Reactive Chemicals and Electrophilic Stress in Cancer: A Minireview
Abstract
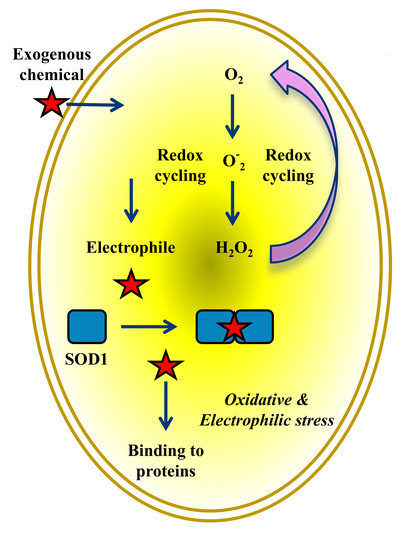
:1. Introduction
2. Epidermal Growth Factor Receptor as a Paradigm for Screening Small Molecule Modulators of Cell Signalling
3. Superoxide Dismutase 1 Plays a Pivotal Role in Cell Signalling Induced with Nitro-Benzoxadiazole Compounds
4. Lipophilic Electrophiles Rapidly Bind to Multiple Proteins in Cancer Cells
5. Electrophilic and Oxidative Stress Are Mutually and Tightly Related Processes
6. Perspectives
Acknowledgments
Conflicts of Interest
References
- Klaunig, J.E.; Kamendulis, L.M. The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 239–267. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Oxygen: How do we stand it? Med. Princ. Pract. 2013, 22, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Rad. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Nozierè, B.; Kalberer, M.; Claeys, M.; Allan, J.; D’Anna, B.; Decesari, S.; Finessi, E.; Glasius, M.; Grgić, I.; Hamilton, J.F.; et al. The molecular identification of organic compounds in the atmosphere: State of the art and challenges. Chem. Rev. 2015, 115, 3919–3983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emanuele, S.; D’Anneo, A.; Calvaruso, G.; Cernigliaro, C.; Giuliano, M.; Lauricella, M. The double-edged sword profile of redox signaling: Oxidative events as molecular switches in the balance between cell physiology and cancer. Chem. Res. Toxicol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Suzuki, T.; Hiramoto, K.; Asami, S.; Naganuma, E.; Suda, H.; Iso, T.; Yamamoto, H.; Morita, M.; Baird, L.; et al. Characterizations of three major cysteine sensors of Keap1 in stress response. Mol. Cell. Biol. 2015, 36, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Munnia, A.; Giese, R.W.; Polvani, S.; Galli, A.; Cellai, F.; Peluso, M.E.M. Bulky DNA adducts, tobacco smoking, genetic susceptibility, and lung cancer risk. Adv. Clin. Chem. 2017, 81, 231–277. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; De Maria, F.; Antonini, G.; Turella, P.; Bullo, A.; Stella, L.; Filomeni, G.; Federici, G.; Caccuri, A.M. 7-Nitro-2,1,3-benzoxadiazole derivatives, a new class of suicide inhibitors for glutathione S-transferases. Mechanism of action of potential anticancer drugs. J. Biol. Chem. 2005, 280, 26397–26405. [Google Scholar] [CrossRef] [PubMed]
- Schlessinger, J. Receptor Tyrosine Kinases: Legacy of the first two decades. Cold Spring Harb. Perspect. Biol. 2014, 6, a008912. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S.; Kang, S.W.; Seo, M.S.; Baines, I.C.; Tekle, E.; Chock, P.B.; Rhee, S.G. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 1997, 272, 217–221. [Google Scholar] [CrossRef] [PubMed]
- DeYulia, G.J., Jr.; Cárcamo, J.M.; Bórquez-Ojeda, O.; Shelton, C.C.; Golde, D.W. Hydrogen peroxide generated extracellularly by receptor-ligand interaction facilitates cell signaling. Proc. Natl. Acad. Sci. USA 2005, 102, 5044–5049. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Truong, T.H.; Garcia, F.J.; Homann, A.; Gupta, V.; Leonard, S.E.; Carroll, K.S. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat. Chem. Biol. 2012, 8, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Tinti, M.; Costa, S.; Corallino, S.; Nardozza, A.P.; Chatraryamontri, A.; Ceol, A.; Cesareni, G.; Castagnoli, L. Identification of new substrates of the protein-tyrosine phosphatase PTP1B by Bayesian integration of proteome evidence. J. Biol. Chem. 2011, 286, 4173–4185. [Google Scholar] [CrossRef] [PubMed]
- Burdick, A.D.; Davis, J.W., 2nd; Liu, K.J.; Hudson, L.G.; Shi, H.; Monske, M.L.; Burchiel, S.W. Benzo(a)pyrene quinones increase cell proliferation, generate reactive oxygen species, and transactivate the epidermal growth factor receptor in breast epithelial cells. Cancer Res. 2003, 63, 7825–7833. [Google Scholar] [PubMed]
- Blanchet, S.; Ramgolam, K.; Baulig, A.; Marano, F.; Baeza-Squiban, A. Fine Particulate Matter Induces Amphiregulin Secretion by Bronchial Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2004, 30, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Pourazar, J.; Blomberg, A.; Kelly, F.J.; Davies, D.E.; Wilson, S.J.; Holgate, S.T.; Sandström, T. Diesel exhaust increases EGFR and phosphorylated C-terminal Tyr 1173 in the bronchial epithelium. Part. Fibre Toxicol. 2008, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Avila-Tang, E.; Samet, J.M. Lung cancer in never smokers: A call to action. Clin. Cancer Res. 2009, 15, 5622–5625. [Google Scholar] [CrossRef] [PubMed]
- MacBeath, G.; Koehler, A.N.; Schreiber, S.L. Printing small molecules as microarrays and detecting protein ligand interactions en masse. J. Am. Chem. Soc. 1999, 121, 7967–7968. [Google Scholar] [CrossRef]
- Sakanyan, V.A. Molecular interactions on microarrays. Eur. BioPharm. Rev. 2008, Autumn, 74–78. [Google Scholar]
- Gyulkhandanyan, A.; Gyulkhandanyan, L.; Ghazaryan, R.; Fleury, F.; Angelini, M.; Gyulkhandanyan, G.; Sakanyan, V. Assessment of new cationic porphyrin binding to plasma proteins by planar microarray and spectroscopic methods. J. Biomol. Struct. Dyn. 2013, 31, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Sakanyan, V.; Angelini, M.; Le Béchec, M.; Lecocq, M.F.; Benaiteau, F.; Rousseau, B.; Gyulkhandanyan, A.; Gyulkhandanyan, L.; Logé, C.; Reiter, E.; et al. Screening and discovery of nitro-benzoxadiazole compounds activating epidermal growth factor receptor (EGFR) in cancer cells. Sci. Rep. 2014, 4, 3977. [Google Scholar] [CrossRef] [PubMed]
- Toyo’ka, T. Development of benzofurazan-bearing fluorescence labeling reagents for separation and detection in high-performance liquid chromatography. Chromatography 2012, 33, 1–17. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Sakanyan, V.; Hulin, P.; Alves de Sousa, R.; Silva, V.; Hambardzumyan, A.; Nedellec, S.; Tomasoni, C.; Logé, C.; Pineau, C.; Roussakis, C.; et al. Activation of EGFR by small compounds through coupling the generation of hydrogen peroxide to stable dimerization of Cu/Zn SOD1. Sci. Rep. 2016, 6, 21088. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.A.O.; Lafont, F.; Benhelli-Mokrani, H.; LeBreton, M.; Hulin, P.; Chabot, T.; Paris, F.; Sakanyan, V.; Fleury, F. Rapid diminution in the level and activity of DNA-dependent protein kinase in cancer cells by a reactive nitro-benzoxadiazole compound. Int. J. Mol. Sci. 2016, 17, 703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culotta, V.C.; Yang, M.; O’Halloran, T.V. Activation of superoxide dismutases: Putting the metal to the pedal. Biochim. Biophys. Acta 2006, 1763, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Crisp, M.J.; Mawuenyega, K.G.; Patterson, B.W.; Reddy, N.C.; Chott, R.; Self, W.K.; Weihl, C.C.; Jockel-Balsarotti, J.; Varadhachary, A.S.; Bucelli, R.C.; et al. In vivo kinetic approach reveals slow SOD1 turnover in the CNS. J. Clin. Investig. 2015, 125, 2772–2780. [Google Scholar] [CrossRef] [PubMed]
- Burma, S.; Chen, B.P.; Chen, D.J. Role of non-homologous end joining (NHEJ) in maintaining genomic integrity. DNA Repair 2006, 5, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; Lee, J.H.; Kwon, D.Y.; Genovesio, A.; Fenistein, D.; Ogier, A.; Brondani, V.; Grailhe, R. Dimerization, oligomerization, and aggregation of human amyotrophic lateral sclerosis copper/zinc superoxide dismutase 1 protein mutant forms in live cells. J. Biol. Chem. 2014, 289, 15094–15103. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I.; Spence, M.T.Z. The Molecular Probes Handbook: A Guide to Fluorescent Probes and Labeling Technologies, 11 ed.; Life Technologies Corporation: Carlsbad, CA, USA, 2010. [Google Scholar]
- Sakanyan, V.; Benaiteau, F.; Alves de Sousa, R.; Pineau, C.; Artaud, I. Straightforward detection of reactive compound binding to multiple proteins in cancer cells: Towards a better understanding of electrophilic stress. Ann. Clin. Exp. Metabol. 2016, 1, 1006. [Google Scholar]
- Federici, L.; Lo Sterzo, C.; Pezzola, S.; Di Matteo, A.; Scaloni, F.; Federici, G.; Caccuri, A.M. Structural basis for the binding of the anticancer compound 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol to human glutathione S-transferases. Cancer Res. 2009, 69, 8025–8034. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.; Prochownik, E.V. Small-molecule inhibitors of the Myc oncoprotein. Biochim. Biophys. Acta. 2015, 1849, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Asanuma, M.; Nakanishi, S.; Saito, Y.; Okazaki, M.; Dodo, K.; Sodeoka, M. Turn-ON fluorescent affinity labeling using a small bifunctional O-nitrobenzoxadiazole unit. Chem Sci. 2014, 5, 1021–1029. [Google Scholar] [CrossRef]
- Marnett, L.J.; Riggins, J.N.; West, J.D. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J. Clin. Investig. 2003, 111, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Liebler, D.C. Protein damage by reactive electrophiles: Targets and consequences. Chem. Res. Toxicol. 2008, 21, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, T.K.; Freeman, B.A. Transduction of redox signaling by electrophile-protein reactions. Sci. Signal. 2009, 2, re7. [Google Scholar] [CrossRef] [PubMed]
- Wall, S.B.; Smith, M.R.; Ricart, K.; Zhou, F.; Vayalil, P.K.; Oh, J.Y.; Landar, A. Detection of electrophile-sensitive proteins. Biochim. Biophys. Acta 2014, 1840, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.T.; Marnett, L.J. Systems analysis of protein modification and cellular responses induced by electrophilic stress. Acc. Chem. Res. 2010, 43, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, H.A.; Woo, C.M. Mapping the small molecule interactome by mass spectrometry. Biochemistry 2018, 57, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamamoto, M. Stress-sensing mechanisms and the physiological roles of the Keap1–Nrf2 system during cellular stress. J. Biol. Chem. 2017, 292, 16817–16824. [Google Scholar] [CrossRef] [PubMed]
- Sau, A.; Pellizari Tregno, F.P.; Valentino, F.; Federici, G.; Caccuri, A.M. Glutathione transferases and development of new principles to overcome drug resistance. Arch. Biochem. Biophys. 2010, 500, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Patridge, E.V.; Eriksson, E.S.; Penketh, P.G.; Baumann, R.P.; Zhu, R.; Shyam, K.; Eriksson, L.A.; Sartorelli, A.C. 7-Nitro-4-(phenylthio)benzofurazan is a potent generator of superoxide and hydrogen peroxide. Arch. Toxicol. 2012, 86, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Paluch, E.K.; Raz, E. The role and regulation of blebs in cell migration. Curr. Opin. Cell Biol. 2013, 25, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Milane, L.; Duan, Z.; Amiji, M. Role of hypoxia and glycolysis in the development of multi-drug resistance in human tumor cells and the establishment of an orthotopic multi-drug resistant tumor model in nude mice using hypoxic pre-conditioning. Cancer Cell. Int. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, J.L.; Nissink, J.W.; Strasser, J.M.; Francis, S.; Higgins, L.; Zhou, H.; Zhang, Z.; Walters, M.A. PAINS in the assay: Chemical mechanisms of assay interference and promiscuous enzymatic inhibition observed during a sulfhydryl-scavenging HTS. J. Med. Chem. 2015, 58, 2091–2113. [Google Scholar] [CrossRef] [PubMed]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991, 51, 794–798. [Google Scholar] [PubMed]
- Castaldo, S.A.; Freitas, J.R.; Conchinha, N.V.; Madureira, P.A. The Tumorigenic Roles of the Cellular REDOX Regulatory Systems. Oxid. Med. Cell. Longev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wellington, K.W. Understanding cancer and the anticancer activities of naphthoquinones—A review. RSC Adv. 2015, 5, 20309–20338. [Google Scholar] [CrossRef]
- Devarie-Baez, N.O.; Silva Lopez, E.I.; Furdui, C.M. Biological chemistry and functionality of protein sulfenic acids and related thiol modifications. Free Radic. Res. 2016, 50, 172–194. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Davies, M.J.; Kramer, A.C.; Miotto, G.; Zaccarin, M.; Zhang, H.; Ursini, F. Protein cysteine oxidation in redox signaling: Caveats on sulfenic acid detection and quantification. Arch. Biochem. Biophys. 2017, 617, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Yang, J.; Liebler, D.C.; Carroll, K.S. Diverse redoxome reactivity profiles of carbon nucleophiles. J. Am. Chem. Soc. 2017, 139, 5588–5595. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Liu, K.; Sun, M.; Tian, C.; Sun, R.; Morales Betanzos, C.; Tallman, K.A.; Porter, N.A.; Yang, Y.; Guo, C.; et al. Systematic and quantitative assessment of hydrogen peroxide reactivity with cysteines across human proteomes. Mol. Cell. Proteom. 2017, 16, 1815–1828. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, H.; Edmands, W.M.B.; Lan, Q.; Carlsson, H.; Vermeulen, R.; Zhang, L.; Yin, S.-N.; Li, G.-L.; Smith, M.T.; Rothman, N.; et al. Adductomic signatures of benzene exposure provide insights into cancer induction. Carcinogenesis 2018. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Finch, C.E. A critical review of assays for hazardous components of air pollution. Free Radic. Biol. Med. 2018, 117, 202–217. [Google Scholar] [CrossRef] [PubMed]
- Dennis, K.L.; Marder, E.; Balshaw, D.M.; Cui, Y.; Lynes, M.A.; Patti, G.J.; Rappaport, S.M.; Shaughnessy, D.T.; Vrijheid, M.; Barr, D.B. Biomonitoring in the era of the Exposome. Environ. Health Perspect. 2017, 125, 502–510. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakanyan, V. Reactive Chemicals and Electrophilic Stress in Cancer: A Minireview. High-Throughput 2018, 7, 12. https://doi.org/10.3390/ht7020012
Sakanyan V. Reactive Chemicals and Electrophilic Stress in Cancer: A Minireview. High-Throughput. 2018; 7(2):12. https://doi.org/10.3390/ht7020012
Chicago/Turabian StyleSakanyan, Vehary. 2018. "Reactive Chemicals and Electrophilic Stress in Cancer: A Minireview" High-Throughput 7, no. 2: 12. https://doi.org/10.3390/ht7020012