A Facile Method Using a Flux to Improve Quantum Efficiency of Submicron Particle Sized Phosphors for Solid-State Lighting Applications
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Reagents
2.2. Preparation of Ca0.94Eu0.06MgSi2O6 with and without a Flux
2.3. Characterization
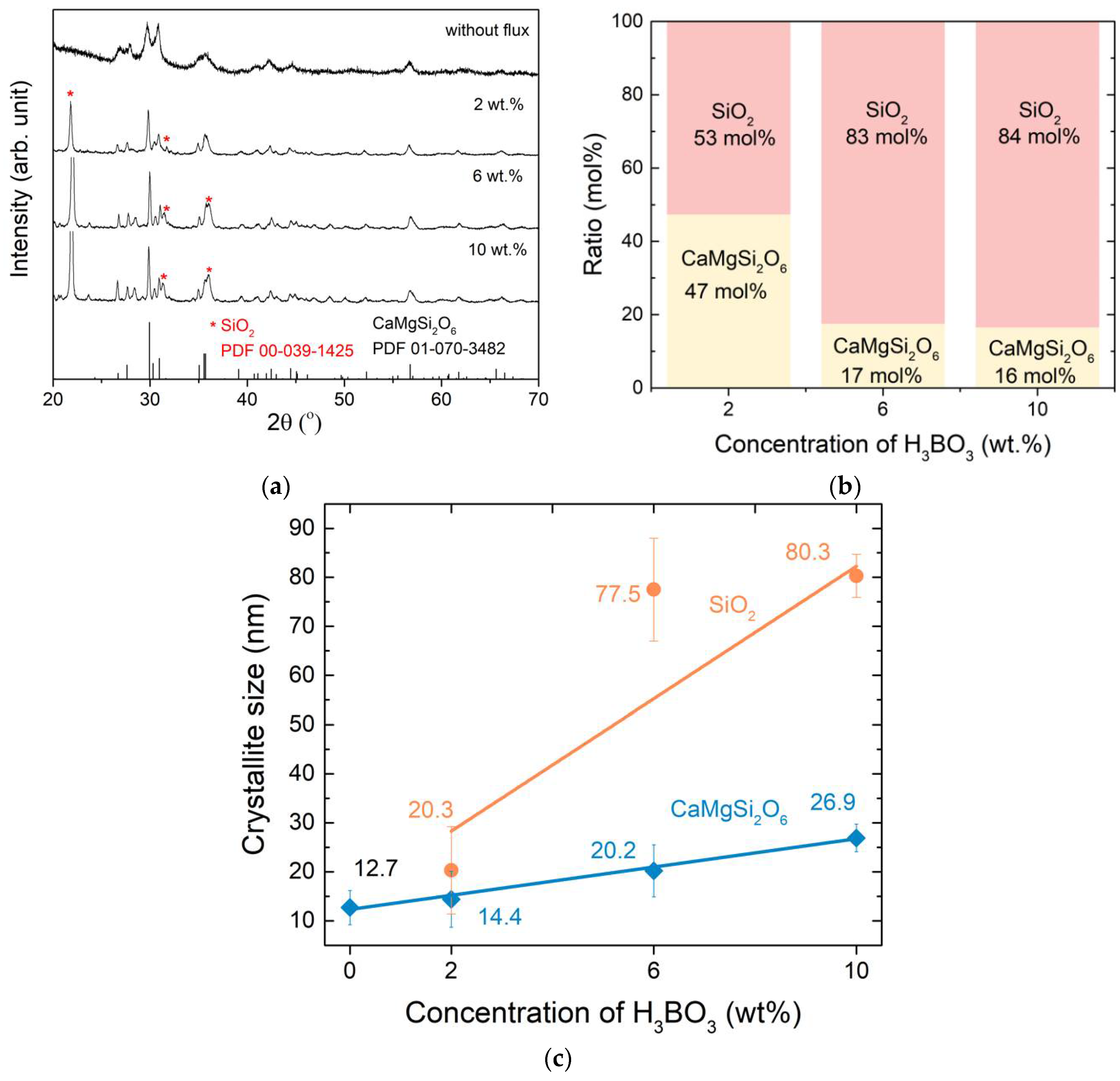
3. Results and Discussion
3.1. Crystal Structure and Lattice Parameters
3.2. Scanning Electron Microscopy and Dynamic Lighting Scattering Analysis
3.3. Photoluminescence Spectra and Quantum Efficiency
3.4. Effect of High Concentration of NH4Cl
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blasse, G.; Grabmaier, B.C. Luminescent Materials; Springer: Berlin/Heidlberg, Germany, 1994. [Google Scholar]
- Han, J.K.; Choi, J.I.; Piquette, A.; Hannah, M.; Anc, M.; Galvez, M.; Talbot, J.B.; McKittrick, J. Phosphor development and integration for near-UV LED solid state lighting. ECS J. Solid State Sci. Technol. 2012, 2, R3138–R3147. [Google Scholar] [CrossRef]
- Luo, H.; Liu, J.; Zheng, X.; Han, L.; Ren, K.; Yu, X. Enhanced photoluminescence of Sr3SiO5:Ce3+ and tuneable yellow emission of Sr3SiO5:Ce3+, Eu2+ by Al3+ charge compensation for W-LEDs. J. Mater. Chem. 2012, 22, 15887–15893. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, J.I.; Kim, Y.J.; Han, J.K.; Ha, J.; Novitskaya, E.; Talbot, J.B.; McKittrick, J. Comparison of luminescent properties of Y2O3:Eu3+ and LaPO4:Ce3+, Tb3+ phosphors prepared by various synthetic methods. Mater. Charact. 2015, 103, 162–169. [Google Scholar] [CrossRef]
- Terraschke, H.; Wickleder, C. UV, blue, green, yellow, red, and small: Newest developments on Eu-doped nanophosphors. Chem. Rev. 2015, 115, 11352–11378. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.Y.; Lee, C.H.; Kang, Y.C. Effect of surface area and crystallite size on luminescent intensity of Y2O3:Eu phosphor prepared by spray pyrolysis. Mater. Lett. 2005, 59, 2451–2456. [Google Scholar] [CrossRef]
- Chiang, C.H.; Liu, T.H.; Lin, H.Y.; Kuo, H.Y.; Chu, S.Y. Effects of flux additives on the characteristics of Y2.95Al5O12:0.05Ce3+ phosphor: Particle growth mechanism and luminescence. J. Appl. Phys. 2013, 114, 243517. [Google Scholar] [CrossRef]
- Zhang, Q.; Ni, H.; Wang, L.; Xiao, F. Effects of BaF2 flux on the synthesis of green emitting phosphor CaSc2O4:Ce3+. ECS J. Solid State Sci. Technol. 2014, 4, R23–R26. [Google Scholar] [CrossRef]
- Dai, P.; Zhang, X.; Sun, P.; Yang, J.; Wang, L.; Yan, S.; Liu, Y.; Ballato, J. Influence of flux on morphology and luminescence properties of phosphors: A case study on Y1.55Ti2O7:0.45Eu3+. J. Am. Ceram. Soc. 2012, 95, 1447–1453. [Google Scholar] [CrossRef]
- Lee, G.-H.; Yoon, C.; Kang, S. Role of flux in the production process of red phosphors for white LEDs. J. Mater. Sci. 2008, 43, 6109–6115. [Google Scholar] [CrossRef]
- Pamplin, B.R. Crystal Growth, 2nd ed.; Pergamon Press: Beccles/London, UK, 1980. [Google Scholar]
- Stoll, S.L.; Stacy, A.M. Single-crystal growth, alkali metal ordering, and superconductivity in La2−xMxCuO4 (M = Na, K). Inorg. Chem. 1994, 33, 2761–2765. [Google Scholar] [CrossRef]
- Chen, Y.B.; Gong, M.L.; Cheah, K.W. Effects of fluxes on the synthesis of Ca3Sc2Si3O12:Ce3+ green phosphors for white light-emitting diodes. Mater. Sci. Eng. B Adv. Funct. Solid State Mater. 2010, 166, 24–27. [Google Scholar] [CrossRef]
- Tang, J.Y.; He, Y.M.; Hao, L.Y.; Xu, X.; Agathopoulos, S. Fine-sized BaSi3Al3O4N5:Eu2+ phosphors prepared by solid-state reaction using BaF2 flux. J. Mater. Res. 2013, 28, 2598–2604. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.H.; Shi, P.L.; Guan, W.M.; Zhang, H.Y. High dispersibility and enhanced luminescence properties of BaMgAl10O17:Eu2+ phosphors derived from molten salt synthesis. Opt. Mater. 2015, 46, 432–437. [Google Scholar] [CrossRef]
- Liu, J.Q.; Wang, X.J.; Xuan, T.T.; Wang, C.B.; Li, H.L.; Sun, Z. Lu3(Al,Si)5(O,N)12:Ce3+ phosphors with broad emission band and high thermal stability for white LEDs. J. Lumin. 2015, 158, 322–327. [Google Scholar] [CrossRef]
- Dong, K.; Li, Z.L.; Xiao, S.G.; Xiang, Z.F.; Zhang, X.A.; Yang, X.L.; Jin, X.L. Yellowish-orange luminescence in Sr8Al12O24S2:Eu2+ phosphor. J. Alloys Compd. 2012, 543, 105–108. [Google Scholar] [CrossRef]
- Lee, S.H.; Jung, D.S.; Han, J.M.; Koo, H.Y.; Kang, Y.C. Fine-sized Y3Al5O12:Ce phosphor powders prepared by spray pyrolysis from the spray solution with barium fluoride flux. J. Alloys Compd. 2009, 477, 776–779. [Google Scholar] [CrossRef]
- Kang, H.S.; Kang, Y.C.; Jung, K.Y.; Park, S.B. Eu-doped barium strontium silicate phosphor particles prepared from spray solution containing NH4Cl flux by spray pyrolysis. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2005, 121, 81–85. [Google Scholar] [CrossRef]
- Wang, J.S.; Zhu, D.-C.; Zheng, Q.; Han, T. Effect of flux on the composition and luminescent properties of Ca0.68Mg0.2SiO3:0.12Eu3+ red phosphor. J. Lumin. 2016, 179, 183–188. [Google Scholar] [CrossRef]
- Zhang, B.; Feng, L.; Qiang, Y. Preparation and photoluminescence properties of the Sr1.56Ba0.4SiO4:0.04Eu2+ phosphor. J. Lumin. 2012, 132, 1274–1277. [Google Scholar] [CrossRef]
- Pires, A.M.; Davolos, M.R. Luminescence of europium (III) and manganese (II) in barium and zinc orthosilicate. Chem. Mater. 2001, 13, 21–27. [Google Scholar] [CrossRef]
- Pawar, A.U.; Jadhav, A.P.; Pal, U.; Kim, B.K.; Kang, Y.S. Blue and red dual emission nanophosphor CaMgSi2O6:Eun+; crystal structure and electronic configuration. J. Lumin. 2012, 132, 659–664. [Google Scholar] [CrossRef]
- Ha, J.; Wang, Z.; Novitskaya, E.; Hirata, G.A.; Graeve, O.A.; Ong, S.P.; McKittrick, J. An integrated first principles and experimental investigation of the relationship between structural rigidity and quantum efficiency in phosphors for solid state lighting. J. Lumin. 2016, 179, 297–305. [Google Scholar] [CrossRef]
- Cahill, J.T.; Ruppert, J.N.; Wallis, B.; Liu, Y.; Graeve, O.A. Development of mesoporosity in scandia-stabilized zirconia: Particle size, solvent, and calcination effects. Langmuir 2014, 30, 5585–5591. [Google Scholar] [CrossRef] [PubMed]
- Graeve, O.A.; Fathi, H.; Kelly, J.P.; Saterlie, M.S.; Sinha, K.; Rojas-George, G.; Kanakala, R.; Brown, D.R.; Lopez, E.A. Reverse micelle synthesis of oxide nanopowders: Mechanisms of precipitate formation and agglomeration effects. J. Colloid Interface Sci. 2013, 407, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Saterlie, H.S.M.S.; Kavlicoglu, B.; Liu, Y.; Graeve, O.A. Surfactant effects on dispersion characteristics of copper-based nanofluids: A dynamic light scattering study. Chem. Mater. 2012, 24, 3299–3306. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 2008, 41, 653–658. [Google Scholar] [CrossRef]
- Knyazev, A.V.; Bulanov, E.N.; Korshunov, A.O.; Krasheninnikova, O.V. Synthesis and thermal expansion of some lanthanide-containing apatites. Inorg. Mater. 2013, 49, 1133–1137. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Marler, B.; Borowski, M.; Wodara, U.; Schreyer, W. Synthetic tourmaline (olenite) with excess boron replacing silicon in the tetrahedral site: II. Structure analysis. Eur. J. Mineral. 2002, 14, 763–771. [Google Scholar] [CrossRef]
- Xia, Z.; Molokeev, M.S.; Im, W.B.; Unithrattil, S.; Liu, Q. Crystal structure and photoluminescence evolution of La5(Si2+xB1−x)(O13−xNx):Ce3+ solid solution phosphors. J. Phys. Chem. C 2015, 119, 9488–9495. [Google Scholar] [CrossRef]
- Denton, A.R.; Ashcroft, N.W. Vegard’s law. Phys. Rev. A 1991, 43, 3161–3164. [Google Scholar] [CrossRef] [PubMed]
- Prencipe, D.D.I.; Zani, A.; Rizzo, D.; Passoni, M. Energy dispersive X-ray spectroscopy for nanostructured thin film density evaluation. Sci. Technol. Adv. Mater. 2015, 16, 025007. [Google Scholar] [CrossRef] [PubMed]
- Dantelle, G.; Salaün, M.; Bruyère, R.; Kodjikian, S.; Ibanez, A. Luminescent coatings prepared from optimized YAG:Ce nanoparticles. Thin Solid Films 2017, 643, 36–42. [Google Scholar] [CrossRef]







| Flux/Tm/Tb (°C) | Phosphor Composition | Synthesis Method | Annealing Temperature (°C) | Results | Ref. |
|---|---|---|---|---|---|
| CaF2/1418/2533 | (Ca0.99Ce0.01)3Sc2Si3O12 | Solid state reaction | 1100–1450 | Reduced impurities, decreased formation temperature, no reported crystallite size and Φ, emission intensity increased 2×, narrow particles distribution, removed flux by sublimation after reaction | [13] |
| BaF2/1368/2260 | Y2.965Ce0.035Al5O12 | Spray pyrolysis | 1300–1600 | Enlarged, regular morphology, and non-aggregated particles, no reported crystallite size and Φ, emission intensity increased 1.4× | [18] |
| Y2.95Ce0.05Al5O12 | Solid state reaction | 1000–1500 | Able to reduce annealing temperature BaAl2O4, byproduct from BaF2 Spherical shape and smooth surface Φ external) increased 1.3× over commercial sample | [7] | |
| Ba0.85Eu0.15Si3Al3O4N5 | Solid state reaction | 1550 | Enlarged crystallite size (no specific number) and particles size, narrow particles distribution, emission intensity increased slightly, no reported Φ | [14] | |
| Ca0.99Ce0.01Sc2O4 | Solid state reaction | 1550 and 1450 | Φ external) increased 1.1×, no reported crystallite size, enlarged and regular particles | [8] | |
| LiF/845/1673 | Ba0.9Eu0.1Mg0.98Mn0.02Al10O17 | Molten salt synthesis | 1100–1400 | Particles size enlarged, Li+ into the host lattice analyzed by lattice parameter, no report crystallite size from XRD, no reported Φ, emission intensity increased 2× | [15] |
| NaF/993/1695 | Lu2.925Ce0.075Al4.79Si0.21O11.79N0.21 | Solid state reaction | 1500 | Emission intensity increased 1.3×, regular morphology of particles, no report crystallite size and Φ | [16] |
| NaF/993/1695 LiF/845/1675 H3BO3/171/300 NH4F/100/decomposes | Y1.55Eu0.45Ti2O7 | Solid state reaction | 1350 | Crystallite size enlarged (no specific number), emission intensity increased 11× (NaF), 9× (LiF), 5× (H3BO3), 2.5× (NH4F), 39% of Φ (NaF), no reported Φ without flux, enlarged particles size | [9] |
| NH4Cl/338/decomposes | Ba1.488Sr0.5Eu0.012SiO4 | Spray pyrolysis | 900–1400 | Enlarged particles, enlarged crystallite size (no specific number), no reported Φ, emission intensity increased 1.3×, optimum annealing temperature decreased | [19] |
| K2CO3/891/decomposes | Ca0.68Eu0.12Mg0.2SiO3 | Co-precipitation | 1200 | Charge compensation, crystallite size increased 1.1×, Φ increased 2.5×, no phase composition change, no reported particles size | [20] |
| Li2CO3/734/1310 | (Sr0.92Eu0.08)8Al12O24S2 | Solid state reaction | 900 | Improved purity, but still impurities remained. No report crystallite size and Φ | [17] |
| SrCl2/874/1250 | Sr1.56Eu0.04Ba0.4SiO4 | Combustion | 800–950 | Crystallite size increased (no specific number), emission intensity increased 2.7×, no reported Φ, similar particles size | [21] |
| Flux | Melting Point (°C) | Boiling Point (°C) |
|---|---|---|
| NH4F | 100 | Decomposition * |
| NH4Cl | 338 | Decomposition ** |
| H3BO3 | 171 | 300 *** |
| wt.% Flux | NH4F (mol.%) | NH4Cl (mol.%) | H3BO3 (mol.%) |
|---|---|---|---|
| 2 | 12 | 8 | 7 |
| 6 | 28 | 22 | 19 |
| 10 | 40 | 31 | 28 |
| Ions | 4-Coordinated | 6-Coordinated | 8-Coordinated |
|---|---|---|---|
| Ca2+ | - | - | 0.112 |
| Mg2+ | - | 0.072 | - |
| Si4+ | 0.026 | - | - |
| O2− | 0.138 | 0.140 | 0.142 |
| B3+ | 0.011 | 0.027 | - |
| F− | 0.131 | 0.133 | - |
| Cl− | - | 0.181 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, J.; Novitskaya, E.; Hirata, G.A.; Zhou, C.; Ridley, R.E.; Graeve, O.A.; McKittrick, J. A Facile Method Using a Flux to Improve Quantum Efficiency of Submicron Particle Sized Phosphors for Solid-State Lighting Applications. Ceramics 2018, 1, 38-53. https://doi.org/10.3390/ceramics1010005
Ha J, Novitskaya E, Hirata GA, Zhou C, Ridley RE, Graeve OA, McKittrick J. A Facile Method Using a Flux to Improve Quantum Efficiency of Submicron Particle Sized Phosphors for Solid-State Lighting Applications. Ceramics. 2018; 1(1):38-53. https://doi.org/10.3390/ceramics1010005
Chicago/Turabian StyleHa, Jungmin, Ekaterina Novitskaya, Gustavo A. Hirata, Chenhui Zhou, Robyn E. Ridley, Olivia A. Graeve, and Joanna McKittrick. 2018. "A Facile Method Using a Flux to Improve Quantum Efficiency of Submicron Particle Sized Phosphors for Solid-State Lighting Applications" Ceramics 1, no. 1: 38-53. https://doi.org/10.3390/ceramics1010005





