On the Synthesis and Characterization of Lanthanide Metal-Organic Frameworks
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
3.1. XRD Analysis
3.2. Infrared (FTIR) Spectroscopy
3.3. Thermal (TG/DTG) Analysis
3.4. Luminescent Properties
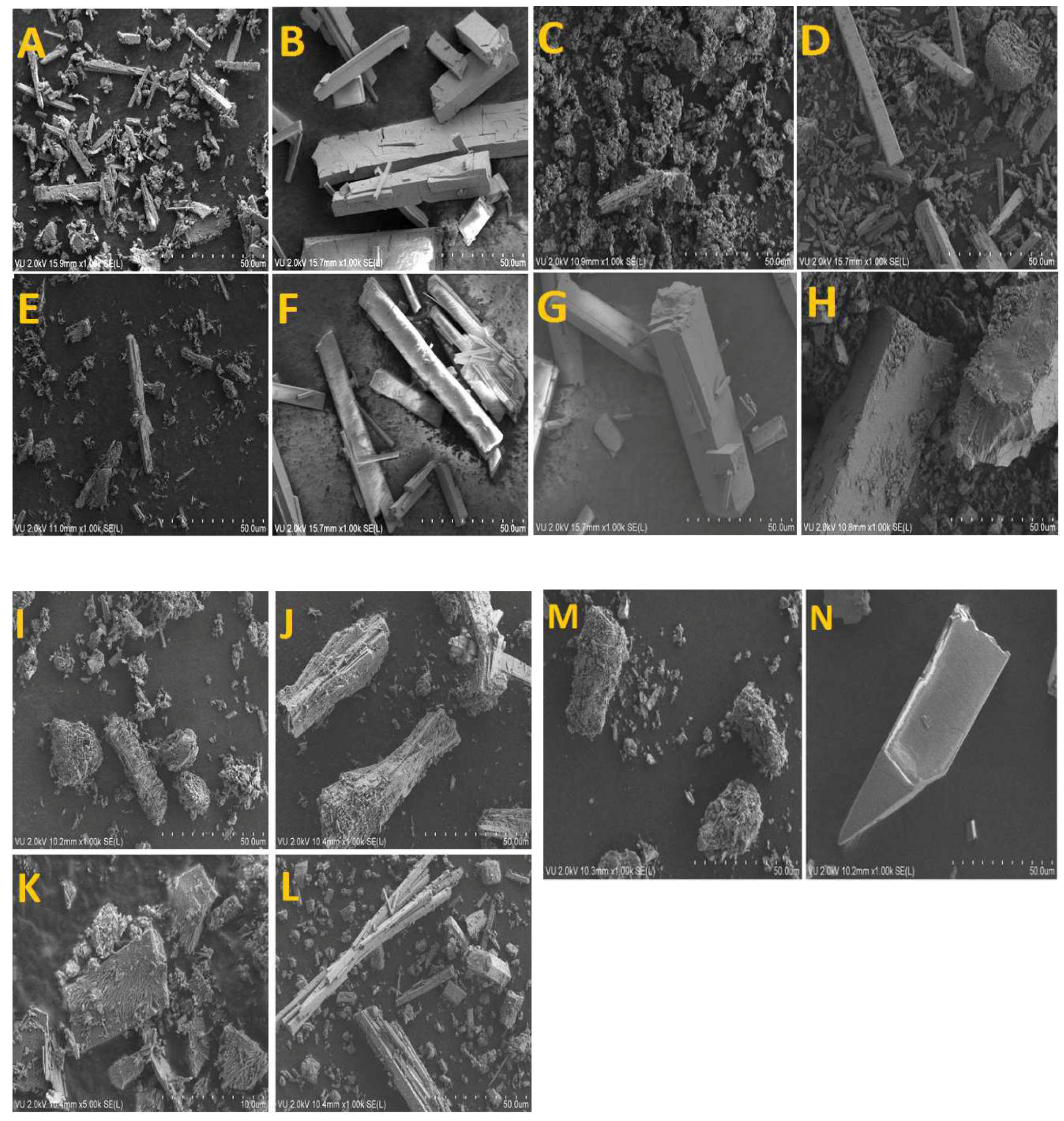
3.5. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, X.D.; Zhu, G.S.; Fang, Q.R.; Xue, M.; Tian, G.; Sun, J.Y.; Li, X.T.; Qiu, S.L. Synthesis, Structure and Luminescent Properties of Rare Earth Coordination Polymers Constructed from Paddle-Wheel Building Blocks. Inorg. Chem. 2005, 44, 3850–3855. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Li, P.F. Synthesis, Structure, and Catalytic Activity of A New Mn(II) Complex with 1,4-Phenylenediacetic Acid and 1,10-Phenanthroline. Bull. Chem. React. Eng. Catal. 2018, 13, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Rosi, N.L.; Kim, J.; Eddaoudi, M.; Chen, B.L.; O’Keeffe, M.; Yaghi, O.M. Rod Packings and Metal−Organic Frameworks Constructed from Rod-Shaped Secondary Building Units. J. Am. Chem. Soc. 2005, 127, 1504–1518. [Google Scholar] [CrossRef] [PubMed]
- Laurikenas, A.; Katelnikovas, A.; Skaudzius, R.; Kareiva, A. Synthesis and characterization of Tb3+ and Eu3+ metal-organic frameworks with TFBDC2− linkers. Opt. Mater. 2018, in press. [Google Scholar]
- Li, Y.L.; Yu, C.; Yang, B.; Liu, Z.R.; Xia, P.Y.; Wang, Q. Target-catalyzed hairpin assembly and metal-organic frameworks mediated nonenzymatic co-reaction for multiple signal amplification detection of miR-122 in human serum. Biosens. Bioelctron. 2018, 102, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Zhang, J.L.; Han, Y.; Zhu, M.Y.; Shang, S.S.; Li, W. MOF-derived various morphologies of N-doped carbon composites for acetylene hydrochlorination. J. Mater. Sci. 2018, 53, 4913–4926. [Google Scholar] [CrossRef]
- Liu, X.M.; Tang, B.; Long, J.L.; Zhang, W.; Liu, X.H.; Mirza, Z. The development of MOFs-based nanomaterials in heterogeneous organocatalysis. Sci. Bull. 2018, 63, 502–524. [Google Scholar] [CrossRef]
- Valizadeh, B.; Nguyen, T.N.; Stylianou, K.C. Shape engineering of metal-organic frameworks. Polyhedron 2018, 145, 1–15. [Google Scholar] [CrossRef]
- Stackhouse, C.A.; Ma, S.Q. Azamacrocyclic-based metal organic frameworks: Design strategies and applications. Polyhedron 2018, 145, 154–165. [Google Scholar] [CrossRef]
- Echaide-Gorriz, C.; Clement, C.; Cacho-Bailo, F.; Tellez, C.; Coronas, J. New strategies based on microfluidics for the synthesis of metal-organic frameworks and their membranes. J. Mater. Chem. A 2018, 6, 5485–5506. [Google Scholar] [CrossRef]
- Van Vleet, M.J.; Weng, T.T.; Li, X.Y.; Schmidt, J.R. In Situ, Time-Resolved, and Mechanistic Studies of Metal-Organic Framework Nucleation and Growth. Chem. Rev. 2018, 118, 3681–3721. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Qiu, L.-G.; Wang, W.; Li, Z.-Q.; Xu, T.; Wu, Z.-Y.; Jiang, X. Kinetics of oxidation of hydroquinone to p-benzoquinone catalyzed by microporous metal-organic frameworks M3(BTC)2 [M = copper(II), cobalt(II), or nickel(II); BTC = benzene-1,3,5-tricarboxylate] using molecular oxygen. Transit. Met. Chem. 2009, 34, 263–268. [Google Scholar] [CrossRef]
- Faustini, M.; Kim, J.; Jeong, G.-Y.; Kim, J.Y. Microfluidic Approach toward Continuous and Ultrafast Synthesis of Metal–Organic Framework Crystals and Hetero Structures in Confined Microdroplets. J. Am. Chem. Soc. 2013, 135, 14619–14626. [Google Scholar] [CrossRef] [PubMed]
- Almáši, M.; Zeleňák, V.; Opanasenko, M.; Císařová, I. Ce(III) and Lu(III) metal–organic frameworks with Lewis acid metal sites: Preparation, sorption properties and catalytic activity in Knoevenagel condensation. Catal. Today 2015, 243, 184–194. [Google Scholar] [CrossRef]
- Peng, M.M.; Jeon, U.J.; Ganesh, M.; Aziz, A.; Vinodh, R.; Palanichamy, M.; Jang, H.T. Oxidation of Ethylbenzene Using Nickel Oxide Supported Metal Organic Framework Catalyst. Bull. Korean Chem. Soc. 2014, 35, 3213–3218. [Google Scholar] [CrossRef] [Green Version]
- Da Luz, L.L.; Viana, B.F.; Oliveira da Silva, G.; Gatto, C.C.; Fontes, A.M. Controlling the energy transfer in lanthanide–organic frameworks for the production of white-light emitting materials. CrystEngCom 2014, 16, 6914–6918. [Google Scholar] [CrossRef]
- Kareiva, A.; Karppinen, M.; Niinistö, L. Sol-gel synthesis of superconducting YBa2Cu4O8 using acetate and tartrate precursors. J. Mater. Chem. 1994, 4, 1267–1270. [Google Scholar] [CrossRef]





| LaMOF | CeMOF | PrMOF | NdMOF | SmMOF | EuMOF | GdMOF | |
| Empirical Formula | C15H19N2O9La | C15H19N2O9Ce | C15H19N2O9Pr | C15H19N2O9Nd | C15H19N2O9Sm | C15H19N2O9Eu | C15H19N2O9Gd |
| Formula Weight | 509.9 | 511.12 | 511.91 | 515.24 | 521.36 | 522.96 | 528.25 |
| Crystal System | monoclinic | monoclinic | monoclinic | monoclinic | monoclinic | monoclinic | monoclinic |
| Space Group | C2/c | C2/c | C2/c | C2/c | C2/c | C2/c | C2/c |
| a (Å) | 18.896 | 18.847 | 18.798 | 18.749 | 18.7 | 18.651 | 18.602 |
| b (Å) | 11.649 | 11.682 | 11.673 | 11.601 | 11.615 | 11.66 | 11.59 |
| c (Å) | 19.866 | 19.906 | 19.851 | 19.897 | 19.849 | 19.795 | 19.688 |
| β (deg) | 75.447 | 72.783 | 73.13 | 74.4 | 72.97 | 73.11 | 72.9 |
| V (Å3) | 4232.6 | 4186.34 | 4168.43 | 4129.64 | 4122.17 | 4119.14 | 4057.03 |
| Z | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| TbMOF | DyMOF | HoMOF | ErMOF | TmMOF | YbMOF | LuMOF | |
| Empirical Formula | C15H19N2O9Tb | C15H19N2O9Dy | C15H19N2O9Ho | C15H19N2O9Er | C15H19N2O9Tm | C15H19N2O9Yb | C15H19N2O9Lu |
| Formula Weight | 530.24 | 533.82 | 536.25 | 538.58 | 540.25 | 544.36 | 545.96 |
| Crystal System | monoclinic | monoclinic | monoclinic | monoclinic | monoclinic | monoclinic | monoclinic |
| Space Group | C2/c | C2/c | C2/c | C2/c | C2/c | C2/c | C2/c |
| a (Å) | 18.568 | 18.493 | 18.44 | 18.401 | 18.382 | 18.299 | 18.258 |
| b (Å) | 11.63 | 11.58 | 11.647 | 11.649 | 11.592 | 11.592 | 11.578 |
| c (Å) | 19.741 | 19.51 | 19.698 | 19.68 | 19.632 | 19.557 | 19.592 |
| β (deg) | 72. 97 | 72.79 | 72.945 | 73.03 | 72.92 | 72.98 | 72.58 |
| V (Å3) | 4072.3 | 3991 | 4044.5 | 4034.8 | 3998.8 | 3966.9 | 3951.62 |
| Z | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Band Wavenumber, cm−1 | 689 and 780 | 1382 | 1556 | 1678 | 2915 | 3420 |
|---|---|---|---|---|---|---|
| Assignment | Vibrations of 1,4-substituted benzene ring | Sym. stretching of BTC carboxylic groups | Assym. stretching of BTC carboxylic groups | ν(=co) of DMF | Assym. Stretch. of –CH3 in DMF | ν(-OH) of absorbed, residual water |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurikenas, A.; Beganskiene, A.; Kareiva, A. On the Synthesis and Characterization of Lanthanide Metal-Organic Frameworks. Ceramics 2018, 1, 54-64. https://doi.org/10.3390/ceramics1010006
Laurikenas A, Beganskiene A, Kareiva A. On the Synthesis and Characterization of Lanthanide Metal-Organic Frameworks. Ceramics. 2018; 1(1):54-64. https://doi.org/10.3390/ceramics1010006
Chicago/Turabian StyleLaurikenas, Andrius, Aldona Beganskiene, and Aivaras Kareiva. 2018. "On the Synthesis and Characterization of Lanthanide Metal-Organic Frameworks" Ceramics 1, no. 1: 54-64. https://doi.org/10.3390/ceramics1010006




