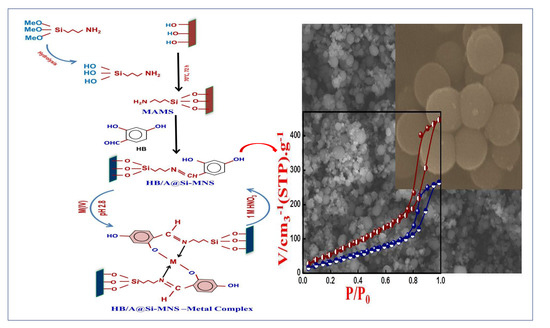
Fabrication of Silica Microspheres (HB/A@SI-MNS) for Hafnium and Zirconium Recovery from Zirconyl Leach Liquor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Zircon Mineral Concentrates Processing
2.3. Recycling of Silicate Waste for Fabrication of Mesoporous Silica Microspheres
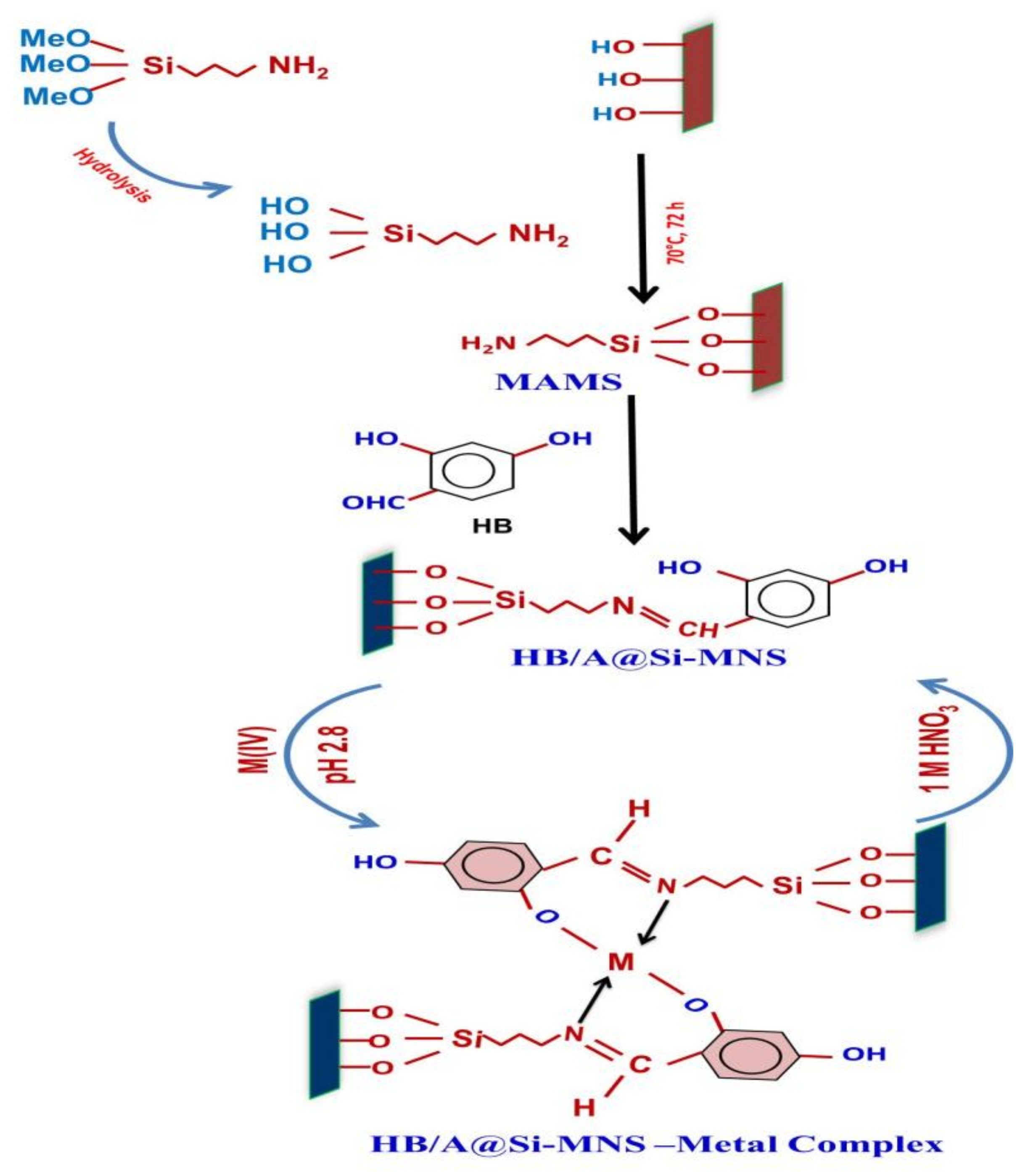
2.4. Synthesis of HB/A@Si-MNS Meso-sorbent
2.5. Characterization of Fabricated Materials
2.6. Adsorption Experiments
3. Results and Discussions
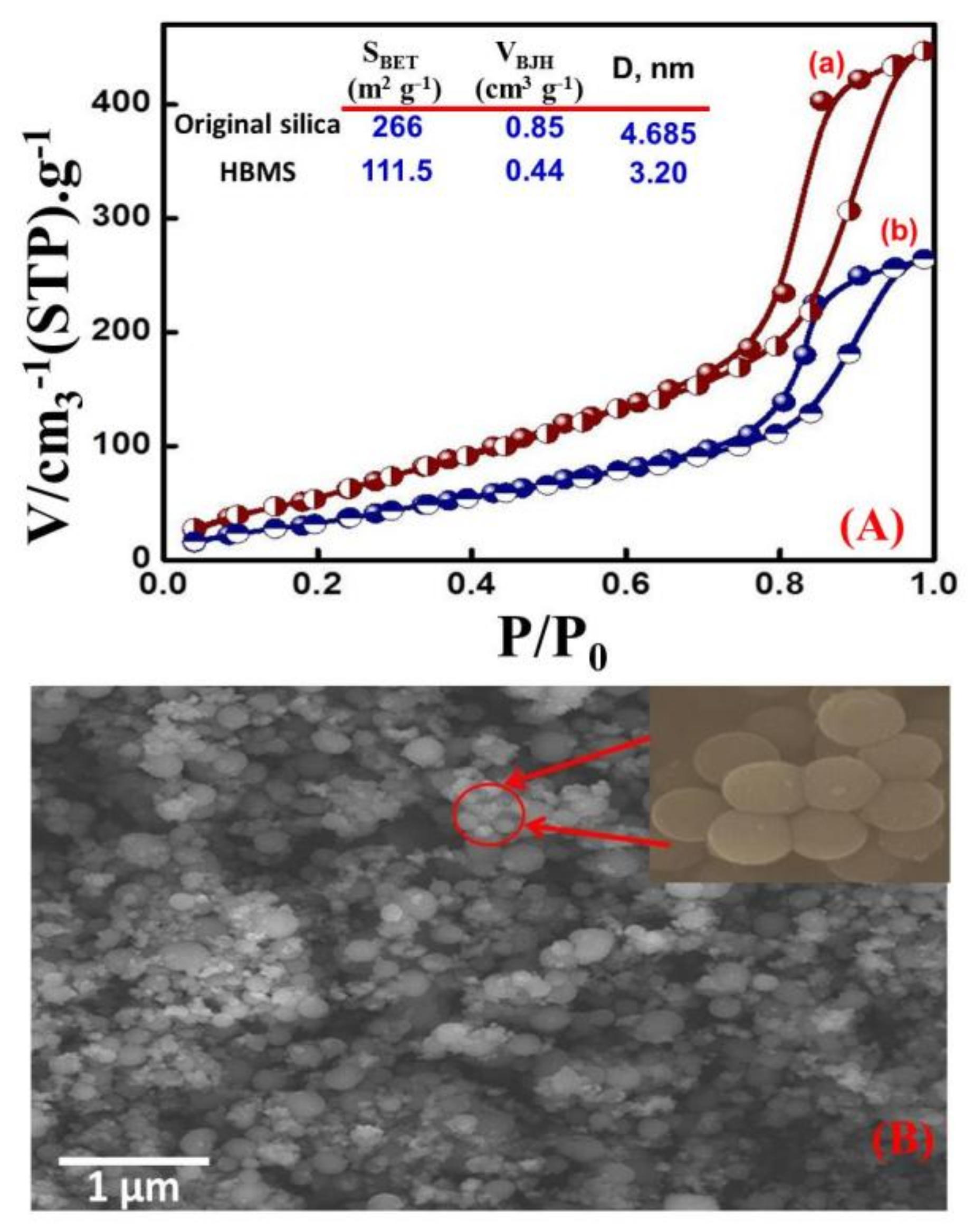
3.1. Characterization of HB/A@Si-MNS
3.2. Adsorption Assays of Zr(IV) and Hf(IV) Ions
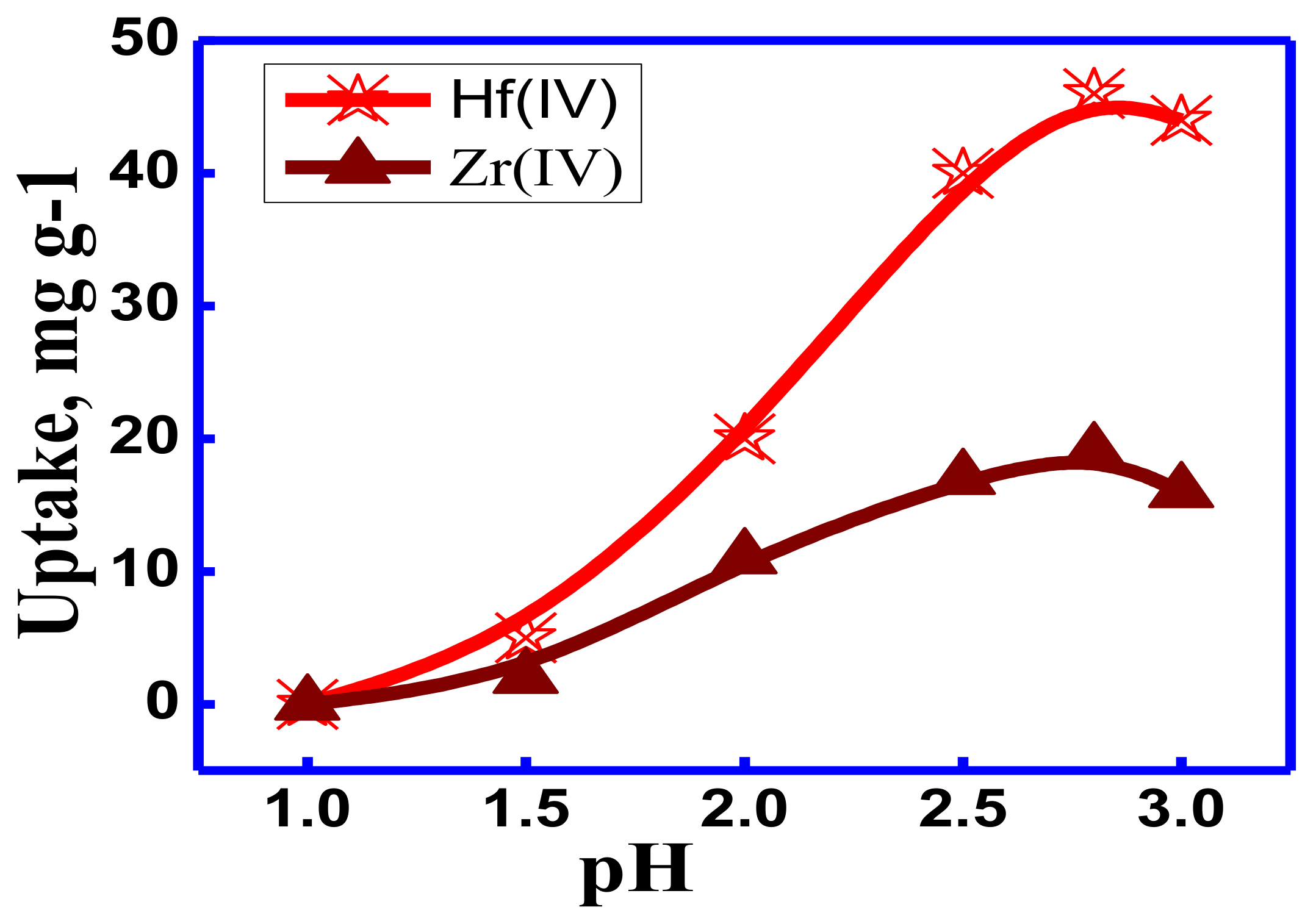
3.2.1. Effect of pH on the Batch Adsorption Process
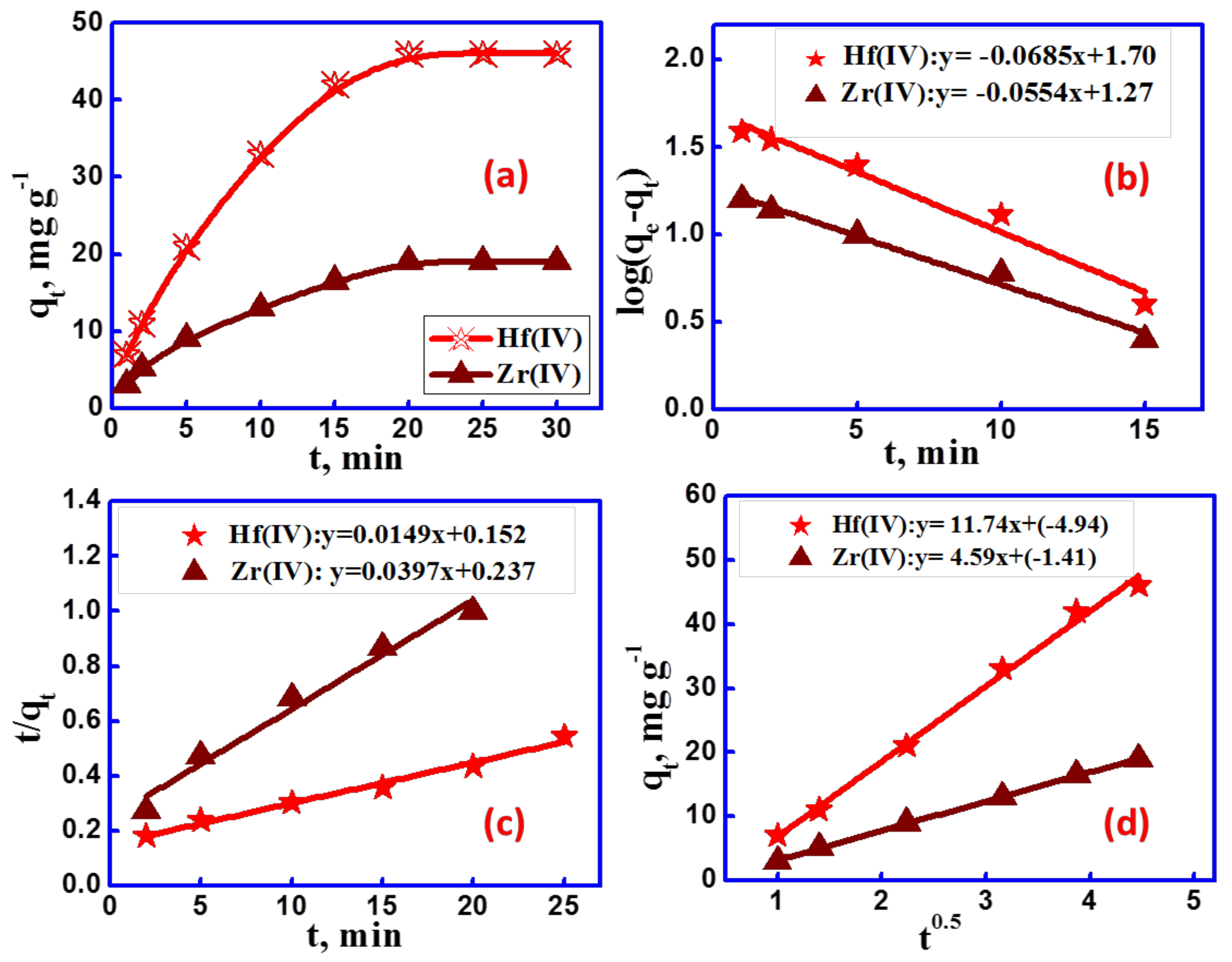
3.2.2. Adsorption Kinetics
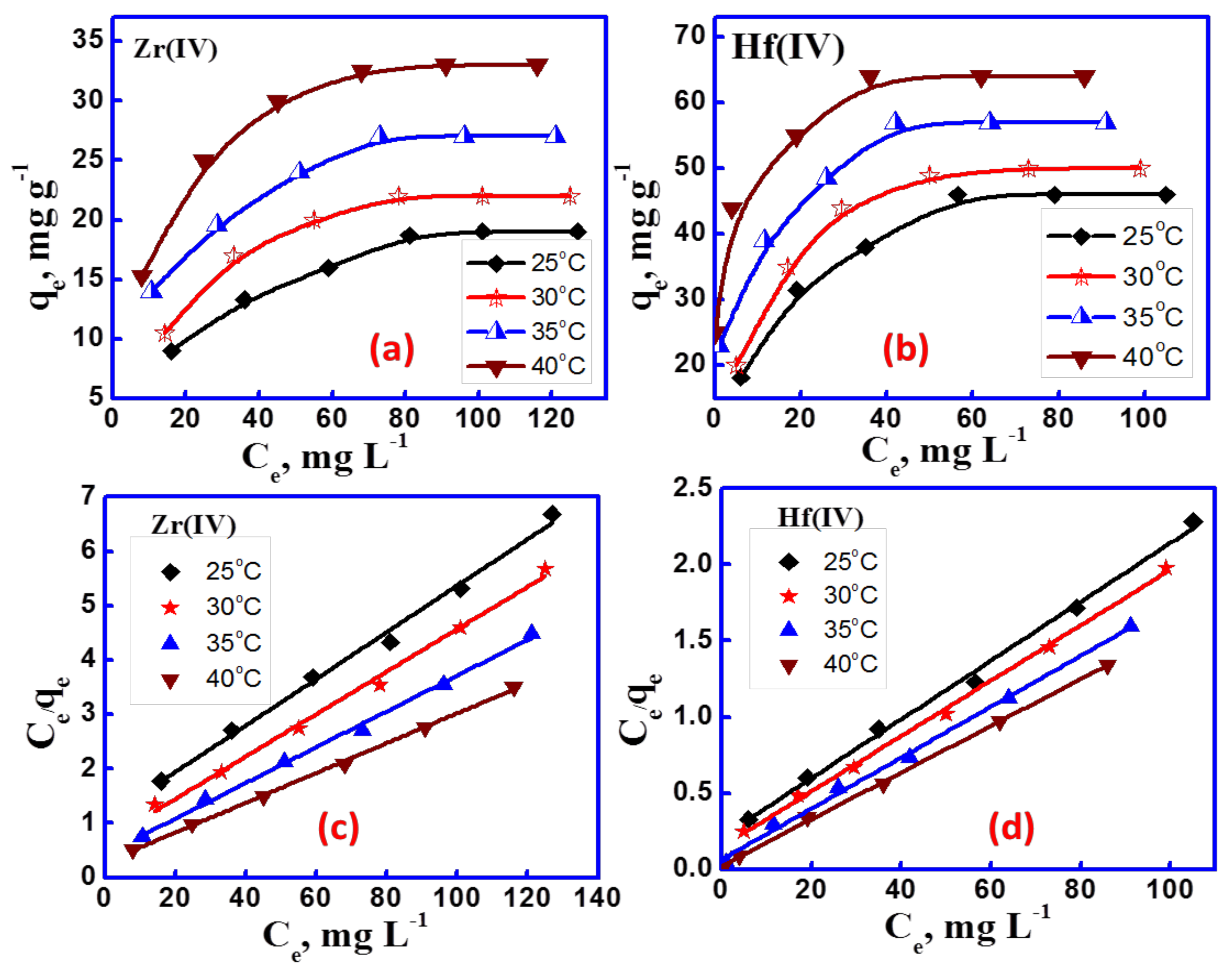
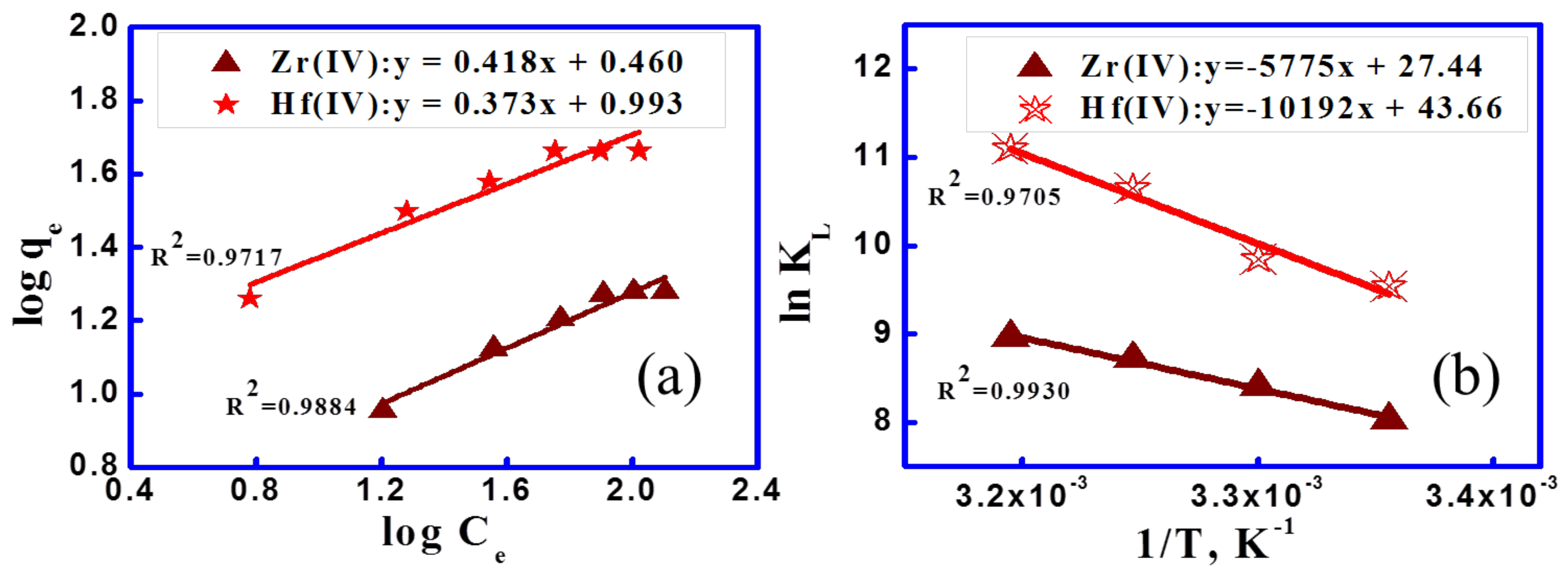
3.2.3. Adsorption Isotherms and Thermodynamic Studies
3.3. Elution and Regeneration
3.4. Selective Separation of Hf(IV) from Zr(IV) Metal Ions
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Donia, A.M.; Atia, A.A.; Daher, A.M.; Desouky, O.; Elshehy, E.A. Extraction and separation of Zr(IV) from hydrochloric acid solution using modified silica gel produced from waste solution of sodium silicate. Sep. Sci. Technol. 2011, 46, 1329–1336. [Google Scholar] [CrossRef]
- Donia, A.M.; Atia, A.A.; Daher, A.M.; Elshehy, E.A. Extraction and separation of zirconium(IV) and hafnium(IV) from chloride media using magnetic resin with phosphoric acid functionality. J. Dispers. Sci. Technol. 2011, 2, 193–202. [Google Scholar] [CrossRef]
- Mohammed, N.; Daher, A. Preparation of high-purity zirconia from Egyptian zircon: An anion-exchange purification process. Hydrometallurgy 2002, 65, 103–107. [Google Scholar] [CrossRef]
- Daher, A.M.; Abdelkader, A.; El-Kashef, E. Novel decomposition method for zircon. J. Alloys Compd. 2008, 460, 577–580. [Google Scholar]
- Barin, I.; Knacke, O.; Kubaschewski, O. Thermochemical Properties of Inorganic Substances; Springer: Berlin, Germany, 1973. [Google Scholar]
- Hyde, B.G.; Andersson, S. Inorganic Crystal Structures; John Wiley & Sons: New York, NY, USA, 1989. [Google Scholar]
- El-Barawy, K.A.; El Tawil, S.Z.; Francis, A.A. Alkali fusion of zircon sand. Trans. Inst. Min. Metall. Sect. C 2000, 109, 49–56. [Google Scholar] [CrossRef]
- Poriel, L.; Pellet-Rostaing, S.; Lamotte, V.; Lemaire, M.; Favre-Reguillon, A. Zirconium and hafnium separation, part 2. solid/liquid extraction in hydrochloric acid aqueous solution with anion exchange resins. Sep. Sci. Technol. 2006, 41, 2711–2722. [Google Scholar] [CrossRef]
- Donia, A.M.; Atia, A.A.; Daher, A.M.; Desouky, O.; Elshehy, E.A. synthesis of amine/thiol magnetic resin and study of its interaction with Zr(IV) and Hf(IV) ions in their aqueous solutions. J. Dispers. Sci. Technol. 2011, 32, 634–641. [Google Scholar] [CrossRef]
- Shenashen, M.; Elshehy, E.A.; El-Safty, S.A.; Khairy, M. Visual monitoring and removal of divalent copper, cadmium, and mercury ions from water by using mesoporous cubic Ia3d aluminosilica sensors. Sep. Purif. Technol. 2013, 116, 73–86. [Google Scholar] [CrossRef]
- Gao, M.; Ma, Q.; Lin, Q.; Chang, J.; Ma, H. A novel approach to extract SiO2 from fly ash and its considerable adsorption properties. Mater. Des. 2017, 116, 666–675. [Google Scholar] [CrossRef]
- Seaton, K.; Little, I.; Tate, C.; Mohseni, R.; Roginskay, M.; Povazhniy, V.; Vasiliev, A. Adsorption of cesium on silica gel containing embedded phosphotungstic acid. Microporous Mesoporous Mater. 2017, 244, 55–66. [Google Scholar] [CrossRef]
- Liu, S.; Cui, H.-Z.; Li, Y.-L.; Yang, A.-L.; Zhang, J.-F.; Zhong, R.; Zhou, Q.; Lin, M.; Hou, X.-F. Bis-pyrazolyl functionalized mesoporous SBA-15 for the extraction of Cr(III) and detection of Cr(VI) in artificial jewelry samples. Microchem. J. 2017, 131, 130–136. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Shenashen, M.A.; Sakai, M.; Elshehy, E.; Halada, K. Detection and recovery of palladium, gold and cobalt metals from the urban mine using novel sensors/adsorbents designated with nanoscale wagon-wheel-shaped pores. J. Vis. Exp. 2015, 106, 53044. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, D.; Cegłowski, M.; Smoluch, M.; Reszke, E.; Silberring, J.; Schroeder, G. Magnetic mesoporous silica Fe3O4@SiO2@meso-SiO2 and Fe3O4@SiO2@meso-SiO2-NH2 as adsorbents for the determination of trace organic compounds. Microporous Mesoporous Mater. 2017, 240, 80–90. [Google Scholar] [CrossRef]
- Ghosh, S.; Vandana, V. Nano-structured mesoporous silica/silver composite: Synthesis, characterization and targeted application towards water purification. Mater. Res. Bull. 2017, 88, 291–300. [Google Scholar] [CrossRef]
- Qin, W.; Xu, S.; Xu, G.; Xie, Q.; Wang, C.; Xu, Z. Preparation of silica gel bound crown ether and its extraction performance towards zirconium and hafnium. Chem. Eng. J. 2013, 225, 528–534. [Google Scholar] [CrossRef]
- Guo, X.; Feng, Y.; Ma, L.; Gao, D.; Jing, J.; Yu, J.; Sun, H.; Gong, H.; Zhang, Y. Phosphoryl functionalized mesoporous silica for uranium adsorption. App. Surf. Sci. 2017, 402, 53–60. [Google Scholar]
- Guo, X.; Feng, Y.; Ma, L.; Gao, D.; Jing, J.; Yu, J.; Sun, H.; Gong, H.; Zhang, Y. Adsorbents based on crown ether functionalized composite mesoporous silica for selective extraction of trace silver. Chem. Eng. J. 2017, 313, 1278–1287. [Google Scholar]
- Zhou, Y.; Li, X.; Chen, Z. Rapid synthesis of well-ordered mesoporous silica from sodium silicate. Powder Technol. 2012, 226, 239–245. [Google Scholar] [CrossRef]
- Marczenko, Z. Separation and Spectrophotometric Determination of Elements; Ellis Harwood: Chichester, UK, 1986. [Google Scholar]
- Abd El-Magied, M.O.; Galhoum, A.A.; Atia, A.A.; Tolba, A.A.; Maize, M.S.; Vincent, T.; Guibal, E. Cellulose and chitosan derivatives for enhanced sorption of erbium(III). Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 580–593. [Google Scholar] [CrossRef]
- Böhmer, V.; Dozol, J.F.; Grüttner, C.; Liger, K.; Matthews, S.E.; Rudershausen, S.; Saadioui, M.; Wang, P.; Org, P. Separation of lanthanides and actinides using magnetic silica particles bearing covalently attached tetra-CMPO-calix[4]arenes. Org. Biomol. Chem. 2004, 2, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Elshehy, E.A.; Shenashen, M.A.; Abd El-Magied, M.O.; El-Nahas, A.M.; Tolan, D.A.; Halada, K.; Atia, A.A.; El-Safty, S.A. Selective recovery of silver (I) ions from e-waste using cubically multi-thiolated cage mesoporous monoliths. Eur. J. Inorg. Chem. 2017, 2017, 4823–4833. [Google Scholar] [CrossRef]
- Abd El-Magied, M.O.; Elshehy, E.A.; Manaa, E.; Tolba, A.; Atia, A.A. Kinetics and thermodynamics studies on the recovery of thorium ions using amino resins with magnetic properties. Ind. Eng. Chem. Res. 2016, 55, 11338–11345. [Google Scholar] [CrossRef]
- Poriel, L.; Favre-Réguillon, A.; Pellet-Rostaing, S.; Lemaire, M. Zirconium and hafnium separation, part 1. liquid/liquid extraction in hydrochloric acid aqueous solution with aliquat 336. Sep. Sci. Technol. 2006, 41, 1927–1940. [Google Scholar] [CrossRef]
- Abdel-Fattah, A.A.; Ali, S.M.; El-Sweify, F.H. Thermodynamics of the solvent extraction of Zr(IV) by Amberlite LA-2, TBP and HDEHP from different aqueous media. J. Radioanal. Nucl. Chem. 2002, 253, 465–475. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, H.Y. Distribution of Zr(IV) ion species in aqueous solution. J. Korean Inst. Resour. Recycl. 2011, 20, 56–62. [Google Scholar] [CrossRef]
- Wang, L.Y.; Lee, M.S. A review on the aqueous chemistry of Zr(IV) and Hf(IV) and their separation by solvent extraction. J. Ind. Eng. Chem. 2016, 39, 1–9. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Sayed, M.A.; Amine, M.M.; Abd El-Magied, M.O. Selective solid-phase extraction of U(VI) by amine functionalized glycidyl methacrylate. J. Environ. Chem. Eng. 2014, 2, 293–303. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Moussa, E.M.M.; El-Sayed, M.A.; Amine, M.M.; Abd El-Magied, M.O. Uranium(VI) and Thorium(IV) adsorption studies on chelating resin containing pentaethylenehexamine as a functional group. J. Dispers. Sci. Technol. 2014, 35, 926–933. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Abd El-Magied, M.O.; Tolba, A.; El-Gendy, H.; Zaki, S.; Atia, A.A. Studies on the recovery of Th(IV) ions from nitric acid solutions using amino-magnetic glycidyl methacrylate resins and application to granite leach liquors. Hydrometallurgy 2017, 169, 89–98. [Google Scholar] [CrossRef]

| Major Constituents | Trace Constituents | ||
|---|---|---|---|
| Constituent | Concentration, % | Constituent | Concentration, % |
| ZrO2 | 64.42 | U3O8 | 0.04 |
| HfO2 | 1.46 | ThO2 | 0.02 |
| SiO2 | 32.23 | REO | 0.07 |
| Fe2O3 | 0.14 | MgO | 0.02 |
| TiO2 | 0.22 | CaO | 0.01 |
| P2O5 | 0.13 | Al2O3 | 0.06 |
| - | - | K2O | <0.01 |
| - | - | Na2O | <0.01 |
| Metal Ion | qe, mg g−1 | 1st Order | 2nd Order | IPD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qmax | k1 | R2 | Qmax | k2 | R2 | kip | I | R2 | ||
| Zr(IV) | 19 | 18.56 | 0.16 | 0.972 | 25.19 | 0.001 | 0.988 | 11.74 | −4.95 | 0.996 |
| Hf(IV) | 46 | 50.37 | 0.13 | 0.986 | 67.11 | 0.006 | 0.989 | 4.95 | −1.41 | 0.999 |
| Metal Ion | qe mg g−1 | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|---|
| Qmax | KL | R2 | RL | KF | n | R2 | ||
| Zr(IV) | 19 | 24.75 | 0.034 | 0.9911 | 0.196 | 2.89 | 2.39 | 0.9884 |
| Hf(IV) | 46 | 54.05 | 0.078 | 0.9968 | 0.091 | 9.83 | 2.68 | 0.9716 |
| T, °C | Zr(IV) | Hf(IV) | ||||||
|---|---|---|---|---|---|---|---|---|
| ∆H°, kJ/mol | ∆S°, kJ/(mol K) | ∆G°, kJ/mol | T∆S°, kJ/mol | ∆H°, kJ/mol | ∆S°, kJ/(mol K) | ∆G°, kJ/mol | T∆S°, kJ/mol | |
| 25 | 84.74 | 0.36 | −23.43 | 108.17 | 48.01 | 0.23 | −19.97 | 67.98 |
| 30 | −25.24 | 109.98 | −21.11 | 69.12 | ||||
| 35 | −27.06 | 111.80 | −22.25 | 70.27 | ||||
| 40 | −28.87 | 113.61 | −23.39 | 71.41 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Magied, M.O.; Salem, W.M.; Daher, A.A.; Elshehy, E.A. Fabrication of Silica Microspheres (HB/A@SI-MNS) for Hafnium and Zirconium Recovery from Zirconyl Leach Liquor. Colloids Interfaces 2018, 2, 14. https://doi.org/10.3390/colloids2020014
Abd El-Magied MO, Salem WM, Daher AA, Elshehy EA. Fabrication of Silica Microspheres (HB/A@SI-MNS) for Hafnium and Zirconium Recovery from Zirconyl Leach Liquor. Colloids and Interfaces. 2018; 2(2):14. https://doi.org/10.3390/colloids2020014
Chicago/Turabian StyleAbd El-Magied, Mahmoud O., Waheed M. Salem, Ahmad A. Daher, and Emad A. Elshehy. 2018. "Fabrication of Silica Microspheres (HB/A@SI-MNS) for Hafnium and Zirconium Recovery from Zirconyl Leach Liquor" Colloids and Interfaces 2, no. 2: 14. https://doi.org/10.3390/colloids2020014