Comparison of the Critical Coagulation Concentrations of Allophane and Smectites
Abstract
:1. Introduction
2. Materials and Methods
3. Results
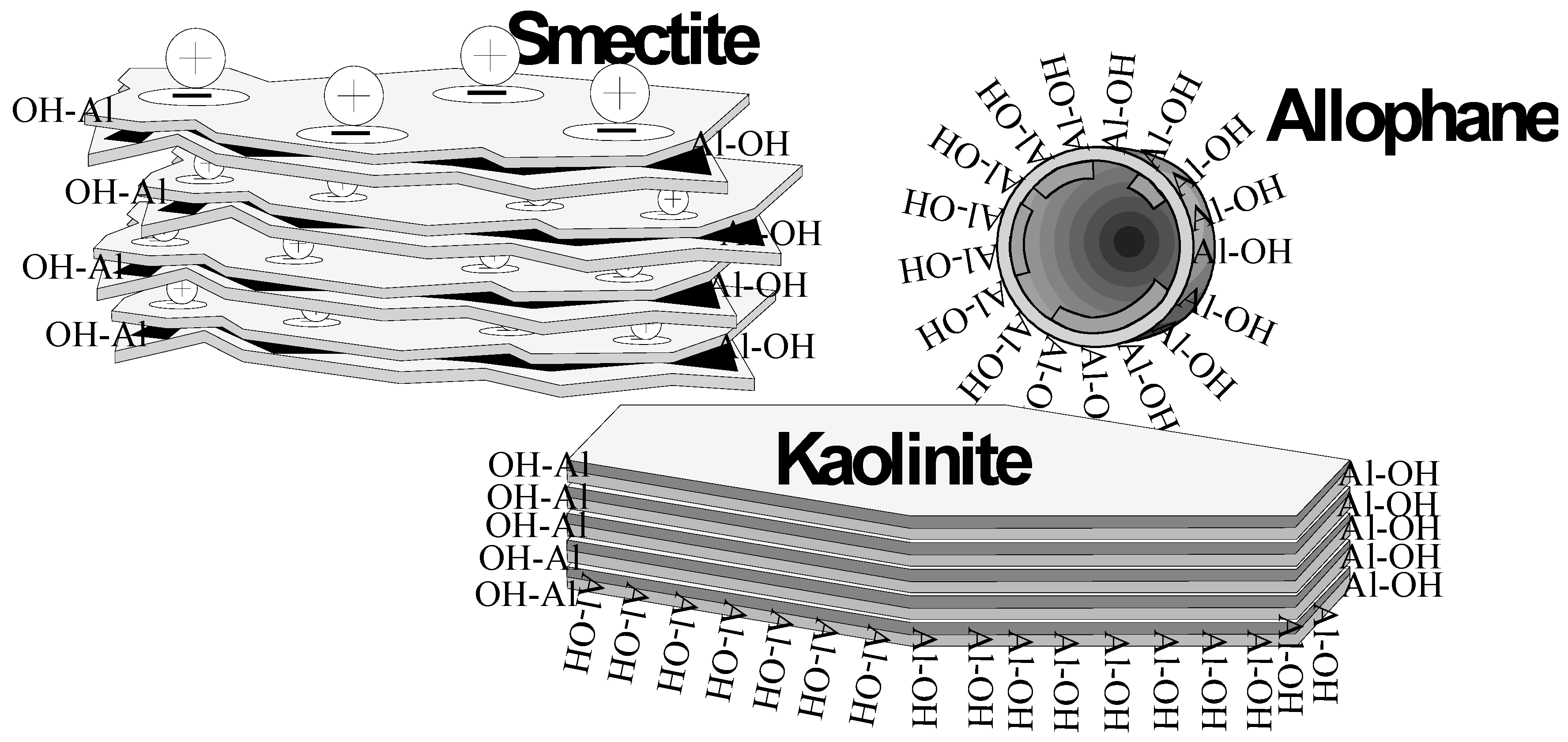
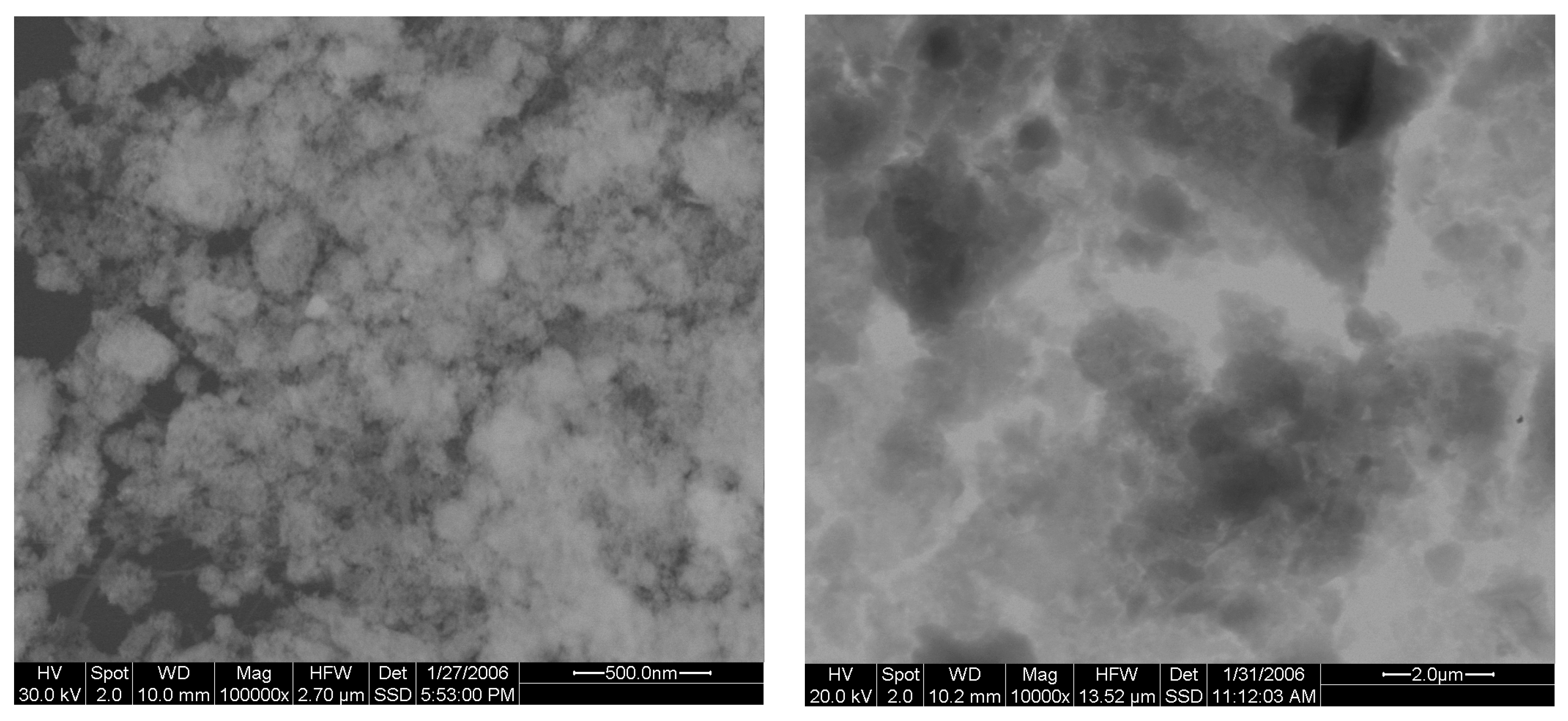
3.1. Allophane
3.2. Bentonites
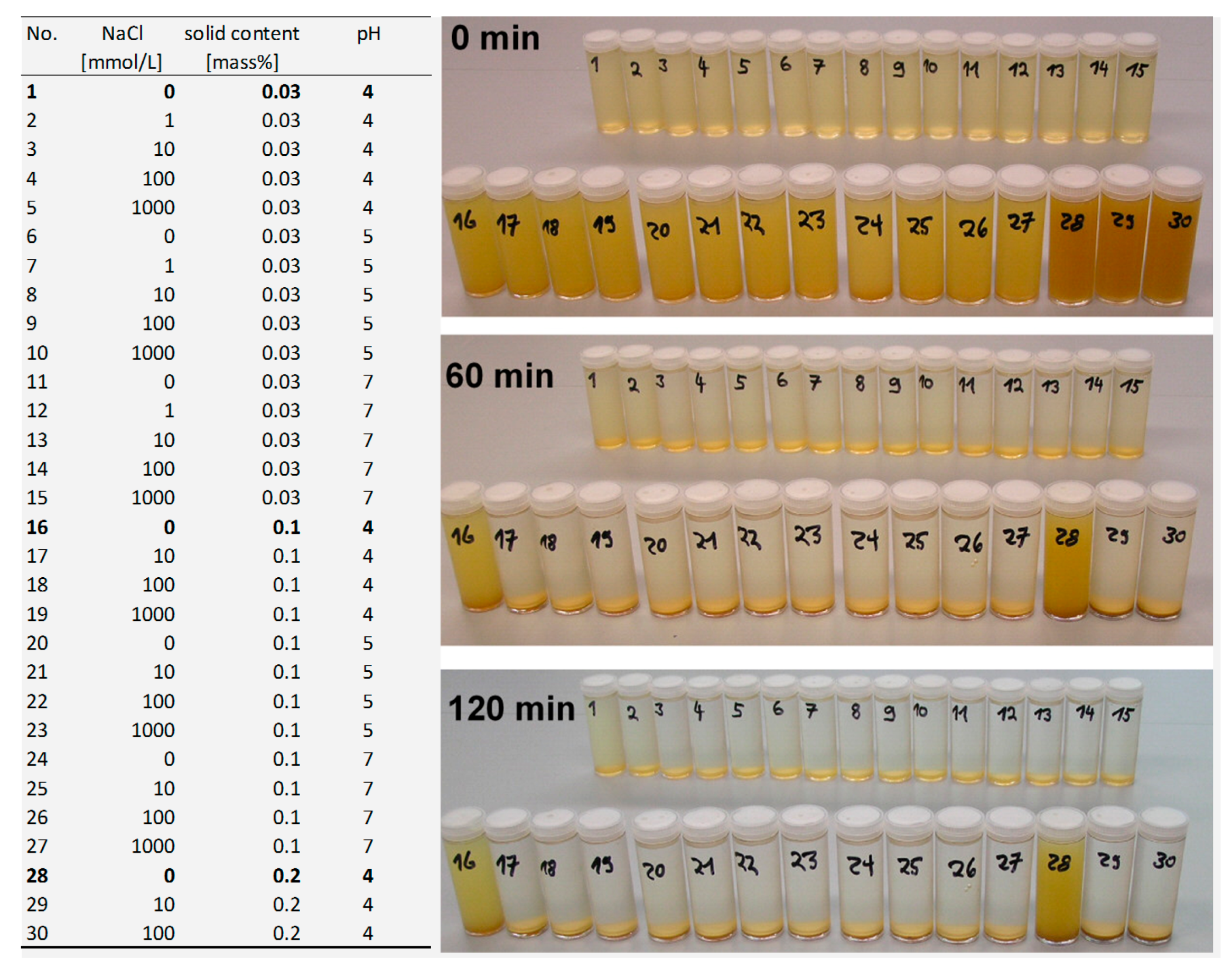
3.2.1. Solid/Liquid Ratio
3.2.2. pH Effect
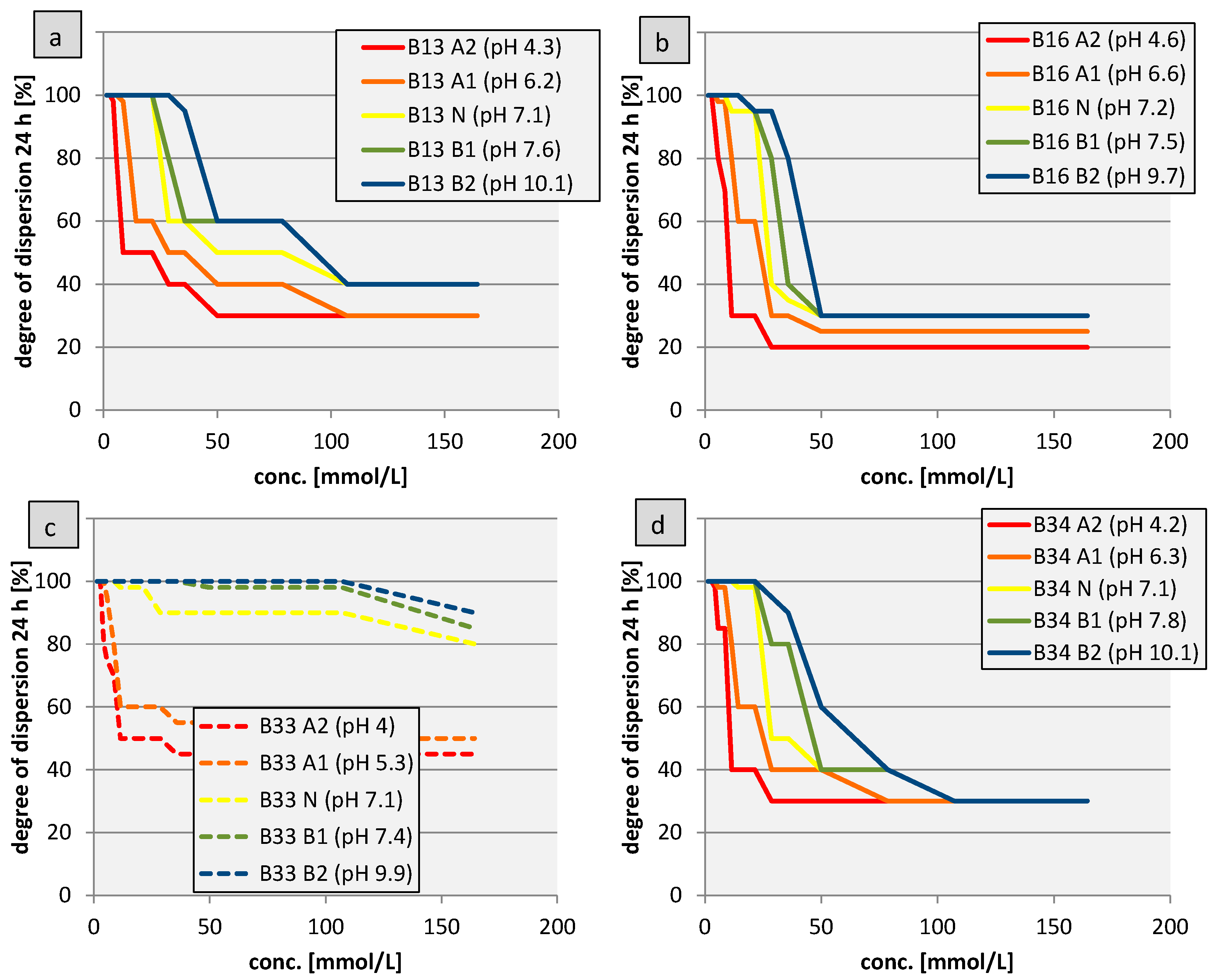
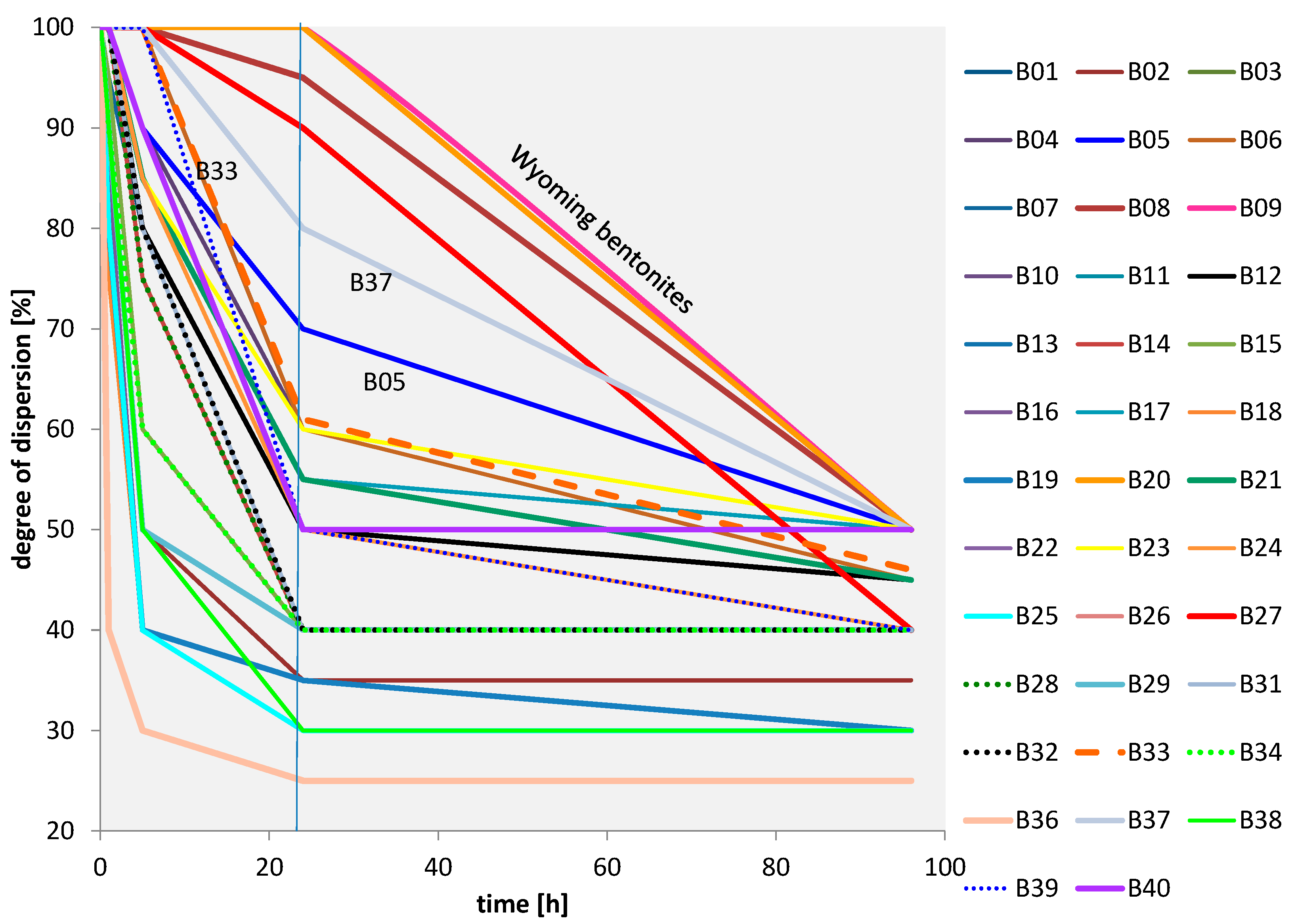
3.2.3. Coagulation Kinetics
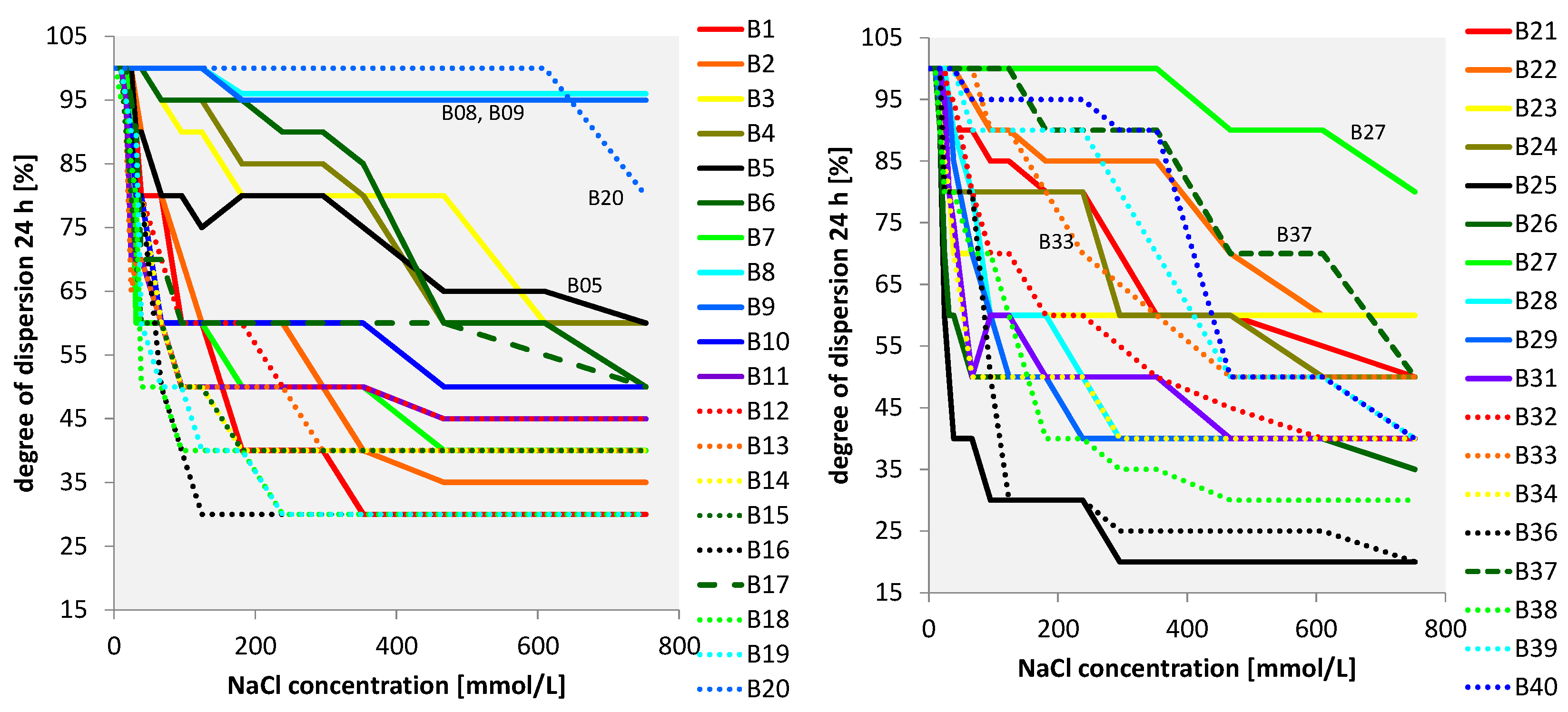
3.2.4. All Bentonites
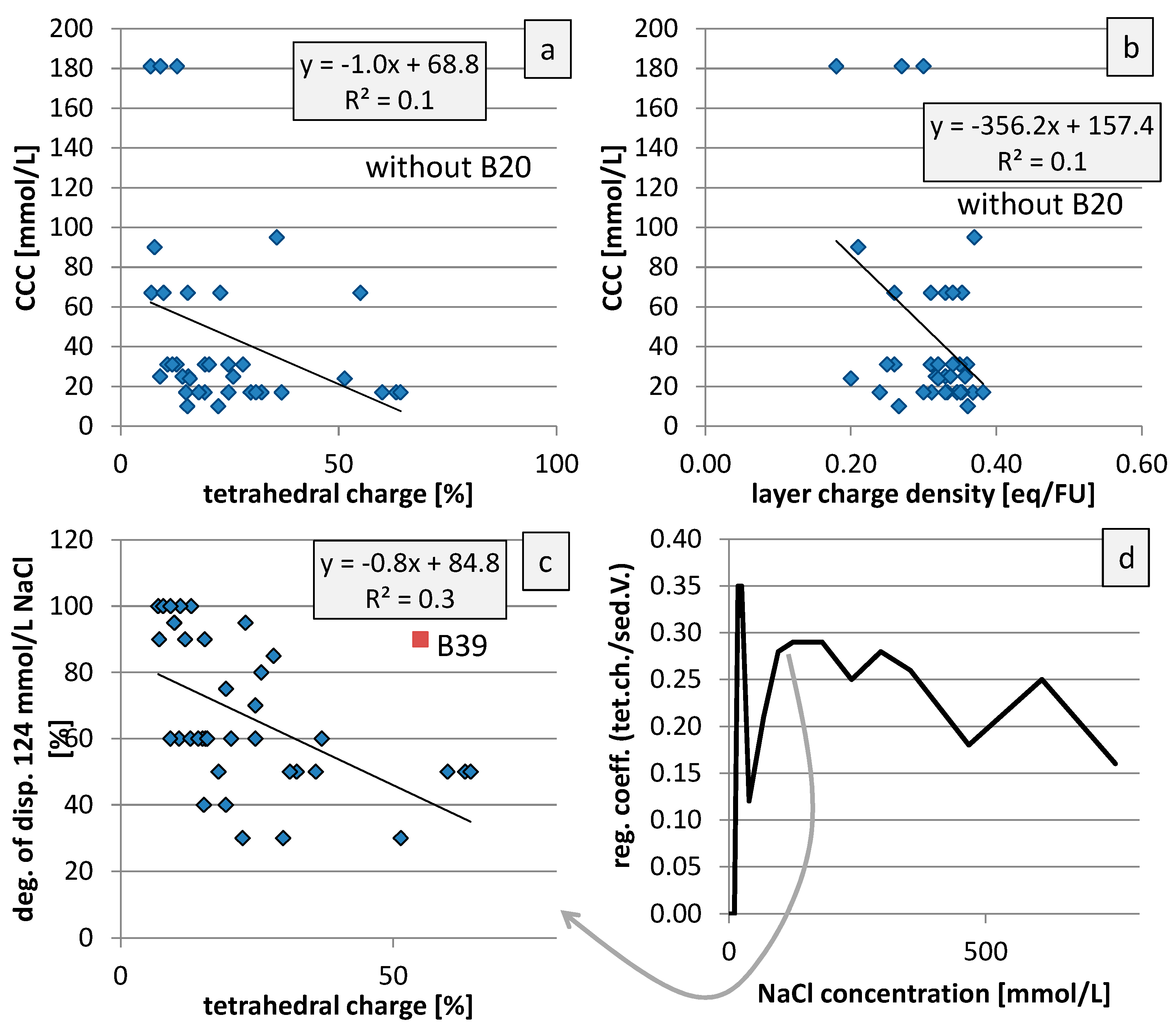
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Overbeek, J.T.G. Recent developments in the understanding of colloid stability. J. Colloid Interface Sci. 1977, 58, 408–422. [Google Scholar] [CrossRef]
- Van Olphen, H. An Introduction to Clay Colloid Chemistry; Wiley: New York, NY, USA, 1977. [Google Scholar]
- Lagaly, G. Ullmann’s Encyclopedia of Industrial Chemistry; VCH-Verlagsgesellschaft: Weinheim, Germany, 1986; Volume A7, pp. 341–367. [Google Scholar]
- Lagaly, G. From clay mineral crystals to colloidal clay mineral dispersions. In Coagulation and Flocculation: Theory and Application; Dobias, B., Ed.; Marcel Dekker: New York, NY, USA, 1992; pp. 427–494. [Google Scholar]
- Lagaly, G.; Dékány, I. Colloid Clay Science. In Handbook of Clay Science–Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 243–345. [Google Scholar]
- Missana, T.; Adell, A. On the Applicability of DLVO Theory to the Prediction of Clay Colloids Stability. J. Colloid Interface Sci. 2000, 230, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Lagaly, G. Reaktionen der Tonminerale. In Tonminerale und Tone–Struktur, Eigenschaften, Anwendung und Einsatz in Industrie und Umwelt; Jasmund, K., Lagaly, G., Eds.; Springer: Berlin, Germany, 1993; pp. 89–158. [Google Scholar]
- Neretnieks, I.; Liu, L.; Moreno, L. Mechanisms and Models for Bentonite Erosion; SKB Technical Report TR-09-35; Department of Chemical Engineering and Technology, Royal Institute of Technology, KTH: Stockholm, Sweden, 2009. [Google Scholar]
- SKB. Long-Term Safety for KBS-3 Repository at Forsmark and LAXMAR—A First Evaluation; Technical Report TR-06-09; SKB: Stockholm, Sweden, 2006. [Google Scholar]
- Kaufhold, S.; Dohrmann, R. Detachment of colloidal particles from bentonites in water. Appl. Clay Sci. 2008, 39, 50–59. [Google Scholar] [CrossRef]
- Kretzschmar, R.; Holthoff, H.; Sticher, H. Influence of pH and Humic Acid on Coagulation Kinetics of Kaolinite: A Dynamic Light Scattering Study. J. Colloid Interface Sci. 1998, 202, 95–103. [Google Scholar] [CrossRef]
- Novich, B.E.; Ring, T.A. Colloid Stability of Clays Using Photon Correlation Spectroscopy. Clays Clay Miner. 1984, 32, 400–407. [Google Scholar] [CrossRef]
- Hetzel, F.; Donner, H.E. Some colloidal properties of Beidellite: Comparison with low and high charge Montmorillonites. Clays Clay Miner. 1993, 41, 453–460. [Google Scholar] [CrossRef]
- Lagaly, G.; Ziesmer, S. Colloid chemistry of clay minerals: The coagulation of montmorillonite dispersions. Adv. Colloid Interface Sci. 2003, 100, 105–128. [Google Scholar] [CrossRef]
- Chheda, P.; Grasso, D.; van Oss, C.J. Impact of ozone on stability of montmorillonite suspensions. J. Colloid Interface Sci. 1992, 153, 226–236. [Google Scholar] [CrossRef]
- Tombácz, E.; Szekeres, M. Colloidal behavior of aqueous montmorillonite suspensions: The specific role of pH in the presence of indifferent electrolytes. Appl. Clay Sci. 2004, 27, 75–94. [Google Scholar] [CrossRef]
- Tombácz, E.; Ábrahám, I.; Gilde, M.; Szántó, F. The pH-dependent colloidal stability of aqueous montmorillonite suspensions. Colloids Surf. 1990, 49, 71–80. [Google Scholar] [CrossRef]
- Frey, E.; Lagaly, G. Selective coagulation in mixed colloidal suspensions. J. Colloid Interface Sci. 1979, 70, 46–55. [Google Scholar] [CrossRef]
- Wada, K. Allophane and imogolite. In Minerals in Soil Environments; Dixon, J.B., Weed, S.B., Eds.; Soil Science Society of America: Madison, WI, USA, 1989; pp. 1051–1087. [Google Scholar]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides; VCH-Verlagsgesellschaft: Weinheim, Germany, 1996. [Google Scholar]
- Essington, M.E. Soil and Water Chemistry: An Integrative Approach; CRC Press: Boca Raton, FL, USA, 2003; p. 534. [Google Scholar]
- Abidin, Z.; Matsue, N.; Henmi, T. Nanometer-scale chemical modification of nano-ball allophane. Clays Clay Miner. 2007, 55, 443–449. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R. The variable charge of dioctahedral clay minerals. J. Colloid Interface Sci. 2013, 390, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Kaufhold, A. Eigenschaften von Allophan aus Ecuador (Santo Domingo de los Colorados) und Anwendungspotential als Rohstoff. Ph.D. Thesis, Martin Luther University of Halle-Wittenberg, Halle, Germany, 2011; p. 175. [Google Scholar]
- Dahlgren, R.A. Quantification of allophane and imogolite. In Quantitative Methods in Soil Mineralogy; Luxmoore, R.J., Ed.; Miscellaneous Publication: Madison, WI, USA, 1994; pp. 431–451. [Google Scholar]
- Harsh, J. Poorly crystalline Aluminosilicate Clays. In Handbook of Soil Science; Sumner, M.E., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. F169–F182. [Google Scholar]
- Karube, J.; Nakaishi, K.; Sugimoto, H. Fujihara Size and shape of allophane particles in dispersed aqueous systems. Clays Clay Miner. 1996, 44, 485–491. [Google Scholar] [CrossRef]
- Kitagawa, Y. The ‘unit particle’ of allophane. Am. Mineral. 1971, 56, 465–475. [Google Scholar]
- Warkentin, B.P.; Maeda, T. Physical and mechanical characteristics of Andisols. In Soils with Variable Charge; Theng, B.K.G., Ed.; Society of Soil Science: Hamilton, New Zealand; Soil Bureau: Lower Hutt, New Zealand, 1980; pp. 281–301. [Google Scholar]
- Churchman, G.J.; Tate, K.R. Stability of aggregates of different size grades in allophanic soils from volcanic ash in New Zealand. J. Soil Sci. 1987, 38, 19–27. [Google Scholar] [CrossRef]
- Kaufhold, S.; Kaufhold, A.; Jahn, R.; Brito, S.; Dohrmann, R.; Hoffmann, R.; Gliemann, H.; Weidler, P.; Frechen, M. A new massive deposit of allophane raw material in Ecuador. Clays Clay Miner. 2009, 57, 72–81. [Google Scholar] [CrossRef]
- Kaufhold, S.; Ufer, K.; Kaufhold, A.; Stucki, J.; Anastácio, A.; Jahn, R.; Dohrmann, R. Quantification of allophane clay from Ecuador. Clays Clay Miner. 2010, 58, 707–716. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R.; Abidin, Z.; Henmi, T.; Matsue, N.; Eichinger, L.; Kaufhold, A.; Jahn, R. Fluoride adsorption on allophane. Appl. Clay Sci. 2010, 50, 25–33. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R.; Koch, D.; Houben, G. The pH of aqueous bentonite suspensions. Clays Clay Miner. 2008, 56, 338–343. [Google Scholar] [CrossRef]
- Ufer, K.; Stanjek, H.; Roth, G.; Dohrmann, R.; Kleeberg, R.; Kaufhold, S. Quantitative phase analysis of bentonites by the Rietveld method. Clays Clay Miner. 2008, 56, 272–282. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R.; Klinkenberg, M. Water uptake capacity of bentonites. Clays Clay Miner. 2010, 58, 37–43. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R.; Klinkenberg, M.; Siegesmund, S.; Ufer, K. N2-BET specific surface area of bentonites. J. Colloid Interface Sci. 2010, 349, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kaufhold, S. (Ed.) Bentonites–From Mine to Application; 2018; in press; ISBN 978-3-510-96859-6. [Google Scholar]
- Kaufhold, S.; Stucki, J.W.; Finck, N.; Steininger, R.; Zimina, A.; Dohrmann, R.; Ufer, K.; Pentrák, M.; Pentráková, L. Tetrahedral charge and Fe content in dioctahedral smectites. Clay Miner. 2017, 52, 51–55. [Google Scholar] [CrossRef]
- Missana, T.; Alonso, U.; Fernández, A.M.; García-Gutiérrez, M. Colloidal properties of different smectite clays: Significance for the bentonite barrier erosion and radionuclide transport in radioactive waste repositories. Appl. Geochem. 2018. submitted. [Google Scholar]


| Sample | Material | Location | Mineral. Comp. | Chem. Comp. | Tetrahedral Charge (%) | Layer Charge Density (eq/FU) | BET Surface Area (m2/g) | Soda Soluble Silica (mass%) |
|---|---|---|---|---|---|---|---|---|
| PM-4-7 | allophane | Ecuador | [32] | [31] | n.d. | n.d. | 273 | n.d. |
| B01 | bentonite | Sardinia | [35] | [10] | 11 | 0.36 | 14 | 1 |
| B02 | bentonite | Sardinia | [35] | [10] | 13 | 0.35 | 22 | 1 |
| B03 | bentonite | Milos | [35] | [10] | 15 | 0.33 | 63 | 3 |
| B04 | bentonite | Milos | [35] | [10] | 23 | 0.31 | 46 | 0 |
| B05 | bentonite | Milos | [35] | [10] | 19 | 0.31 | 65 | 0 |
| B06 | bentonite | Milos | [35] | [10] | 10 | 0.33 | 42 | 1 |
| B07 | bentonite | Milos | [35] | [10] | 16 | 0.33 | 59 | 1 |
| B08 | bentonite | Wyoming | [35] | [10] | 7 | 0.27 | 15 | 4 |
| B09 | bentonite | Wyoming | [35] | [10] | 13 | 0.18 | 31 | 10 |
| B10 | bentonite | India | [35] | [10] | 37 | 0.33 | 67 | 1 |
| B11 | bentonite | India | [35] | [10] | 32 | 0.31 | 66 | 1 |
| B12 | bentonite | India | [35] | [10] | 15 | 0.35 | 40 | 1 |
| B13 | bentonite | Hungary | [35] | [10] | 63 | 0.37 | 102 | 0 |
| B14 | bentonite | Hungary | [35] | [10] | 60 | 0.33 | 130 | 1 |
| B15 | bentonite | Hungary | [35] | [10] | 64 | 0.38 | 100 | 1 |
| B16 | bentonite | Bavaria | [35] | [10] | 22 | 0.27 | 59 | 1 |
| B17 | bentonite | Georgia | [35] | [10] | 14 | 0.32 | 54 | 1 |
| B18 | bentonite | Spain | [35] | [10] | 15 | 0.36 | 43 | 2 |
| B19 | bentonite | Spain | [35] | [10] | 19 | 0.35 | 49 | 1 |
| B20 | bentonite | Wyoming | [35] | [10] | 11 | 0.25 | 22 | 4 |
| B21 | bentonite | Morocco | [35] | [10] | 28 | 0.34 | 23 | 1 |
| B22 | bentonite | Argentina | [35] | [10] | 7 | 0.35 | 31 | 4 |
| B23 | bentonite | Argentina | [35] | [10] | 9 | 0.36 | 7 | 1 |
| B24 | bentonite | Morocco | [35] | [10] | 26 | 0.34 | n.d. | 1 |
| B25 | bentonite | Bavaria | [35] | [10] | 30 | 0.30 | n.d. | 1 |
| B26 | bentonite | Czech R. | [35] | [10] | 18 | 0.35 | n.d. | 8 |
| B27 | bentonite | Wyoming | [35] | [10] | 8 | 0.21 | n.d. | 3 |
| B28 | bentonite | Indonesia | [35] | [10] | 20 | 0.32 | 72 | 5 |
| B29 | bentonite | Indonesia | [35] | [10] | 31 | 0.33 | 96 | 1 |
| B31 | bentonite | Armenia | [35] | [10] | 16 | 0.32 | 38 | 4 |
| B32 | bentonite | England | [35] | [10] | 25 | 0.26 | 112 | 2 |
| B33 | bentonite | Wyoming | [35] | [10] | 12 | 0.25 | 16 | 2 |
| B34 | bentonite | Russia | [35] | [10] | 36 | 0.37 | 24 | 1 |
| B36 | bentonite | Slovakia | [35] | [10] | 51 | 0.20 | 57 | 1 |
| B37 | bentonite | Slovakia | [35] | [10] | 9 | 0.30 | 58 | 1 |
| B38 | bentonite | Russia | [35] | [10] | 25 | 0.24 | 22 | 1 |
| B39 | bentonite | Romania | [35] | [10] | 55 | 0.26 | 111 | 27 |
| B40 | bentonite | Brazil | [35] | [10] | 10 | 0.34 | 66 | 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaufhold, S.; Kaufhold, A.; Dohrmann, R. Comparison of the Critical Coagulation Concentrations of Allophane and Smectites. Colloids Interfaces 2018, 2, 12. https://doi.org/10.3390/colloids2010012
Kaufhold S, Kaufhold A, Dohrmann R. Comparison of the Critical Coagulation Concentrations of Allophane and Smectites. Colloids and Interfaces. 2018; 2(1):12. https://doi.org/10.3390/colloids2010012
Chicago/Turabian StyleKaufhold, Stephan, Annette Kaufhold, and Reiner Dohrmann. 2018. "Comparison of the Critical Coagulation Concentrations of Allophane and Smectites" Colloids and Interfaces 2, no. 1: 12. https://doi.org/10.3390/colloids2010012





