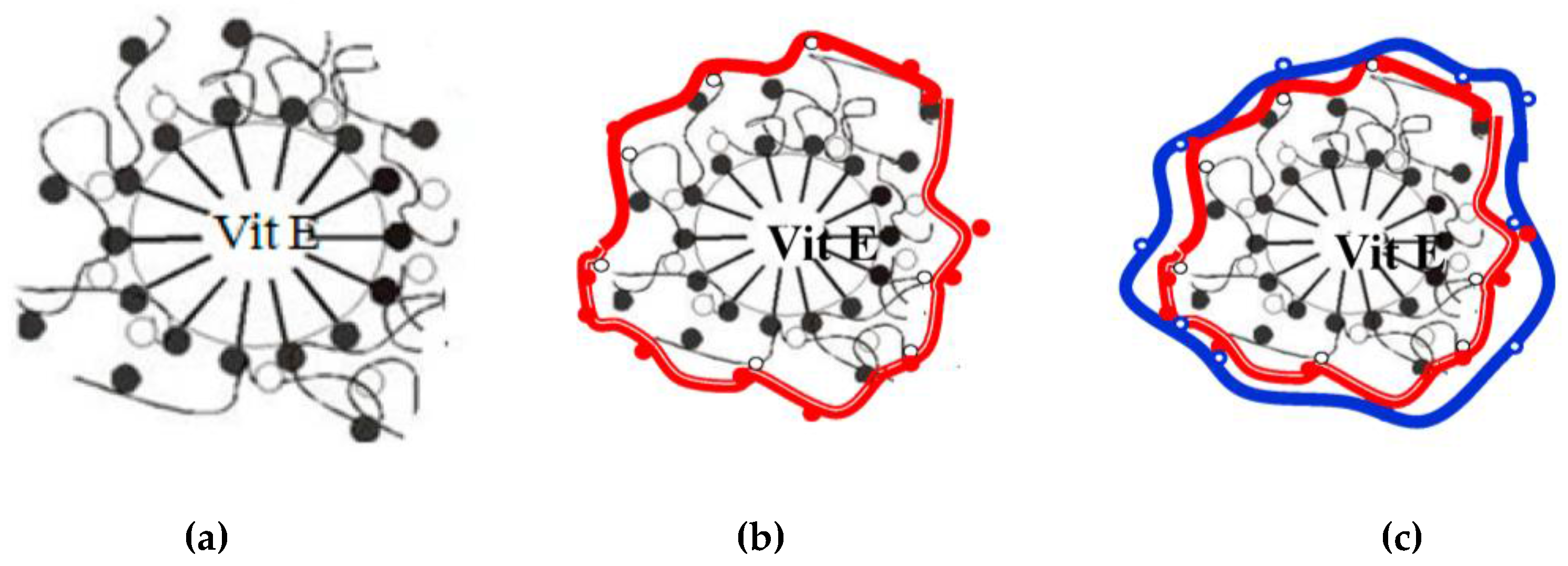
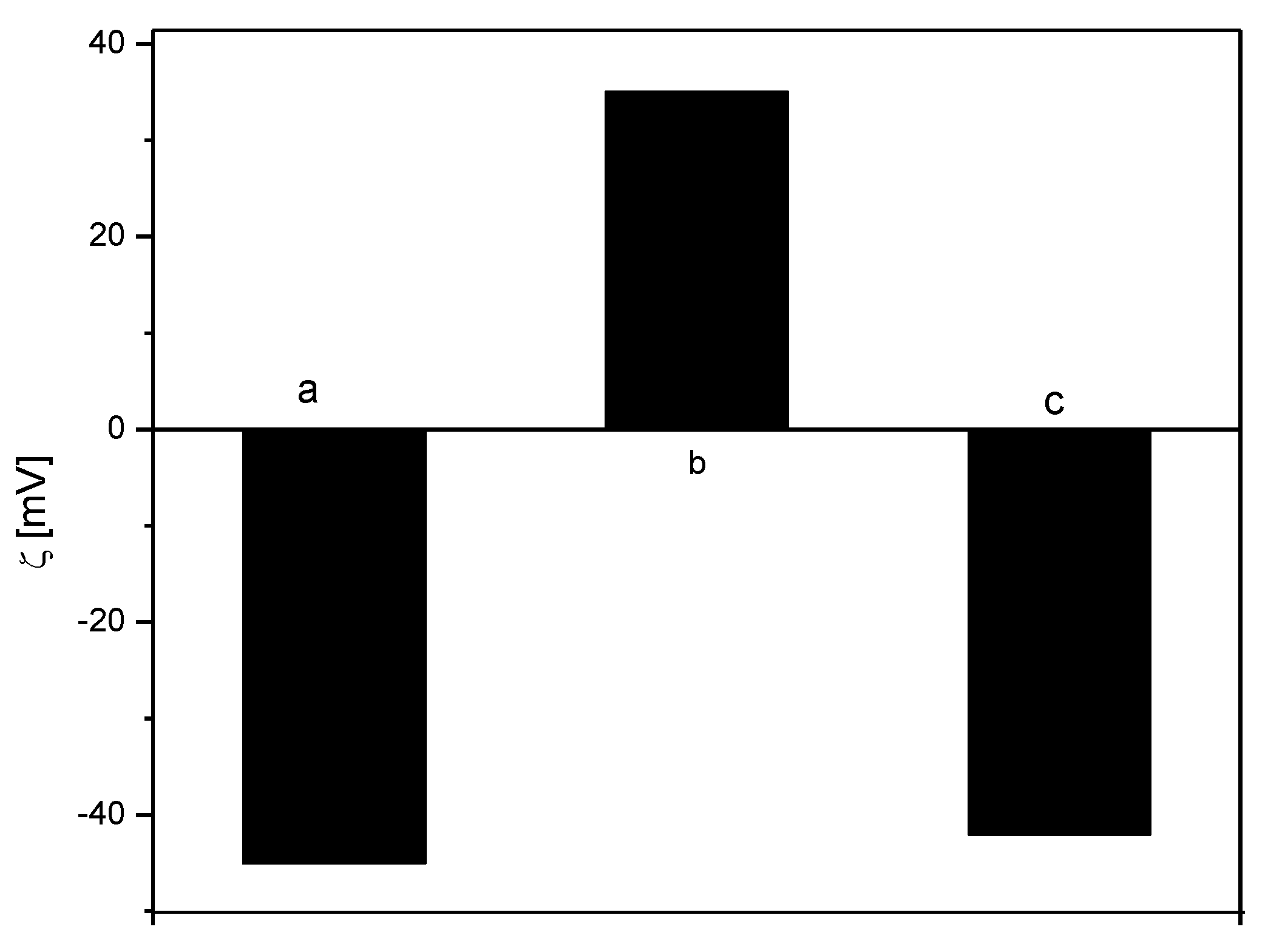
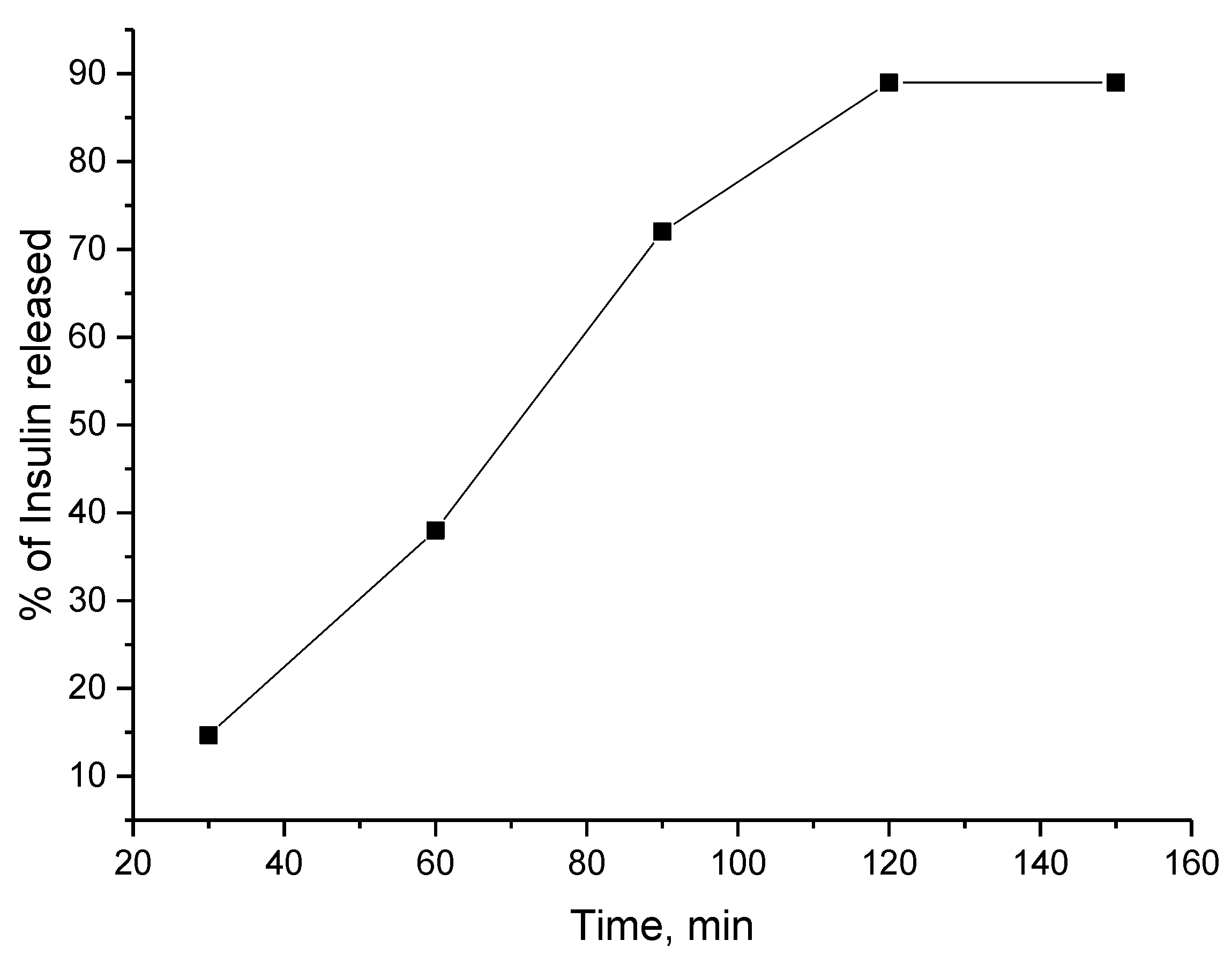
3.2. Encapsulation of Active Ingredients by Double W/O/W Emulsions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Langevin, D. Polyelectrolyte and surfactant mixed solutions. Behavior at surfaces and in thin films. Adv. Colloid Interface Sci. 2001, 89, 467–484. [Google Scholar] [CrossRef]
- Taylor, D.J.F.; Thomas, R.K.; Penfold, J. Polymer/surfactant interactions at the air/water interface. Adv. Colloid Interface Sci. 2007, 132, 69–110. [Google Scholar] [CrossRef] [PubMed]
- Nizri, G.; Lagerge, S.; Kamyshny, A.; Major, D.T.; Magdassi, S. Polymer-surfactant interactions: Binding mechanism of sodium dodecyl sulfate to poly(diallyldimethylammonium chloride). J. Colloid Interface Sci. 2008, 320, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Noskov, B.A.; Grigoriev, D.O.; Lin, S.Y.; Loglio, G.; Miller, R. Dynamic surface properties of polyelectrolyte/surfactant adsorption films at the air/water interface: Poly(diallyldimethylammonium chloride) and sodium dodecylsulfate. Langmuir 2007, 23, 9641–9651. [Google Scholar] [CrossRef] [PubMed]
- Tonigold, K.; Varga, I.; Nylander, T.; Campbell, R.A. Effects of aggregates on mixed adsorption layers of poly(ethylene imine) and sodium dodecyl sulfate at the air/liquid interface. Langmuir 2009, 25, 4036–4046. [Google Scholar] [CrossRef] [PubMed]
- Noskov, B.A.; Loglio, G.; Miller, R. Dilational Viscoelasticity of Polyelectolyte/Surfactant Adsorption Films at the Air/Water Interface: Dodecyltrimethylammonium Bromide and Sodium Poly(styrenesulfonate). J. Phys. Chem. B 2004, 108, 18615–18622. [Google Scholar] [CrossRef]
- Ibragimova, Z.K.; Kasaikin, V.A.; Zezin, A.B.; Kabanov, V.A. Non-stoichiometric complexes of polyacrilic acid with cationic surfactants. Vysokomolekulyarnie soedineniya Seriya A 1986, 28, 1640. [Google Scholar]
- Monteux, C.; Williams, C.E.; Meunier, J.; Anthony, O.; Bergeron, V. Adsorption of Oppositely Charged Polyelectrolyte/Surfactant Complexes at the Air/Water Interface: Formation of Interfacial Gels. Langmuir 2004, 20, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Noskov, B.A.; Loglio, G.; Miller, R. Dilational surface visco-elasticity of polyelectrolyte/surfactant solutions: Formation of heterogeneous adsorption layers. Adv. Colloid Interface Sci. 2011, 168, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Babak, V.G.; Desbrieres, J. Dynamic surface tension and dilational viscoelasticity of adsorption layers of alkylated chitosans and surfactant–chitosan complexes. J. Colloid Polym. Sci. 2006, 284, 745–754. [Google Scholar] [CrossRef]
- Piculell, L.; Lindman, B. Association and segregation in aqueous polymer/polymer, polymer/surfactant, and surfactant/surfactant mixtures: Similarities and differences. Adv. Colloid Interface Sci. 1992, 41, 149–178. [Google Scholar] [CrossRef]
- Benjamins, J.; Lyklema, J.; Lucassen-Reynders, E.H. Compression/expansion rheology of oil/water interfaces with adsorbed proteins. Comparison with the air/water surface. Langmuir 2006, 22, 6181–6188. [Google Scholar] [CrossRef] [PubMed]
- Nylander, T.; Samoshina, Y.; Lindman, B. Formation of polyelectrolyte-surfactant complexes on surfaces. Adv. Colloid Interface Sci. 2006, 123, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Pacios, I.E.; Lindman, B.; Thuresson, K. Polyelectrolyte–surfactant complexes with long range order. J. Colloid Interface Sci. 2008, 319, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.D.; Claesson, P.M.; Langevin, D.; Meszaros, R.; Nylander, T.; Stubenrauch, C.; Titmuss, S.; von Klitzing, R. Complexes of surfactants with oppositely charged polymers at surfaces and in bulk. Adv. Colloid Interface Sci. 2010, 155, 32. [Google Scholar] [CrossRef] [PubMed]
- Sharipova, A.; Aidarova, S.; Fainerman, V.B.; Stocco, A.; Cernoch, P.; Miller, R. Dynamics of adsorption of polyallylamine hydrochloride/sodium docecyl sulphate at water/air and water/hexane interfaces. Colloids Surf. A 2011, 391, 112–118. [Google Scholar] [CrossRef]
- Sharipova, A.; Aidarova, S.; Mucic, N.; Miller, R. Dilational rheology of polymer/surfactant mixtures at water/hexane interface. Colloids Surf. A 2011, 391, 130–134. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Yan, H.; Zhang, J.; Thomas, R.K. Binding of Sodium Dodecyl Sulfate with Linear and Branched Polyethyleneimines in Aqueous Solution at Different pH Values. Langmuir 2006, 22, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.B. Dilational viscoelasticity of anionic polyelectrolyte/surfactant adsorption films at the water–octane interface. Colloid Polym. Sci. 2009, 287, 1131–1137. [Google Scholar] [CrossRef]
- Miller, R.; Ferri, J.K.; Javadi, A.; Krägel, J.; Mucic, N.; Wüstneck, R. Rheology of interfacial layers. Colloid Polym. Sci. 2010, 288, 937–950. [Google Scholar] [CrossRef]
- Kogej, K. Association and structure formation in oppositely charged polyelectrolyte–surfactant mixtures. Adv. Colloid Interface Sci. 2010, 158, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.G.; Le, K.A.; Sadtler, V.; Marchal, P.; Perrin, P.; Tribet, C.; Marie, E.; Durand, A. Enhanced stability of nanoemulsions using mixtures of non-ionic surfactant and amphiphilic polyelectrolyte. Colloids Surf. A 2011, 389, 237–245. [Google Scholar] [CrossRef]
- Stamkulov, N.S.; Mussabekov, K.B.; Aidarova, S.B.; Luckham, P.F. Stabilisation of emulsions by using a combination of an oil soluble ionic surfactant and water soluble polyelectrolytes. I: Emulsion stabilisation and Interfacial tension measurements. Colloids Surf. A 2009, 335, 103–106. [Google Scholar] [CrossRef]
- Sharipova, A.A.; Aidarova, S.B.; Grigoriev, D.O.; Mutalieva, B.Z.; Madybekova, G.M.; Tleuova, A.B.; Miller, R. Polymer–surfactant complexes for microencapsulation of vitamin E and its release. Colloids Surf. B 2016, 137, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Penfold, J.; Tucker, I.; Thomas, R.K.; Taylor, D.J.F. The Interaction between Sodium Alkyl Sulfate Surfactants and the Oppositely Charged Polyelectrolyte, polyDMDAAC, at the Air−Water Interface: The Role of Alkyl Chain Length and Electrolyte and Comparison with Theoretical Predictions. Langmuir 2007, 23, 3128–3136. [Google Scholar] [CrossRef] [PubMed]
- Musabekov, K.B.; Aidarova, S.; Abdiev, K. Adsorption of polyelectrolyte associates at the interfaces. In Progresses of Colloid Chemistry; L. Chemistry: Leningrad, Russia, 1991; pp. 209–223, (Russ); ISBN 5-7245-0765-X. [Google Scholar]
- Sharipova, A.; Aidarova, S.; Cernoch, P.; Miller, R. Effect of surfactant hydrophobicity on the interfacial properties of polyallylamine hydrochloride/sodium alkylsulphate at water/hexane interface. Colloids Surf. A 2013, 438, 141–147. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhang, L.P.; Tang, J.A.; Jiang, L. Interactions between poly(acrylamide) and surfactants of different headgroup charge. Colloids Surf. A 1994, 88, 33–39. [Google Scholar] [CrossRef]
- Kundu, S. Polyelectrolyte–surfactant complexes on solid surface. J. Colloid Interface Sci. 2010, 344, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Terada, E.; Samoshina, Y.; Nylander, T.; Lindman, B. Adsorption of Cationic Cellulose Derivative/Anionic Surfactant Complexes onto Solid Surfaces. II. Hydrophobized Silica Surfaces. Langmuir 2004, 20, 6692–6701. [Google Scholar] [CrossRef] [PubMed]
- Aidarova, S.; Musabekov, K.; Ospanova, Z.; Guden, M. Foaming binary solution mixtures of low molecular surfactant and polyelectrolyte. J. Mater. Sci. 2006, 41, 3979–3986. [Google Scholar] [CrossRef]
- Monteux, C.; Fuller, G.G.; Bergeron, V. Shear and Dilational Surface Rheology of Oppositely Charged Polyelectrolyte/Surfactant Microgels Adsorbed at the Air−Water Interface. Influence on Foam Stability. J. Phys. Chem. B 2004, 108, 16473–16482. [Google Scholar] [CrossRef]
- Langevin, D. Influence of interfacial rheology on foam and emulsion properties. Adv. Colloid Interface Sci. 2000, 88, 209–222. [Google Scholar] [CrossRef]
- Petrovic, L.B.; Sovilj, V.J.; Katona, J.M.; Milanovic, J.L. Influence of polymer–surfactant interactions on O/W emulsion properties and microcapsule formation. J. Colloid Interface Sci. 2010, 342, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Tucker, I.M.; Petkov, J.T.; Jones, C.; Penfold, J.; Thomas, R.K.; Rogers, S.E.; Terry, A.E.; Heenan, R.K.; Grillo, I. Adsorption of Polymer–Surfactant Mixtures at the Oil–Water Interface. Langmuir 2012, 28, 14974–14982. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, G.; Langevin, D. Interfacial Shear Rheology of Mixed Polyelectrolyte−Surfactant Layers. Langmuir 2009, 25, 12201–12207. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.F. Rheology of Dispersions: Principles and Applications; Wiley and Sons: New York, NY, USA, 2010; ISBN 978-3-527-32003-5. [Google Scholar]
- Dukhin, A.S.; Goetz, P.J. Characterization of Liquids, nano- and microparticulates, and porous bodies using ultrasound. In Studies in Interface Science; Möbius, D., Miller, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 24. [Google Scholar]
- Aidarova, S.; Sharipova, A.; Krägel, J.; Miller, R. Polyelectrolyte/surfactant mixtures in the bulk and at water/oil interfaces. Adv. Colloid Interface Sci. 2014, 205, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Penfold, J.; Tucker, I.; Thomas, R.K.; Zhang, J. Adsorption of Polyelectrolyte/Surfactant Mixtures at the Air−Solution Interface: Poly(ethyleneimine)/Sodium Dodecyl Sulfate. Langmuir 2005, 21, 10061–10073. [Google Scholar] [CrossRef] [PubMed]
- Langevin, D. Complexation of oppositely charged polyelectrolytes and surfactants in aqueous solutions. A review. Adv. Colloid Interface Sci. 2009, 147, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Trojer, M.A.; Li, Y.; Abrahamsson, C.; Mohamed, A.; Eastoe, J.; Holmberga, K.; Nyden, M. Charged microcapsules for controlled release of hydrophobic actives. Part I: Encapsulation methodology and interfacial properties. Soft Matter. 2013, 9, 1468–1477. [Google Scholar] [CrossRef]
- Akiyama, E.; Kashimoto, A.; Fukuda, K.; Hotta, H.; Suzuki, T.; Kitsuki, T. Thickening properties and emulsification mechanisms of new derivatives of polysaccharides in aqueous solution. J. Colloid Interface Sci. 2005, 282, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.; Thalberg, K. Polymer-surfactant interactions-recent developments. Interactions of Surfactants with Polymers and Proteins; Goddard, D.E., Ed.; CRC Press: Boca Raton, FL, USA, 1993; p. 203. [Google Scholar]
- Goddard, D.E. Interactions of Surfactants with Polymers and Proteins; Goddard, D.E., Ed.; CRC Press: Boca Raton, FL, USA, 1993; 448р. [Google Scholar]
- Aidarova, S.B.; Fainerman, V.B.; Aksenenko, E.V.; Bekturganova, N.E.; Tarasevich, Y.I.; Miller, R. Effect of electrolyte on adsorption of polyallyl amine hydrochloride/sodium dodecyl sulphate at water/tetradecane interface. Colloids Surf. A 2014, 460, 11–17. [Google Scholar]
- Tasker, A.L.; Hitchcock, J.P.; He, L.; Baxter, E.A.; Biggs, S.; Cayre, O.J. The effect of surfactant chain length on the morphology of poly(methyl methacrylate) microcapsules for fragrance oil encapsulation. J. Colloid Interface Sci. 2016, 484, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Loxley, A.; Vincent, B. Preparation of Poly(methylmethacrylate) Microcapsules with Liquid Cores. J. Colloid Interface Sci. 1998, 208, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Feczkó, T.; Kardos, A.F.; Németh, B.; Trif, L.; Gyenis, J. Microencapsulation of n-hexadecane phase change material by ethyl ccellulose polymer. J. Polym. Bull. 2014, 71, 3289–3304. [Google Scholar] [CrossRef] [Green Version]
- Trojer, M.A.; Mohamed, A.; Eastoe, J. A highly hydrophobic anionic surfactant at oil–water, water–polymer and oil–polymer interfaces: Implications for spreading coefficients, polymer interactions and microencapsulation via internal phase separation. J. Colloids Surf. A 2013, 436, 1048–1059. [Google Scholar] [CrossRef]
- Trongsatitkul, T.; Budhlall, B.M. Multicore–Shell PNIPAm-co-PEGMa Microcapsules for Cell Encapsulation. Langmuir 2011, 207, 13468–13480. [Google Scholar] [CrossRef] [PubMed]
- Staples, E.; Penfold, J.; Tucker, I.J. Adsorption of Mixed Surfactants at the Oil−Water Interface. Phys. Chem. B 2000, 104, 606–614. [Google Scholar] [CrossRef]
- Bumajdad, A.; Eastoe, J.; Nave, S.; Steytler, D.C.; Heenan, R.K.; Grillo, I. Compositions of Mixed Surfactant Layers in Microemulsions Determined by Small-Angle Neutron Scattering. Langmuir 2003, 19, 2560–2567. [Google Scholar] [CrossRef]
- Philip, J.; Gnana Prakash, G.; Jaykumar, T.; Kalyanasundaran, P.; Mondian-Monvial, O.; Raj, B. Interaction between Emulsion Droplets in the Presence of Polymer−Surfactant Complexes. Langmuir 2002, 18, 4625–4631. [Google Scholar] [CrossRef]
- Philip, J.; Gnana Prakash, G.; Jaykumar, T.; Kalyanasundaran, P.; Mondian-Monvial, O.; Raj, B. Three Distinct Scenarios under Polymer, Surfactant, and Colloidal Interaction. Macromolecules 2003, 36, 9230–9236. [Google Scholar] [CrossRef]
- Trojer, M.A.; Li, Y.; Wallin, M.; Holmberg, K.; Nyden, M. Charged microcapsules for controlled release of hydrophobic actives Part II: Surface modification by LbL adsorption and lipid bilayer formation on properly anchored dispersant layers. J. Colloid Interface Sci. 2013, 409, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Gitin, L.; Alexe, P.; Dima, S. Encapsulation of coriander essential oil in alginate and alginate/chitosan microspheres by emulsification external gelation method. In Proceedings of the Inside Food Symposium, Leuven, Belgium, 9–12 April 2013. [Google Scholar]
- Chavanpatil, M.D.; Khdair, A.; Patil, Y.; Handa, H.; Mao, G.; Panyam, J. Polymer-surfactant nanoparticles for sustained release of water-soluble drugs. J. Pharm. Sci. 2007, 96, 3379–3389. [Google Scholar] [CrossRef] [PubMed]
- Maa, Y.F.; Hsu, C.C. Performance of sonication and microfluidization for liquid-liquid emulsification. Pharm. Dev. Technol. 1999, 4, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.S.; He, Y.; Bhandari, B. Nano-Emulsion Production by Sonication and Microfluidization—A Comparison. J. Food Prop. 2006, 9, 475–485. [Google Scholar] [CrossRef]
- Leong, T.S.H.; Wooster, T.J.; Kentish, S.E.; Ashokkumar, M. Minimising oil droplet size using ultrasonic emulsification. Ultrason. Sonochem. 2009, 16, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Che, Z.; Wong, T.N.; Nguyen, N.-T. A simple method for the formation of water-in-oil-in-water (W/O/W) double emulsions. Microfluid. Nanofluid. 2017, 21, 8. [Google Scholar] [CrossRef]
- Mutaliyeva, B.; Grigoriev, D.; Madybekova, G.; Sharipova, A.; Aidarova, S.; Saparbekova, A.; Miller, R. Microencapsulation of insulin and its release using W/O/W double emulsion method. Colloids Surf. A 2017, 521, 147–152. [Google Scholar] [CrossRef]
- Schuch, A.; Helfenritter, C.; Funck, M.; Schuchmann, H.P. Observations on the influence of different biopolymers on coalescence of inner water droplets in W/O/W (water-in-oil-in-water) double emulsions. Colloids Surf. A 2015, 475, 2–8. [Google Scholar] [CrossRef]
- Giri, T.K.; Choudhary, C.; Amit Alexander, A.; Badwaik, H.; Tripathi, D.K. Prospects of pharmaceuticals and biopharmaceuticals loaded microparticles prepared by double emulsion technique for controlled delivery. Saudi Pharm. J. 2013, 21, 125–141. [Google Scholar] [CrossRef] [PubMed]
- The HLB SYSTEM: A time-Saving Guide to Emulsifier Selection; ICI Americas Inc.: Wilmington, DE, USA, 1984; p. 22.
- Chang, L.C.; Yang, C.Y.; Chua, A.C.; Lin, Y.J.; Lai, S.M. Sustained release of transgenic human factor IX: preparation, characterization, and in vivo efficacy. Mol. Pharm. 2011, 8, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.L.; Sun, W.G.; Zhao, W.; Chen, T. Preparation of amifostinepolylactide-co-glycolide microspheres and its irradiation protective to mouse through oral administration. Drug Dev. Ind. Pharm. 2011, 37, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Karal-Ylmaz, O.; Serhat, M.; Baysal, K.; Baysal, B.M. Preparation and in vitro characterization of vascular endot helialgrowth factor (VEGF) loaded poly(d,l-lactic-co-glycolic acid)microspheres using a double emulsion/solvent evaporation technique. J. Microencapsul. 2011, 28, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Dalmoro, A.; Lamberti, G.; Titomanilo, G.; Barba, A.A.; Amore, M. Enteric microparticles for targeted oral drug delivery. Pharm. Sci. Tech. 2010, 11, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Forster, S.; Plantenberg, T. From self-organizing polymers to nanohybrid and biomaterials. Angew. Chem. Int. Ed. 2002, 41, 688–714. [Google Scholar] [CrossRef]
- Sheshala, R.; Peh, K.K.; Darwis, Y. Preparation, characterization, and in vivo evaluation of insulin-loaded PLA-PEG microspheres for controlledparenteral drug delivery. Drug Dev. Ind. Pharm. 2009, 35, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Zhou, J.; Lui, J.; Wu, D. PLGA microspheres with high drug loading and high encapsulation efficiency prepared by a novel solvent evaporation technique. J. Microencapsul. 2006, 23, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Yazan, Y. Formulation and in-vivo evaluation of a cosmetic multipleemulsion containing vitamin C and wheat protein. Pak. J. Pharm. Sci. 2008, 21, 45–50. [Google Scholar] [PubMed]
- Garti, N.; Lutz, R. Recent progress in double emulsions. Interface Sci. Technol. 2004, 4, 557–605. [Google Scholar]
- Yildirim, M.; Sumnu, G.; Sahin, S. The effects of emulsifier type, phase ratio, and homogenization methods on stability of the double emulsion. J. Dispers. Sci. Technol. 2017, 38, 807–814. [Google Scholar] [CrossRef]
- Kanouni, M.; Rosano, H.L.; Naouli, N. Preparation of a stable double emulsion (W1/O/W2): Role ofthe interfacial films on the stability of the system. Adv. Colloid Interface Sci. 2002, 99, 229–254. [Google Scholar] [CrossRef]
- Dickinson, E. Double emulsions stabilized by food biopolymers. Food Biophys. 2011, 6, 1–11. [Google Scholar] [CrossRef]
- Peppas, N.A. Devices based on intelligent biopolymers for oral protein delivery. Int. J. Pharm. 2004, 227, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.C.; Leroux, J.C. Polymeric micelles: A new generation of colloidal drug carrier. Eur. J. Pharm. Biopharm. 2000, 48, 101–111. [Google Scholar] [CrossRef]
- Zielinski, B.A.; Aebischer, P. Chitosan as a matrix for mammalian cell encapsulation. Biomaterials 2002, 15, 1049–1056. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, H.C.; Oh, J.S.; Shin, B.A.; Oh, C.S.; Park, R.D.; Yang, K.S.; Cho, C.S. Polyelectroylte complex composed of chitosan and sodium alginate for wound dressing application. J. Biomater. Sci. Polym. Ed. 2000, 10, 543–556. [Google Scholar] [CrossRef]
- Lee, B.R.; Lee, K.H.; Kang, E.; Kim, D.S.; Lee, S.H. Microfluidic wet spinning of chitosan-alginate microfibers and encapsulation of HepG2 cells in fibers. Biomicrofluidics 2011, 5, 222–308. [Google Scholar] [CrossRef] [PubMed]
- Pays, K.; Giermanska-Kahn, J.; Pouligny, B.; Bibette, J.; Leal-Calderon, F. Double emulsions: How does release occur? J. Control. Release 2002, 79, 193–205. [Google Scholar] [CrossRef]
- Li, J.; Su, L.; Li, J.; Liu, M.-F.; Chen, S.-F.; Li, B.; Zhang, Z.-W.; Liu, Y.-Y. Influence of sucrose on the stability of W1/O/W2 double emulsion droplets. RSC Adv. 2015, 5, 83089–83095. [Google Scholar] [CrossRef]
- Wang, G.; Su, L.; Chen, S.; Yao, H.; Deng, Y.; Li, B.; Wei, J. Stability of large diameter W1/O/W2 emulsion particles. High Power Laser Part. Beams 2012, 24, 389–393. [Google Scholar] [CrossRef]
- Montazeri, L.; Bonakdar, S.; Taghipour, M.; Renaud, P.; Baharvand, H. Modification of PDMS to fabricate PLGA microparticles by a double emulsion method in a single microfluidic device. Lab Chip 2016, 16, 2596–2600. [Google Scholar] [CrossRef] [PubMed]
- Pays, K.; Giermanska-Kahn, J.; Pouligny, B.; Bibette, J.; Leal-Calderon, F. Coalescence in surfactant-stabilized double emulsions. Langmuir 2001, 17, 7758–7769. [Google Scholar] [CrossRef]
- De Araújo, T.M.; Teixeira, Z.; Barbosa-Sampaio, H.C.; Rezende, L.F.; Boschero, A.C.; Durán, N.; Höehr, N.F. Insulin-loaded poly(ε-caprolactone) nanoparticles: Efficient, sustained and safe insulin delivery system. J. Biomed. Nanotechnol. 2013, 9, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Schmidts, T.; Dobler, D.; Nissing, C.; Runkel, F. Influence of hydrophilic surfactants on the properties ofmultiple W/O/W emulsions. J. Colloid Interface Sci. 2009, 338, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Altuntas, O.Y.; Sumnu, G.; Sahin, S. Preparation and characterization of W/O/W type double emulsion containing PGPR–lecithin mixture as lipophilic surfactant. J. Dispers. Sci. Technol. 2017, 38, 486–493. [Google Scholar] [CrossRef]
- Fahad, M.; Almutairi, T.E.; Adams, G.G.; Hayes, M.; McLoughlin, P.; Kök, M.S.; Mackie, A.R.; Rowe, A.J.; Harding, S.E. Hydrodynamic characterisation of chitosan and its interaction withtwo polyanions: DNA and xanthan. Carbohydr. Polym. 2015, 122, 359–366. [Google Scholar]
- Mehrnia, M.-A.; Jafari, S.-M.; Makhmal-Zadeh, B.S.; Maghsoudlou, Y. Rheological and release properties of double nano-emulsions containing crocin prepared with Angum gum, Arabic gum and whey protein. Food Hydrocoll. 2017, 66, 259–267. [Google Scholar] [CrossRef]
- Sanna, V.; Roggio, A.M.; Pal, N.; Marceddu, S.; Lubinu, G.; Mariani, A.; Sechi, M. Effect of chitosan concentration on PLGA microcapsules for controlled release and stability of resveratrol. Int. J. Biolog. Macromol. 2015, 72, 531–536. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharipova, A.A.; Aidarova, S.B.; Mutaliyeva, B.Z.; Babayev, A.A.; Issakhov, M.; Issayeva, A.B.; Madybekova, G.M.; Grigoriev, D.O.; Miller, R. The Use of Polymer and Surfactants for the Microencapsulation and Emulsion Stabilization. Colloids Interfaces 2017, 1, 3. https://doi.org/10.3390/colloids1010003
Sharipova AA, Aidarova SB, Mutaliyeva BZ, Babayev AA, Issakhov M, Issayeva AB, Madybekova GM, Grigoriev DO, Miller R. The Use of Polymer and Surfactants for the Microencapsulation and Emulsion Stabilization. Colloids and Interfaces. 2017; 1(1):3. https://doi.org/10.3390/colloids1010003
Chicago/Turabian StyleSharipova, Altynay A., Saule B. Aidarova, Botagoz Z. Mutaliyeva, Alpamys A. Babayev, Miras Issakhov, Assem B. Issayeva, Galiya M. Madybekova, Dmitry O. Grigoriev, and Reinhard Miller. 2017. "The Use of Polymer and Surfactants for the Microencapsulation and Emulsion Stabilization" Colloids and Interfaces 1, no. 1: 3. https://doi.org/10.3390/colloids1010003