Mechanism of Ampicillin Degradation by Non-Thermal Plasma Treatment with FE-DBD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Sample Preparation
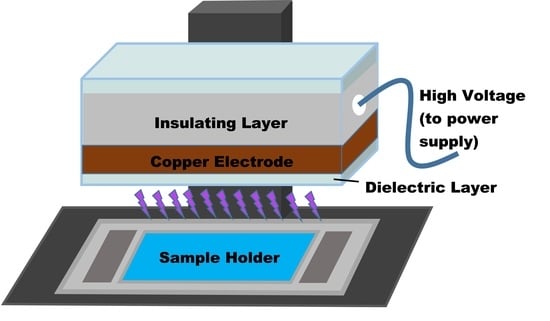
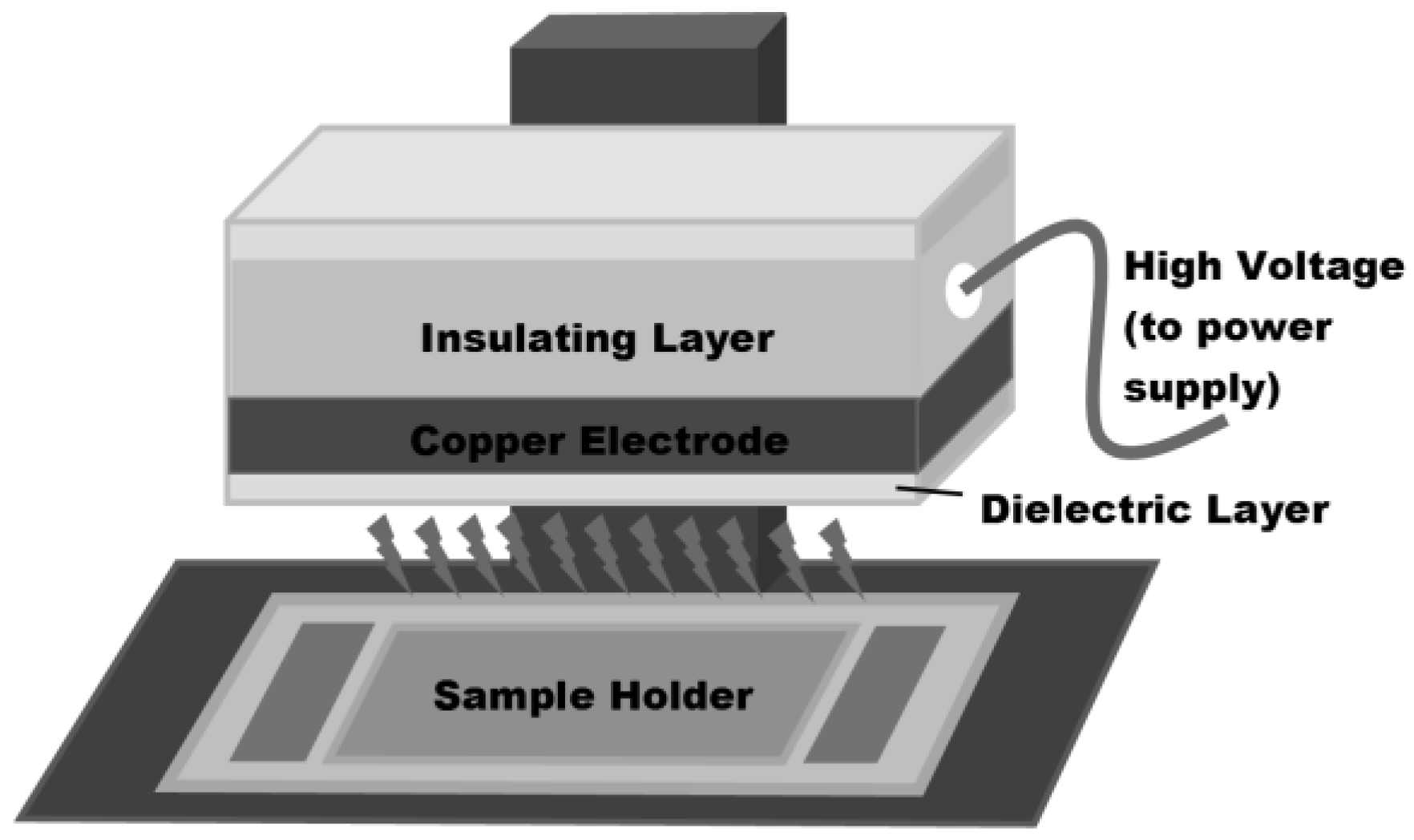
2.2. Plasma Setup and Treatment
2.3. Instrumentation
3. Results and Discussion
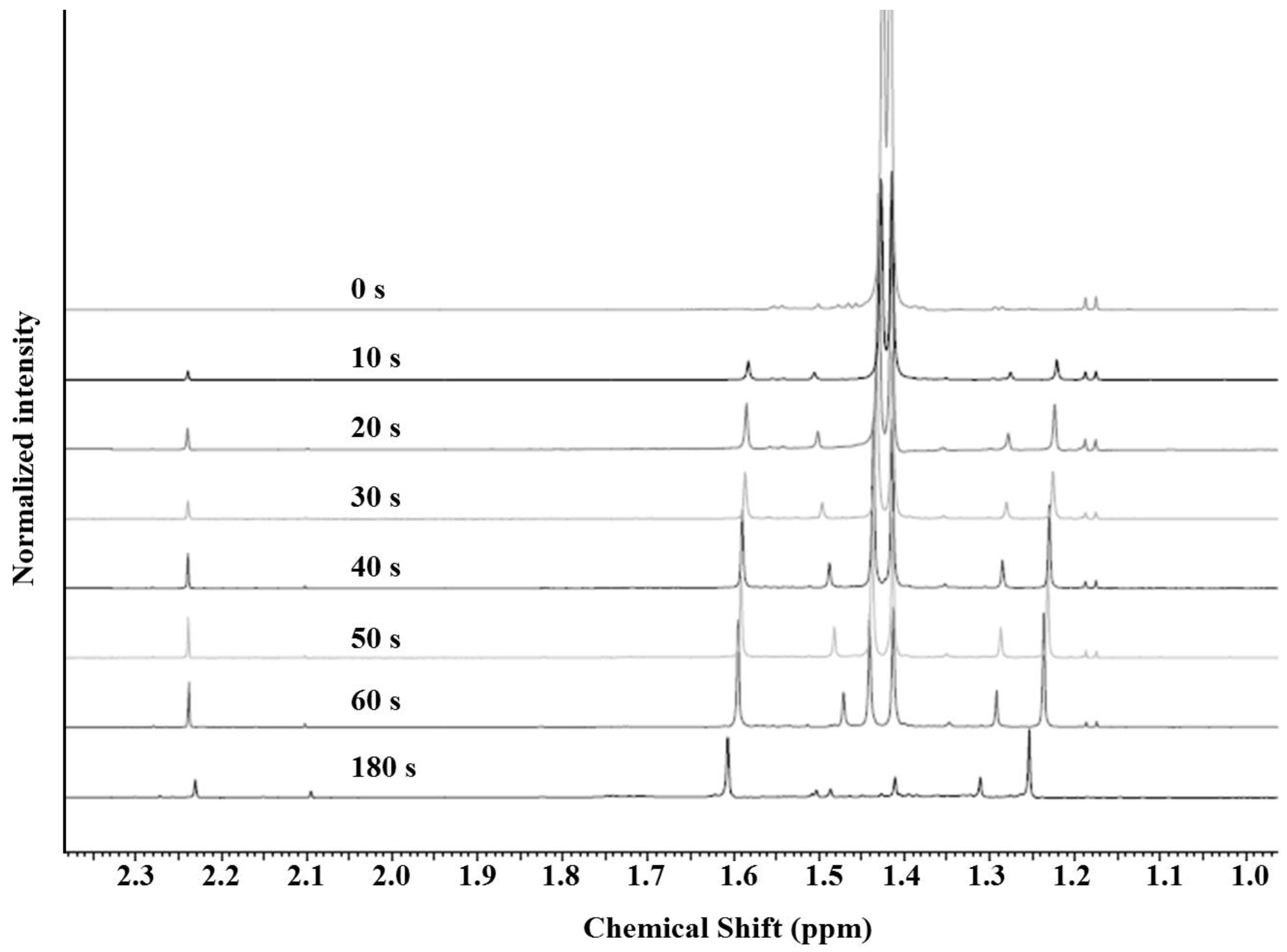
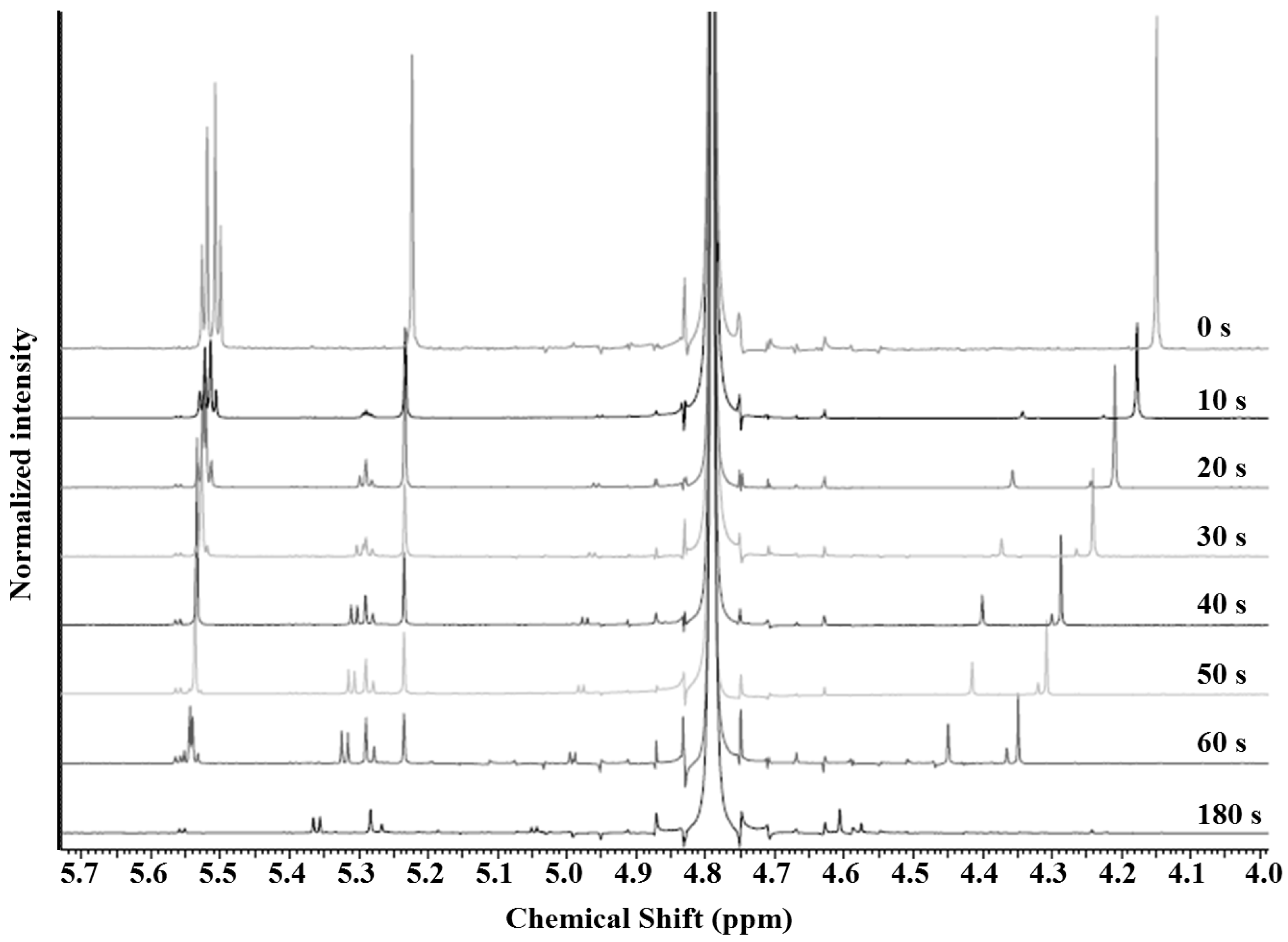
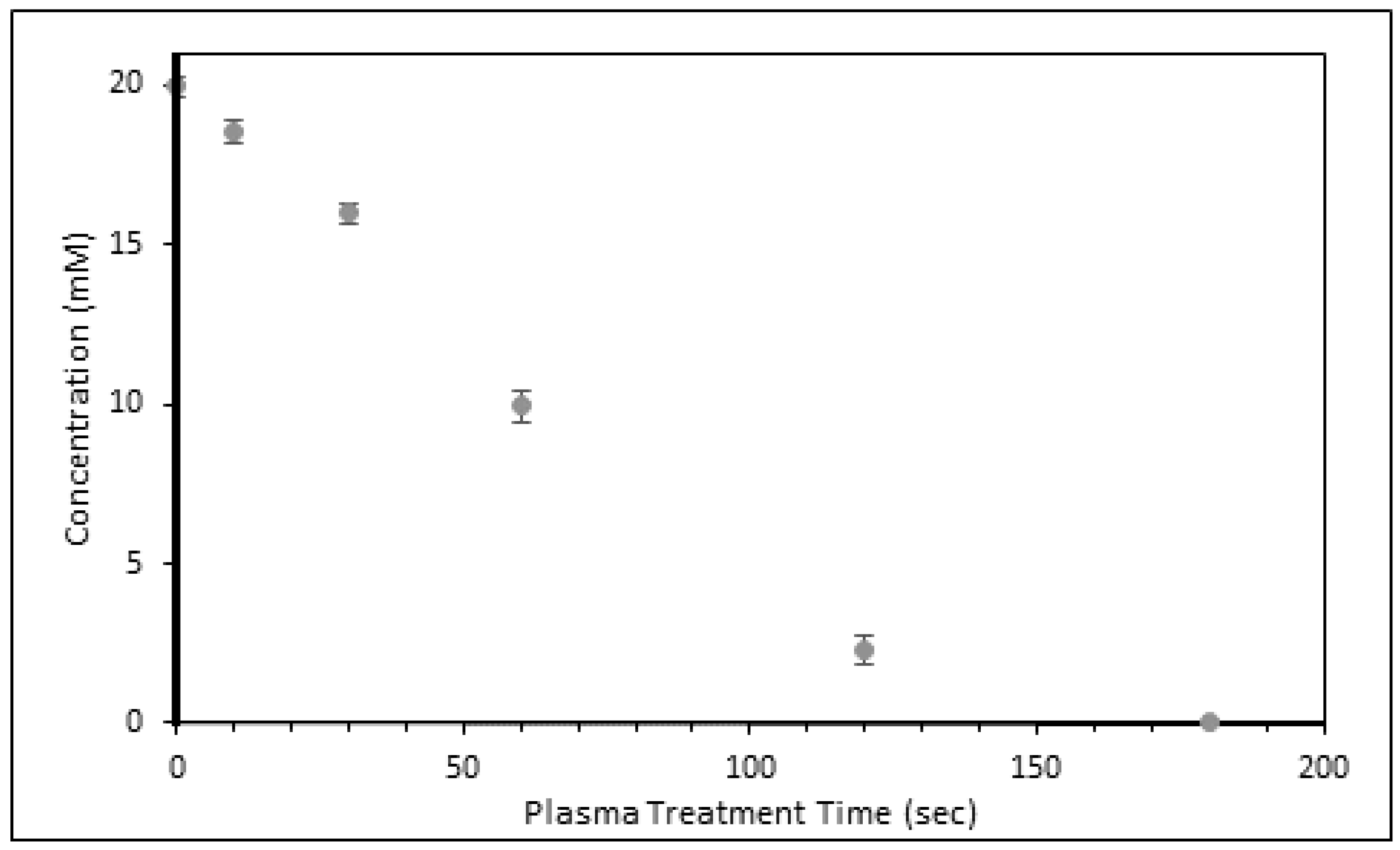
3.1. AMP Degradation
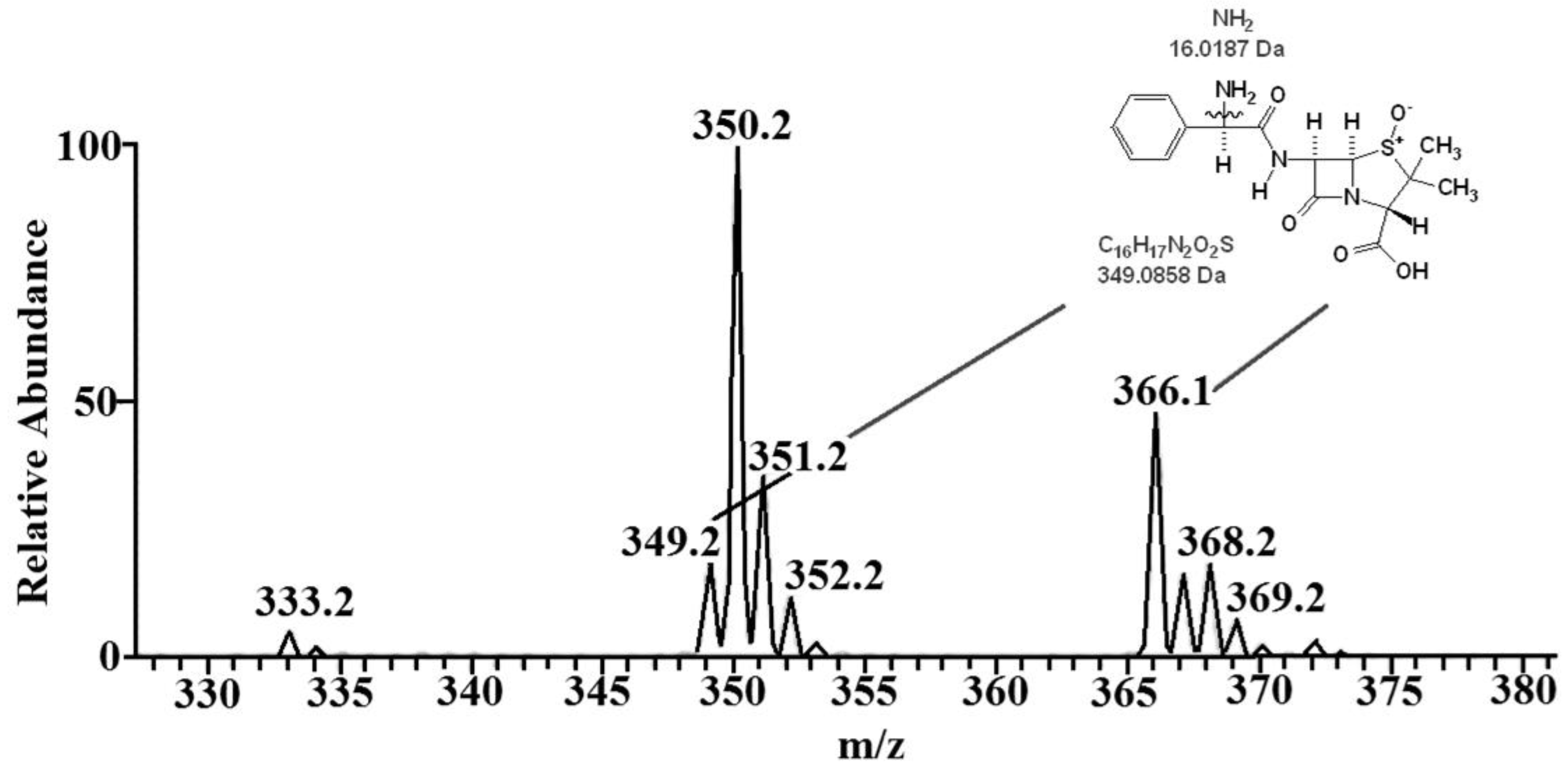
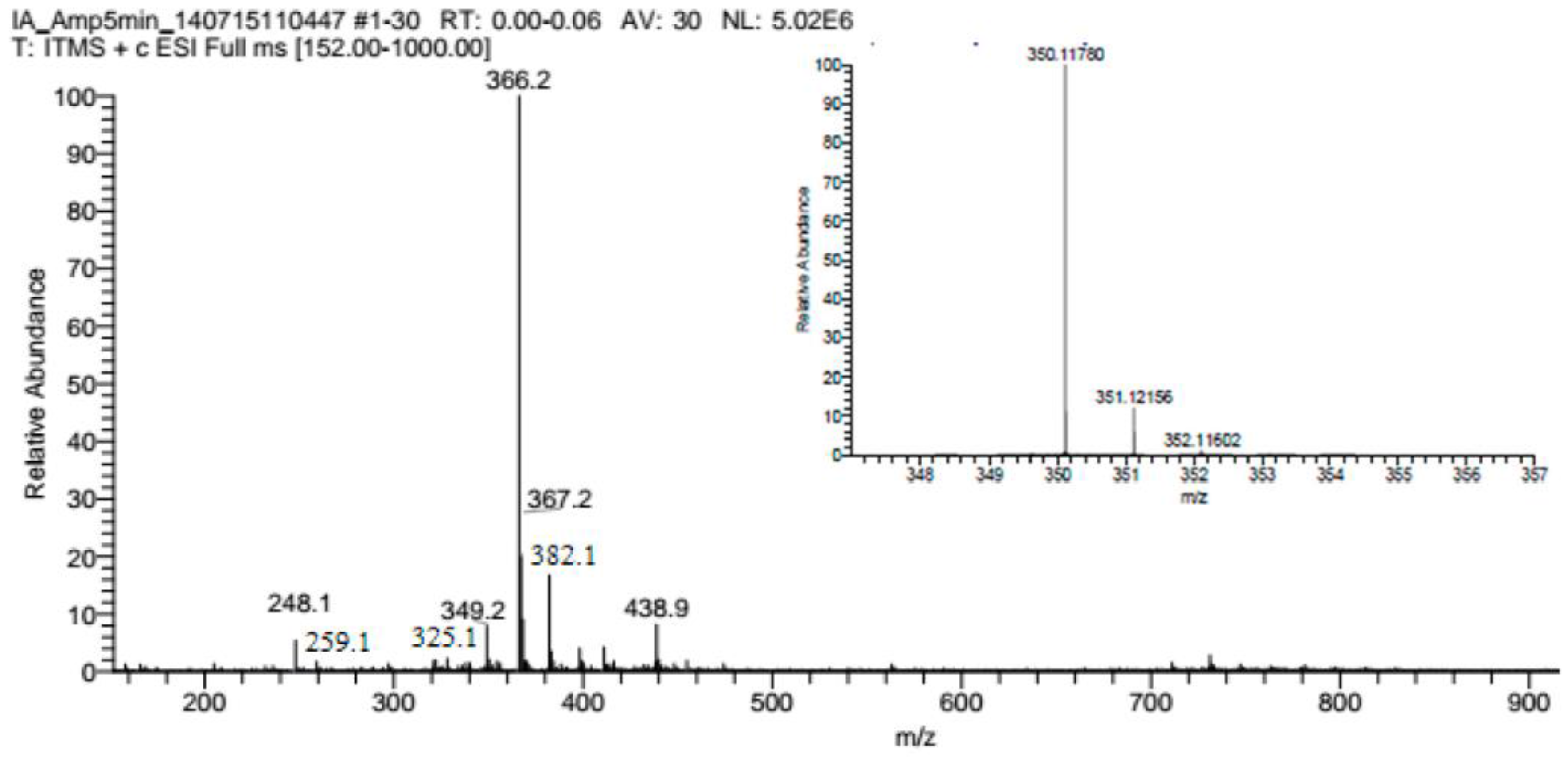
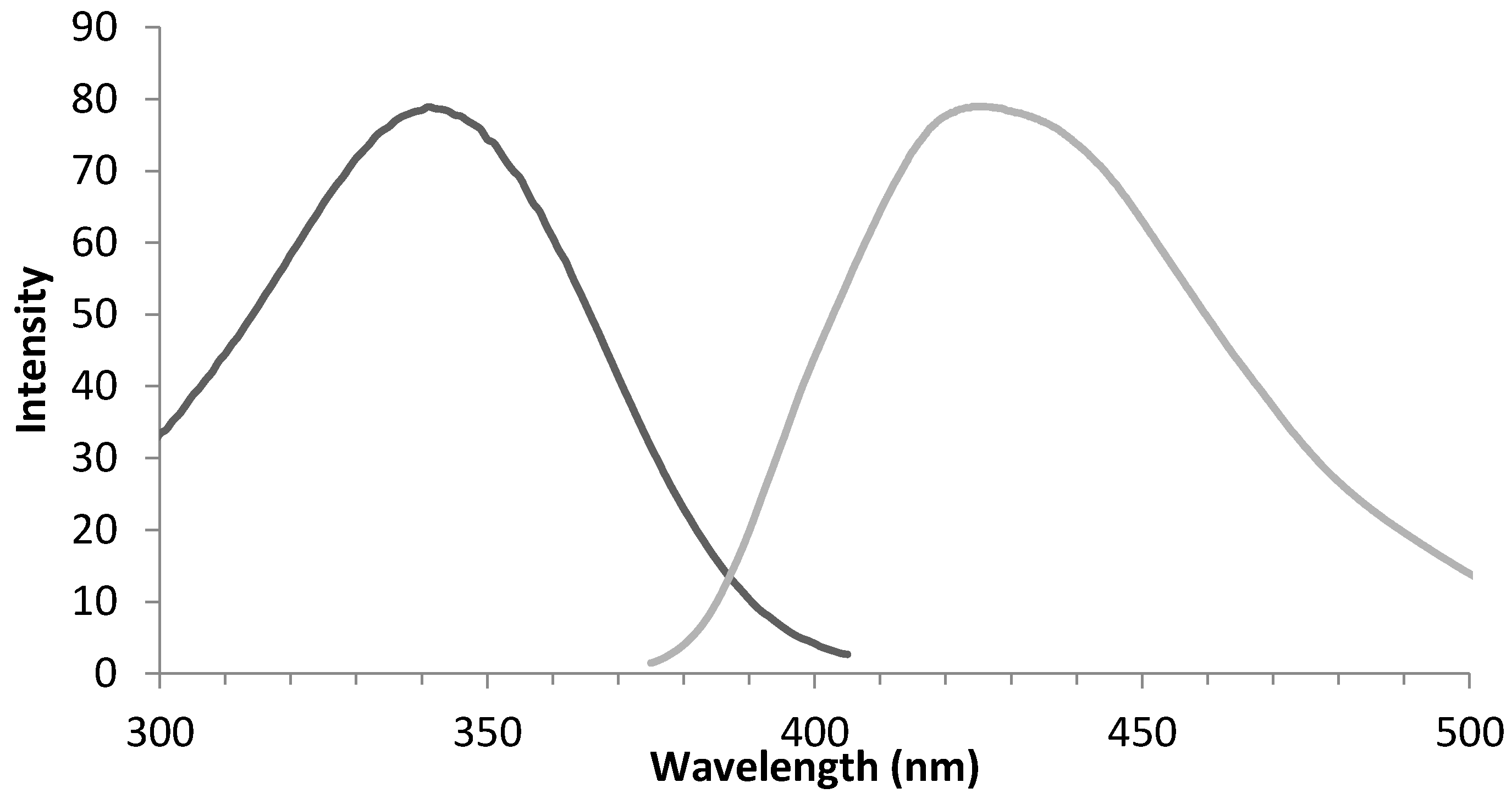
3.2. Mechanism of Plasma Degradation
3.3. Efficiency Comparison
4. Conclusions
Acknowledgement
Author Contributions
Conflicts of Interest
References
- Risk of Birth Defects Due to Certain Antibiotics During Pregnancy. Available online: http://psychomotor4.rssing.com/browser.php?indx=3809892&last=1&item=12 (accessed on 8 August 2017).
- National Action Plan for Combating Antibotic-Resistant Bacteria. Available online: https://www.cdc.gov/drugresistance/pdf/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf (accessed on 8 August 2017).
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part II. Chemosphere 2009, 75, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Fatta-Kassinos, D.; Meric, S.; Nikolaou, A. Pharmaceutical residues in environmental waters and wastewater: Current state of knowledge and future research. Anal. Bioanal. Chem. 2011, 399, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Cruz, M.S.; Lopez de Alda, M.J.; Barcelo, D. Environmental behavior and analysis of veterinary and human drugs in soils, sediments and sludge TrAC. Trends Anal. Chem. 2003, 22, 340–351. [Google Scholar] [CrossRef]
- Brown, K.D.; Kulis, J.; Thomson, B.; Chapman, T.H.; Mawhinney, D.B. Occurrence of antibiotics in hospital, residential, and dairy effluent, municipal wastewater, and the Rio Grande in New Mexico. Sci. Total Environ. 2006, 366, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Czekalski, N.; Berthold, T.; Caucci, S.; Egli, A.; Bürgmann, H. Increased levels of multiresistant bacteria and resistance genes after wastewater treatment and their dissemination into Lake Geneva, Switzerland. Front. Microbiol. 2012, 3, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Antibiotic Resistance from Unexpected Sources—Herbicides, Dust and Metals. Available online: http://www.forbes.com/sites/judystone/2015/04/01/antibiotic-resistance-from-unexpected-sources/ (accessed on 8 August 2017).
- Rogers, H.R. Behavior and fate of organic contaminants during sewage treatment and in sewage sludges. Sci. Total Environ. 1996, 185, 3–26. [Google Scholar] [CrossRef]
- Zhang, G.; Ji, S.; Xi, B. Feasibility study of treatment of amoxillin wastewater with a combination of extraction, Fenton oxidation and reverse osmosis. Desalination 2006, 196, 32–42. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Wang, D. Membrane (RO-UF) filtration for antibiotic wastewater treatment and recovery of antibiotics. Sep. Purif. Technol. 2004, 34, 109–114. [Google Scholar] [CrossRef]
- Aksu, Z.; Tunç, O. Application of biosorption for penicillin G removal: Comparison with activated carbon. Process Biochem. 2005, 40, 831–847. [Google Scholar] [CrossRef]
- Westerhoff, P.; Yoon, Y.; Snyder, S.; Wert, E. Fate of endocrine-disruptor, pharmaceutical, and personal care product chemicals during simulated drinking water treatment processes. Environ. Sci. Technol. 2005, 39, 6649–6663. [Google Scholar] [CrossRef] [PubMed]
- Putra, E.K.; Pranowo, R.; Sunarso, J.; Indraswati, N.; Ismadji, S. Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: Mechanisms, isotherms and kinetics. Water Res. 2009, 43, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Prados-Joya, G.; Sánchez-Polo, M.; Ferro-García, M.A.; Bautista-Toledo, I. Removal of nitroimidazole antibiotics from aqueous solution by adsorption/bioadsorption on activated carbon. J. Hazard. Mater. 2009, 170, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Legrini, O.; Oliveros, E.; Braunm, A.M. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Tekin, H.; Bilkay, O.; Ataberk, S.S.; Balta, T.H.; Ceribasi, I.H.; Sanin, F.D. Use of Fenton oxidation to improve the biodegradability of a pharmaceutical wastewater. J. Hazard. Mater. 2006, 136, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Melero, J.A.; Calleja, G.; Martinez, F.; Molina, R.; Pariente, M.I. Nanocomposite Fe2O3/SBA-15: An efficient and stable catalyst for the catalytic wet peroxidation of phenolic aqueous solutions. Chem. Eng. J. 2007, 131, 245–256. [Google Scholar] [CrossRef]
- Santos, A.; Yustos, P.; Rodriguez, S.; Simon, E.; Garcia-Ochoa, F. Abatement of phenolic mixtures by catalytic wet oxidation enhanced by Fenton’s pretreatment: Effect of H2O2 dosage and temperature. J. Hazard. Mater. 2007, 146, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Dickenson, E.R.V.; Drewes, J.E.; Sedlak, D.L.; Wert, E.C.; Snyder, S.A. Applying surrogates and indicators to assess removal efficiency of trace organic chemicals during chemical oxidation of wastewaters. Environ. Sci. Technol. 2009, 43, 6242–6247. [Google Scholar] [CrossRef] [PubMed]
- Hapeshi, E.; Achilleos, A.; Vasquez, M.I.; Michael, C.; Xekoukoulotakis, N.P.; Mantzavinos, D.; Kassinos, D. Drugs degrading photocatalytically: Kinetics and mechanisms of ofloxacin and atenolol removal on titania suspensions. Water Res. 2010, 44, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Schiavello, M. Some working principles of heterogeneous photocatalysis by semiconductors. Electrochim. Acta 1993, 38, 11–14. [Google Scholar] [CrossRef]
- Naddeo, V.; Meric, S.; Kassinos, D.; Belgiorno, V.; Guida, M. Fate of pharmaceuticals in contaminated urban wastewater effluent under ultrasonic irradiation. Water Res. 2009, 43, 4019–4027. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.; Asce, M.; Wang, Y.; Loftin, K.; Meyer, M. Removal of antibiotics from surface and distilled water in conventional water treatment processes. J. Environ. Eng. 2002, 1282, 253–260. [Google Scholar] [CrossRef]
- Ryan, C.C.; Tan, D.T.; Arnold, W.A. Direct and indirect photolysis of sulfamethoxazole and trimethoprim in wastewater treatment plant effluent. Water Res. 2011, 45, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Balcıoglu, I.A.; Otker, M. Treatment of pharmaceutical wastewater containing antibiotics by O3 and O3/H2O2 processes. Chemosphere 2003, 50, 85–95. [Google Scholar] [CrossRef]
- Arslan, A.I.; Dogruel, S.; Baykal, E.; Gerone, G. Combined chemical and biological oxidation of penicillin formulation effluent. J. Environ. Manag. 2004, 73, 155–163. [Google Scholar]
- Andreozzi, R.; Canterino, M.; Marotta, M.; Paxeus, N. Antibiotic removal from wastewaters: The ozonation of amoxicillin. J. Hazard. Mater. 2005, 122, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices—A review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.C.; Lee, H.W.; Byun, J.H.; Chung, J.; Jeon, Y.C.; Lee, J.K. Dental applications of low-temperature nonthermal plasmas. Plasma Process. Polym. 2013, 10, 199–206. [Google Scholar] [CrossRef]
- Hollender, J.; Zimmermann, S.G.; Koepke, S.; Krauss, M.; McArdell, C.S.; Ort, C.; Singer, H.; von Gunten, U.; Siegrist, H. Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration. Environ. Sci. Technol. 2009, 43, 7862–7869. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Friedman, G.; Brooks, A.; Fridman, A.; Ji, H.-F. Decomposition of Sugars under Non-Thermal Dielectric Barrier Discharge Plasma. Clinical Plasma Medicine. 2014, 2, 56–63. [Google Scholar] [CrossRef]
- Misra, N.N.; Pankaj, S.K.; Walsh, T.; O’Regan, F.; Bourke, P.; Cullen, P.J. In-package nonthermal plasma degradation of pesticides on fresh produce. J. Hazard. Mater. 2014, 271, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Tiede, R.; Hirschberg, J.; Daeschlein, G.; von Woedtke, T.; Vioel, W.; Emmert, S. Plasma Applications: A Dermatological View. Contrib. Plasma Phys. 2014, 54, 118–130. [Google Scholar] [CrossRef]
- Locke, B.R.; Sato, M.; Sunka, P.; Hoffmann, M.R.; Chang, J.-S. Electrohydraulic Discharge and Nonthermal Plasma for Water Treatment. Ind. Eng. Chem. Res. 2006, 45, 882–905. [Google Scholar] [CrossRef]
- Tichonovas, M.; Krugly, E.; Racys, V.; Hippler, R.; Kauneliene, V.; Stasiulaitiene, I.; Martuzevicius, D. Degradation of Various Textile Dyes as Wastewater Pollutants under Dielectric Barrier Discharge Plasma Treatment. Chem. Eng. J. 2013, 229, 9–19. [Google Scholar] [CrossRef]
- Fridman, G.; Peddinghaus, M.; Ayan, H.; Fridman, A.; Balasubramanian, M.; Gutsol, A.; Brooks, A.; Friedman, G. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chem. Plasma Process. 2006, 26, 425–442. [Google Scholar] [CrossRef]
- Fridman, G.; Brooks, A.D.; Balasubramanian, M.; Fridman, A.; Gutsol, A.; Vasilets, V.N.; Ayan, H.; Friedman, G. Comparison of direct and indirect effects of non-thermal atmospheric-pressure plasma on bacteria. Plasma Process. Polym. 2007, 4, 370–375. [Google Scholar] [CrossRef]
- Nehra, V.; Kumar, A.; Dwivedi, H.K. Atmospheric nonthermal plasma sources. Int. J. Eng. 2008, 2, 53–68. [Google Scholar]
- Ayan, H.; Staack, D.; Fridman, G.; Gutsol, A.; Muhkin, Y.; Starikovskii, A.; Fridman, A.; Friedman, G. Application of nanosecond-pulsed dielectric barrier discharge for biomedical treatment of topographically non-uniform surfaces. J. Phys. D 2009, 42, 125202. [Google Scholar] [CrossRef]
- Joshi, S.G.; Cooper, M.; Yost, A.; Paff, M.; Ercan, U.K.; Fridman, G.; Friedman, G.; Fridman, A.; Brooks, A.D. Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative DNA damage and membrane lipid peroxidation in Escherichia coli. Antimicrob. Agents Chemother. 2011, 55, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Yun, G.H. The sterilization of Escherichia coli by dielectric-barrier discharge plasma at atmospheric pressure. Appl. Surf. Sci. 2011, 257, 7065–7070. [Google Scholar] [CrossRef]
- Robinson-Fuentes, V.A.; Jefferies, T.M.; Branch, S.K. Degradation pathways of ampicillin in alkaline solutions. J. Pharm. Pharmacol. 1997, 49, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Massé, D.L.; Cata Saady, N.M.; Gilbert, Y. Potential of Biological Processes to Eliminate Antibiotics in Livestock Manure: An Overview. Animals 2014, 4, 146–163. [Google Scholar] [CrossRef] [PubMed]
- Goossens, H.; Ferech, M.; Vanderstichele, R.; Elseviers, M. Outpatient antibiotic use in Europe and association with ESAC Project Group. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef]
- Branch, S.K.; Casy, A.F.; Ominde, E.M.A. Application of 1H nuclear magnetic resonance spectroscopy to the analysis of beta-lactam antibiotics and their common degradation products. J. Pharm. Biomed. Anal. 1987, 5, 73–103. [Google Scholar] [CrossRef]
- Magureanua, M.; Piroia, D.; Mandachea, N.B.; Davidb, V.; Medvedovicib, A.; Braduc, C.; Parvulescuc, V.I. Degradation of antibiotics in water by non-thermal plasma treatment. Water Res. 2011, 45, 3407–3416. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kojtari, A.; Friedman, G.; Brooks, A.D.; Fridman, A.; Ji, H.F. Decomposition of l-Valine under Nonthermal Dielectric Barrier Discharge Plasma. J. Phys. Chem. B 2014, 118, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dobrynin, D.; Fridman, A. Uniform and non-uniform modes of nanosecond-pulsed dielectric barrier discharge in atmospheric air: Fast imaging and spectroscopic measurements of electric field. J. Phys. D 2014, 47, 252003. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.E.; Steele, B.R.; Boles, M.O.; Gane, P.A.C. Nuclear magnetic resonance and circular dichroism of penicillins derived from disubstituted acetic acids. J. Chem. Soc. Perkin Trans. 1 1982, 563–569. [Google Scholar] [CrossRef]
- Gozlan, I.; Rotstein, A.; Avisar, D. Investigation of an amoxicillin oxidative degradation product formed under controlled environmental conditions. Environ. Chem. 2010, 7, 435–442. [Google Scholar] [CrossRef]
- Micetich, R.G. A Convenient Synthesis of Ampicillin Sulfoxide and 6-Aminopenicillanic Acid Sulfoxide. Synthesis 1976, 1976, 264–265. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Varadhan, S.R.; Karunanidhi, N. Qualitative analysis on the infrared bands of tetracycline and ampicillin. Proc. Indian Natl. Sci. Acad. A 1996, 62, 309–316. [Google Scholar]
- Hou, J.P.; Poole, J.W. Kinetics and mechanism of degradation of ampicillin in solution. J. Pharm. Sci. 1969, 58, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, X.; Fu, S.; Zhang, J.; Zhang, K.; Wang, S.; Zhao, M.; Ding, W.; Wang, Q. Structural elucidation of stress degradation products of ampicillin sodium by liquid chromatography/hybrid triple quadrupole linear ion trap mass spectrometry and liquid chromatography/hybrid quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, M.; Robert, E.; Pesnel, S. Antitumor effect of plasma treatment on U87 glioma xenografts: Preliminary results. Plasma Process Polym. 2010, 7, 264–273. [Google Scholar] [CrossRef]
- Boudam, M.K.; Moisan, M.; Saoudi, B.; Popovici, C.; Gherardi, N.; Massines, F. Bacterial spore inactivation by atmospheric-pressure plasmas in the presence or absence of UV photons as obtained with the same gas mixture. J. Phys. D 2006, 39, 3494. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Degradation of the antibiotics amoxicillin, ampicillin and cloxacillin in aqueous solution by the photo-Fenton process. J. Hazard. Mater. 2009, 172, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Ghauch, A.; Tuqan, A.; Assi, H.A. Antibiotic removal from water: elimination of amoxicillin and ampicillin by microscale and nanoscale iron particles. Environ Pollut. 2009, 157, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Navalon, S.; Alvaro, M.; Garcia, H. Reaction of chlorine dioxide with emergent water pollutants: Product study of the reaction of three beta-lactam antibiotics with ClO2. Water Res. 2008, 42, 1935–1942. [Google Scholar] [CrossRef] [PubMed]


| Concentration | Treatment | Result | Reference |
|---|---|---|---|
| 20 mg/L | Removal using Metallic Iron | Complete removal after 3 h | Ghauch et al. (2009) [61] |
| 105 mg/L | Fenton | Optimal conditions, 2 min degradation | Elmolla and Chaudhuri (2009) [60] |
| 105 mg/L | Photo-Fenton | Optimal conditions, 2 min degradation | Elmolla and Chaudhuri (2009) [60] |
| 105 mg/L | Semiconductor Photocatalysis | With 180 min irradiation time | Elmolla and Chaudhuri (2010) [60] |
| 1.6 mg/L | Chlorination | Possible total degradation after 2 h | Navalon et al. (2008) [62] |
| 6.99 g/L | Air plasma in this work | * complete removal after 3 min |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, J.B.; Adams, I.; Ji, H.-F. Mechanism of Ampicillin Degradation by Non-Thermal Plasma Treatment with FE-DBD. Plasma 2018, 1, 1-11. https://doi.org/10.3390/plasma1010001
Smith JB, Adams I, Ji H-F. Mechanism of Ampicillin Degradation by Non-Thermal Plasma Treatment with FE-DBD. Plasma. 2018; 1(1):1-11. https://doi.org/10.3390/plasma1010001
Chicago/Turabian StyleSmith, Joshua B., Isaac Adams, and Hai-Feng Ji. 2018. "Mechanism of Ampicillin Degradation by Non-Thermal Plasma Treatment with FE-DBD" Plasma 1, no. 1: 1-11. https://doi.org/10.3390/plasma1010001