Plasma Medicine: A Brief Introduction
Abstract
:1. Introduction
- -
- Sterilization, disinfection, and decontamination,
- -
- plasma-aided wound healing
- -
- plasma dentistry
- -
- cancer applications or “plasma oncology,”
- -
- plasma pharmacology,
- -
- plasma treatment of implants for biocompatibility.
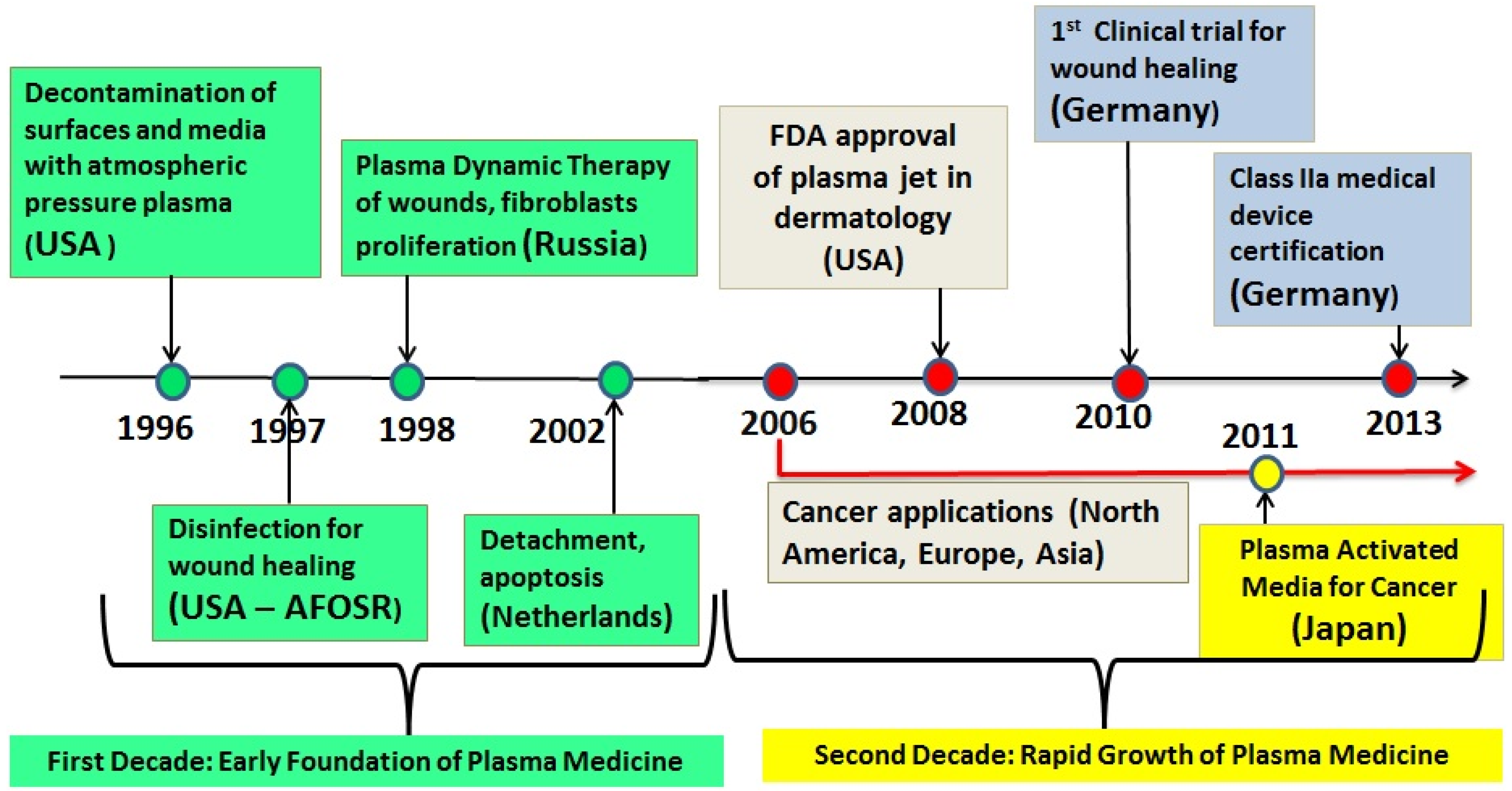
2. LTP Takes on Hygiene and Medical Challenges
3. Mechanisms of Biological Action of LTP: Brief Summary
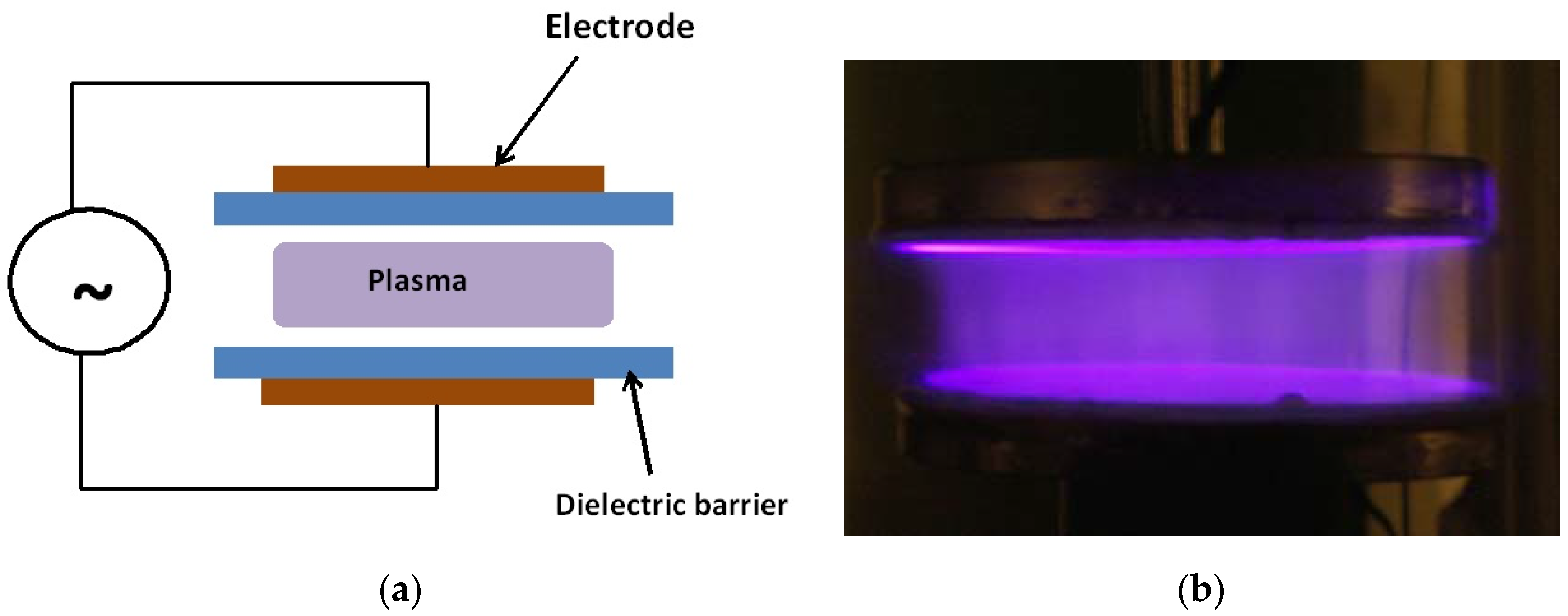
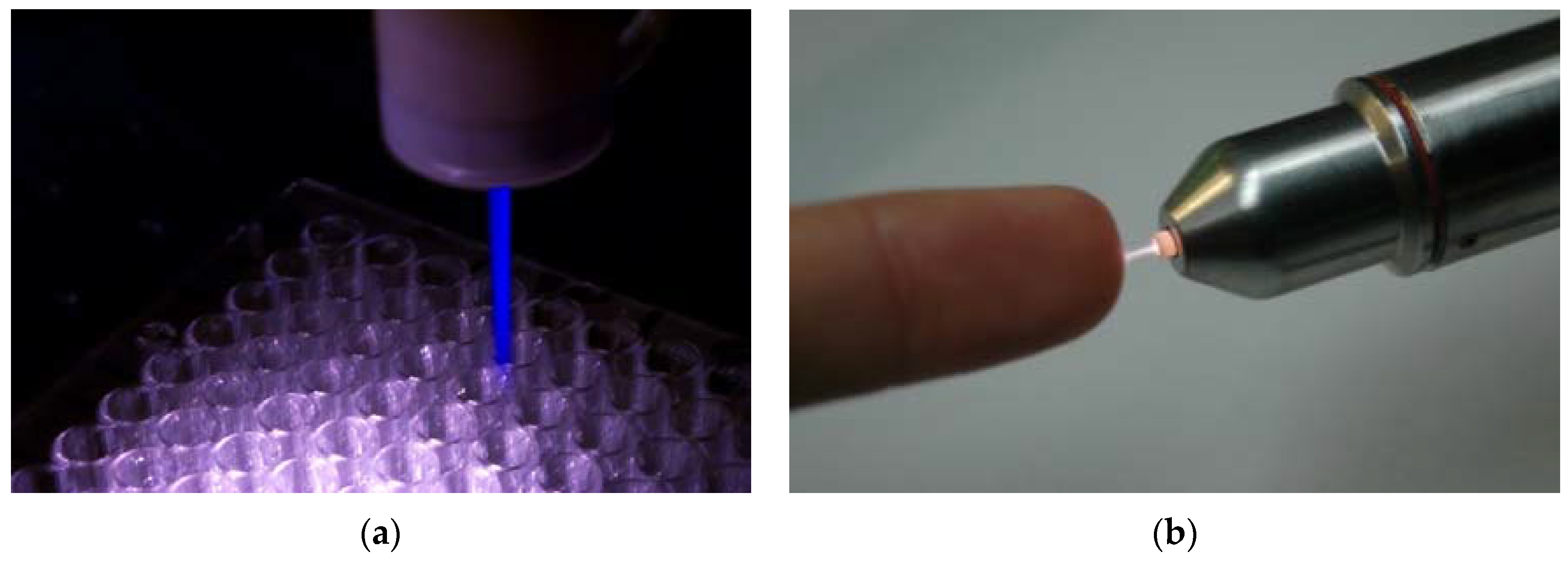
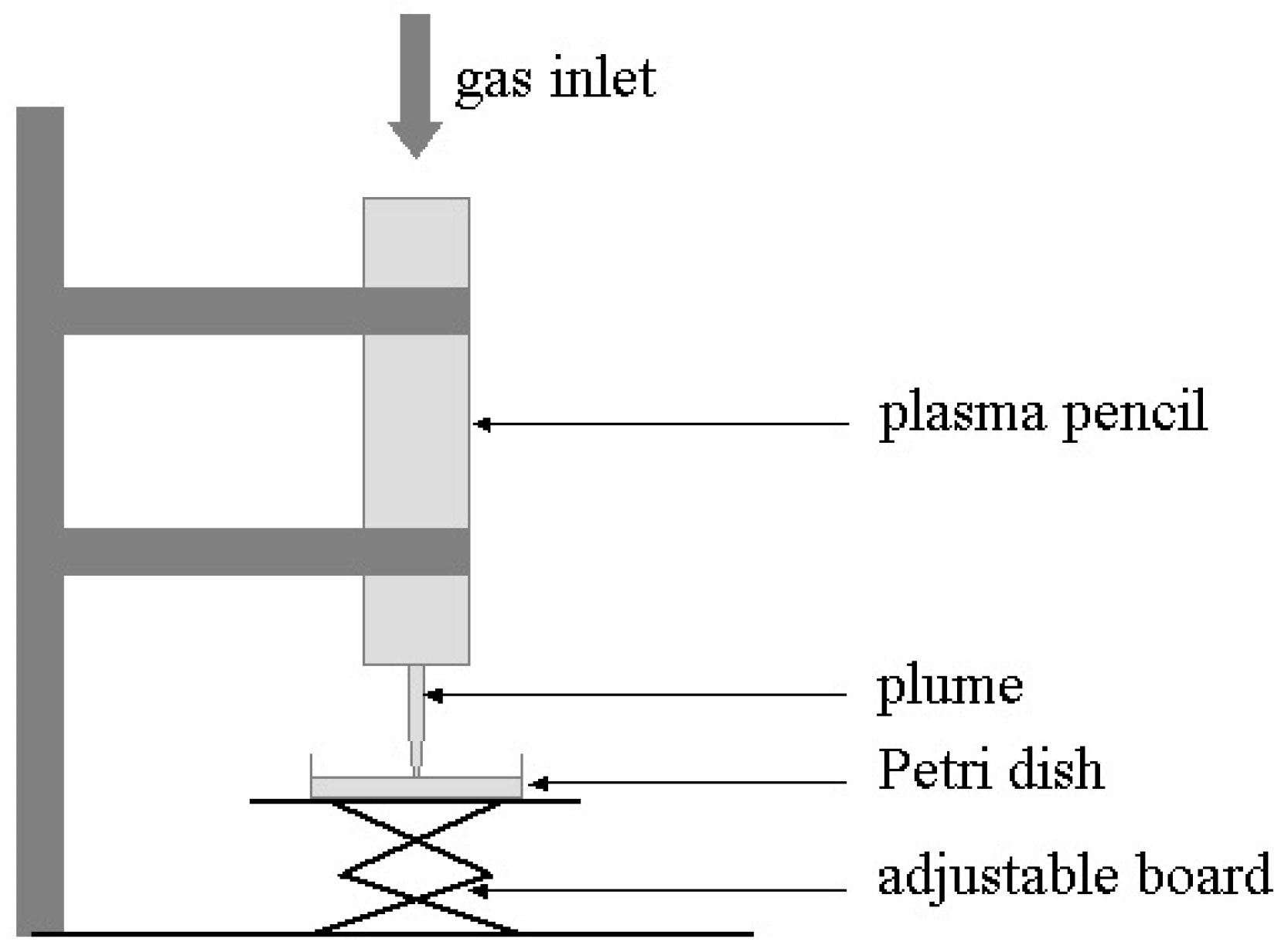
4. Two LTP Sources for Biomedical Applications: Brief Description
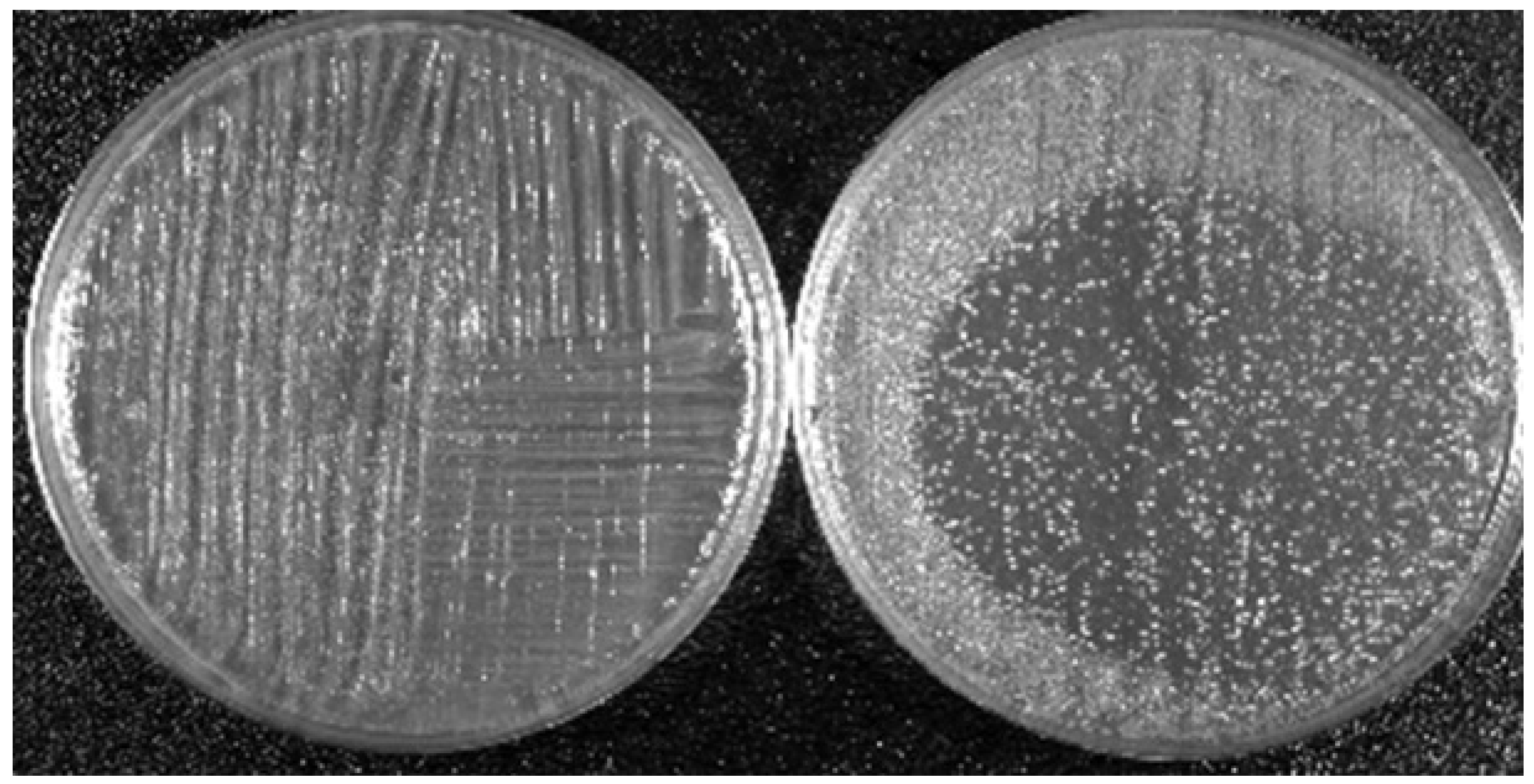
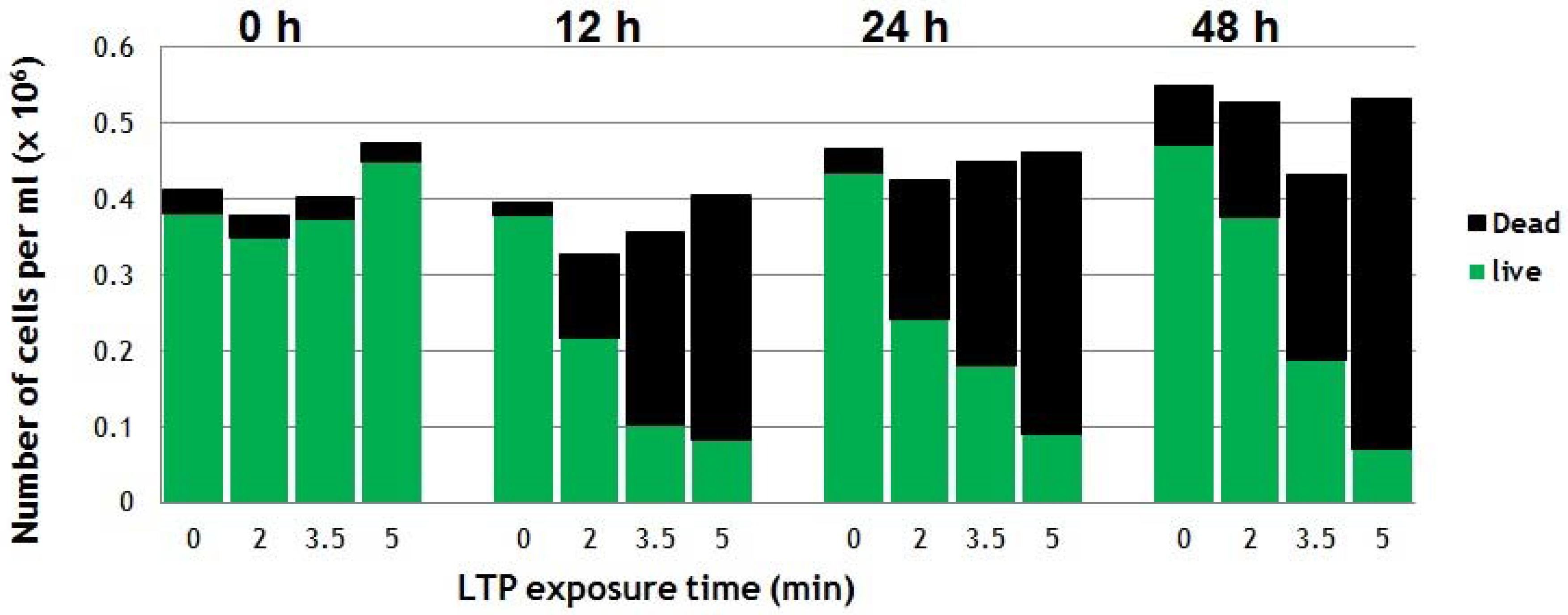
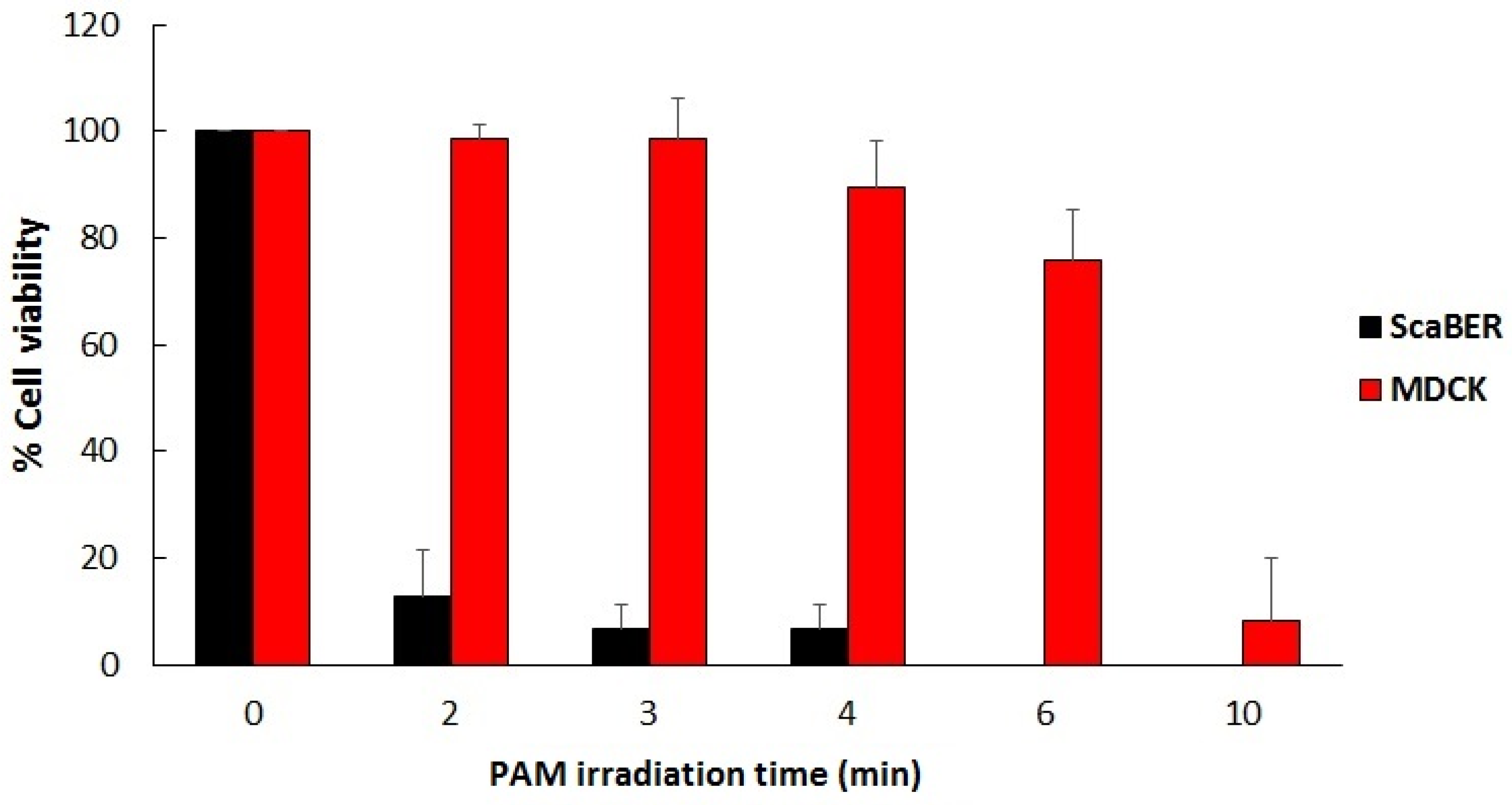
5. Two Biomedical Applications of LTP
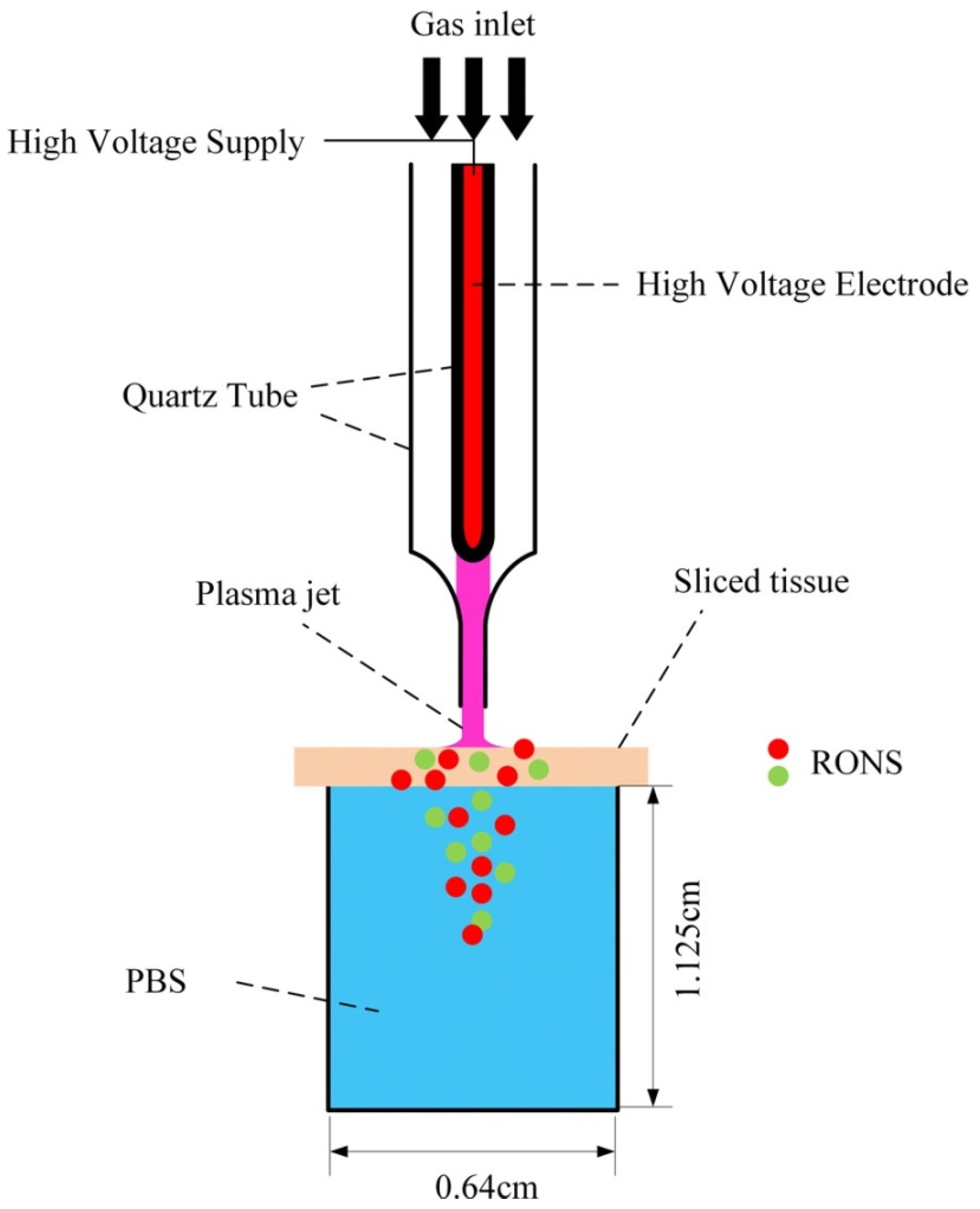
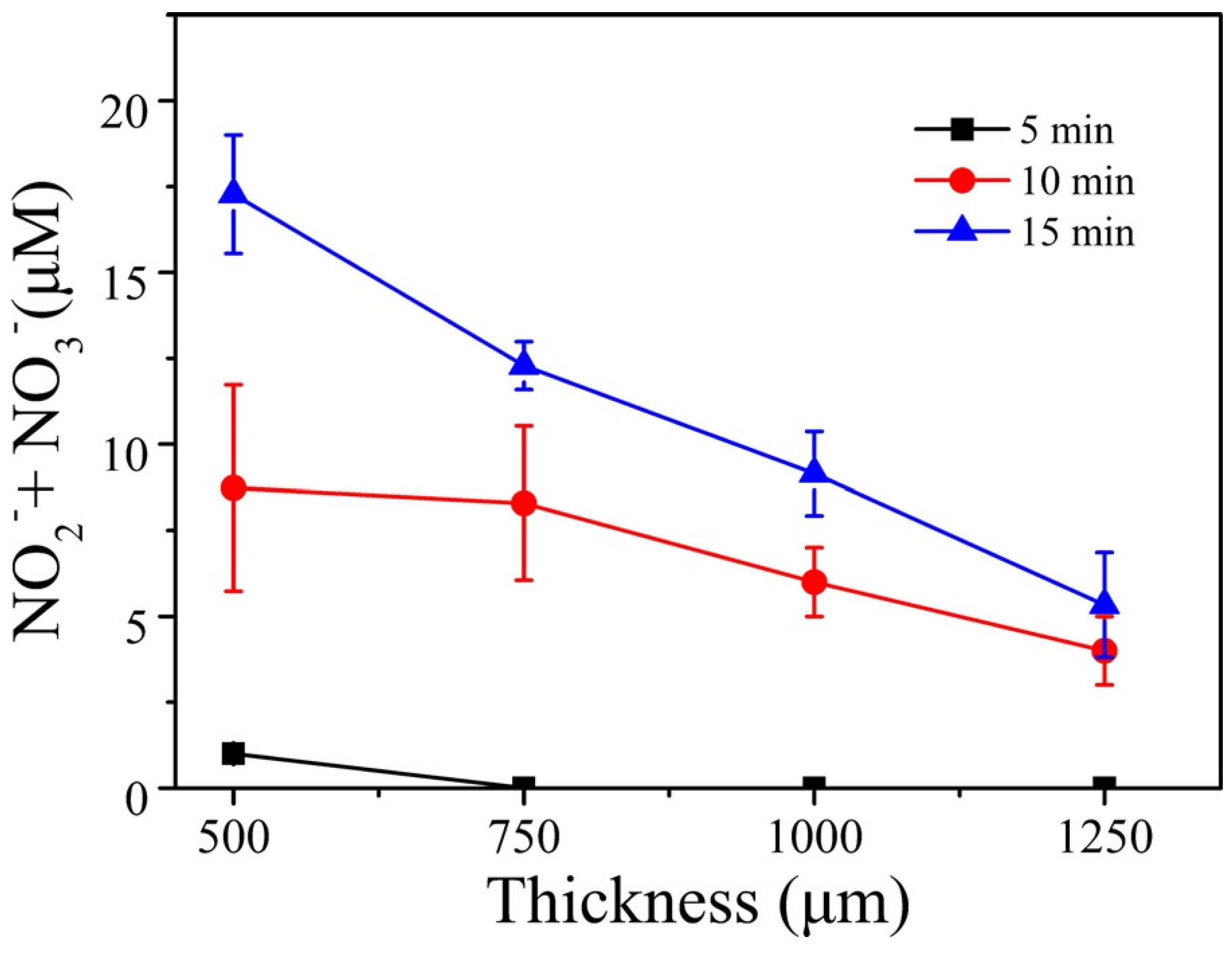
6. Penetration of RONS in Tissues
7. Conclusions
Conflicts of Interest
References
- Laroussi, M. Sterilization of Contaminated Matter with an Atmospheric Pressure Plasma. IEEE Trans. Plasma Sci. 1996, 24, 1188. [Google Scholar] [CrossRef]
- Garate, E.; Evans, K.; Gornostaeva, O.; Alexeff, I.; Kang, W.; Rader, M.; Wood, T. Atmospheric plasma induced sterilization and chemical neutralization. In Proceedings of the IEEE International Conference on Plasma Science, Raleigh, NC, USA, 1–4 June 1998. [Google Scholar] [CrossRef]
- Laroussi, M.; Sayler, G.; Galscock, B.; McCurdy, B.; Pearce, M.; Bright, N.; Malott, C. Images of biological samples undergoing sterilization by a glow discharge at atmospheric pressure. IEEE Trans. Plasma Sci. 1999, 27, 34. [Google Scholar] [CrossRef]
- Hermann, H.W.; Henins, I.; Park, J.; Selwyn, G.S. Decontamination of chemical and biological warfare (CBW) agents using an atmospheric pressure plasma jet (APPJ). Phys. Plasmas 1999, 6, 2284. [Google Scholar] [CrossRef]
- Birmingham, J.G.; Hammerstrom, D.J. Bacterial decontamination using ambient pressure nonthermal discharges. IEEE Trans. Plasma Sci. 2000, 28, 51. [Google Scholar] [CrossRef]
- Laroussi, M.; Alexeff, I.; Kang, W. Biological Decontamination by Non-thermal Plasma. IEEE Trans. Plasma Sci. 2000, 28, 184. [Google Scholar] [CrossRef]
- Montie, T.C.; Kelly-Wintenberg, K.; Roth, J.R. An overview of research using the one atmosphere uniform glow discharge plasma (OAUGDP) for sterilization of surfaces and materials. IEEE Trans. Plasma Sci. 2000, 28, 41. [Google Scholar] [CrossRef]
- Laroussi, M.; Richardson, J.P.; Dobbs, F.C. Effects of Non-Equilibrium Atmospheric Pressure Plasmas on the Heterotrophic Pathways of Bacteria and on their Cell Morphology. Appl. Phys. Lett. 2002, 81, 772. [Google Scholar] [CrossRef]
- Shekhter, A.B.; Kabisov, R.K.; Pekshev, A.V.; Kozlov, N.P.; Perov, Y.L. Experimental and Clinical Validation of Plasmadynamic Therapy of Wounds with Nitric Oxide. Bull. Exp. Biol. Med. 1998, 126, 829. [Google Scholar] [CrossRef]
- Stoffels, E.; Flikweert, A.J.; Stoffels, W.W.; Kroesen, G.M.W. Plasma Needle: A non-destructive Atmospheric Plasma Source for Fine Surface Treatment of Biomaterials. Plasma Sources. Sci. Technol. 2002, 11, 383. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Process. Polym. 2008, 5, 503. [Google Scholar] [CrossRef]
- Laroussi, M. Low Temperature Plasmas for Medicine. IEEE Trans. Plasma Sci. 2009, 37, 714. [Google Scholar] [CrossRef]
- Barekzi, N.; Laroussi, M. Effects of Low Temperature Plasmas on Cancer Cells. Plasma Process. Polym. 2013, 10, 1039. [Google Scholar] [CrossRef]
- Keidar, M.; Shashurin, A.; Volotskova, O.; Stepp, M.A.; Srinivasan, P.; Sandler, A.; Trink, B. Cold atmospheric plasma in cancer therapy. Phys. Plasmas 2013, 20, 057101. [Google Scholar] [CrossRef]
- Laroussi, M.; Mohades, S.; Barekzi, N. Killing of Adherent and non-adherent Cancer Cells by the Plasma Pencil. Biointerphases 2015, 10, 029410. [Google Scholar] [CrossRef]
- Laroussi, M. Non-Thermal Decontamination of Biological Media by Atmospheric Pressure Plasmas: Review, Analysis, and Prospects. IEEE Trans. Plasma Sci. 2002, 30, 1409. [Google Scholar] [CrossRef]
- Laroussi, M. Low Temperature Plasma-Based Sterilization: Overview and State-of-the-Art. Plasma Proc. Polym. 2005, 2, 391. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.-D. Plasma for Medicine. Phys. Rep. 2013, 530, 291. [Google Scholar] [CrossRef]
- Laroussi, M.; Kong, M.; Morfill, G.; Stolz, W. Plasma Medicine: Applications of Low Temperature Gas Plasmas in Medicine and Biology; Cambridge University Press: Cambridge, UK, 2012; ISBN 978-1-107-00643-0. [Google Scholar]
- Fridman, A.; Friedman, G. Plasma Medicine; Wiley: New York, NY, USA, 2013; ISBN 978-0-470-68970-7. [Google Scholar]
- Keidar, M.; Beilis, I.I. Plasma Engineering: Applications from Aerospace to Bio and Nanotechnology; Academic Press: London, UK, 2013; ISBN 978-0-12-385977-8. [Google Scholar]
- Laroussi, M. Interaction of Low Temperature Plasma with Prokaryotic and Eukaryotic cells. In Proceedings of the 61st Gaseous Electronics Conference, Dallas, TX, USA, 13–17 October 2008; American Physical Society: Ridge, NY, USA; p. 29. [Google Scholar]
- Isbary, G.; Morfill, G.; Schmidt, H.U.; Georgi, M.; Ramrath, K.; Heinlin, J. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br. J. Dermatol. 2010, 163, 78. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.D.; McCombs, G.B.; Akan, T.; Hynes, W.; Laroussi, M.; Tolle, S.L. Cold Plasma Technology: Bactericidal Effects on Geobacillus Stearothermophilus and Bacillus Cereus Microorganisms. J. Dental Hygiene 2009, 83, 55. [Google Scholar]
- Claiborne, D.; McCombs, G.B.; Lemaster, M.; Akman, M.A.; Laroussi, M. Low Temperature Atmospheric Pressure Plasma Enhanced Tooth Whitening: The Next Generation Technology. Int. J. Dent. Hygiene 2013. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M.; Barekzi, N. Effects of Low Temperature Plasma on Two Eukaryotic Cell Lines: Epithelial Cells and Prostate Cancer Cells. In Proceedings of the 31st ICPIG, Granada, Spain, 14–19 July 2013. [Google Scholar]
- Barekzi, N.; Laroussi, M. Fibropblasts Cell Morphology Altered by Low Temperature Atmospheric Pressure Plasma. IEEE Trans. Plasma Sci. 2014, 42, 2738. [Google Scholar] [CrossRef]
- Laroussi, M.; Karakas, E.; Hynes, W. Influence of Cell Type, Initial Concentration, and Medium on the Inactivation Efficiency of Low Temperature Plasma. IEEE Trans. Plasma Sci. 2011, 39, 2960. [Google Scholar] [CrossRef]
- Fridman, G.; Brooks, A.; Galasubramanian, M.; Fridman, A.; Gutsol, A.; Vasilets, V.; Ayan, H.; Friedman, G. Comparison of direct and indirect effects of non-thermal atmospheric-pressure plasma on bacteria. Plasma Process. Polym. 2007, 4, 370. [Google Scholar] [CrossRef]
- Shashurin, A.; Keidar, M.; Bronnikov, S.; Jurjus, R.A.; Stepp, M.A. Living tissue under treatment of cold plasma atmospheric jet. Appl. Phys. Lett. 2008, 93, 181501. [Google Scholar] [CrossRef]
- Laroussi, M.; VanWay, L.; Mohades, S.; Barekzi, N. Images of SCaBER Cells Treated by Low Temperature Plasma. IEEE Trans. Plasma Sci. 2014, 42, 2468. [Google Scholar] [CrossRef]
- Xiong, Z.; Cao, Y.; Lu, X.; Du, T. Plasmas in tooth root canal. IEEE Trans. Plasma Sci. 2011, 39, 2968. [Google Scholar] [CrossRef]
- Zimmermann, J.L.; Shimizu, T.; Boxhammer, V.; Morfill, G.E. Disinfection through different textiles using low-temperature atmospheric pressure plasma. Plasma Process. Polym. 2012, 9, 792. [Google Scholar] [CrossRef]
- Babaeva, N.; Kushner, M.J. Reactive fluxes delivered by dielectric barrier discharge filaments to slightly wounded skin. J. Phys. D: Appl. Phys. 2013, 46, 025401. [Google Scholar] [CrossRef]
- Weltmann, K.-D.; Kindel, E.; Brandenburg, R.; Meyer, C.; Bussiahn, C.; Wilke, C.; von Woedtke, T. Atmospheric Pressure Plasma Jet for Medical Therapy: Plasma Parameters and Risk Estimation. Contrib. Plasma Plasma Phys. 2009, 49, 631. [Google Scholar] [CrossRef]
- Ehlbeck, J.; Schnabel, U.; Polak, M.; Winter, J.; von Woedtke, T.; Brandenburg, R.; von dem Hagen, T.; Weltmann, K.-D. Low temperature atmospheric pressure plasma sources for microbial decontamination. J. Phys. D Appl. Phys. 2011, 44, 013002. [Google Scholar] [CrossRef]
- McCombs, G.B.; Darby, M.; Laroussi, M. “Dental Applications”. In Plasma Medicine: Applications of Low Temperature Gas Plasmas in Medicine and Biology; Laroussi, M., Kong, M., Morfill, G., Stolz, W., Eds.; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Utsumi, F.; Kjiyama, H.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Ishikawa, K.; Kondo, H.; Kano, H.; Hori, M.; Kikkawa, F. Effect of Indirect Nonequilibrium Atmospheric Pressure Plasma on Anti-Proliferative Activity against Chronic Chemo-Resistant Ovarian Cancer Cells In Vitro and In Vivo. PLoS ONE 2013, 8, e81576. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Mizuno, M.; Ishikawa, K.; Takeda, K.; Nakamura, K.; Utsumi, F.; Kajiyama, H.; Kano, H.; Okazaki, Y.; Toyokuni, S.; et al. Plasma Medical Science for Cancer Therapy: Toward Cancer Therapy Using Nonthermal Atmospheric Pressure Plasma. IEEE Trans. Plasma Sci. 2014, 42, 3760. [Google Scholar] [CrossRef]
- Mohades, S.; Barekzi, N.; Laroussi, M. Efficacy of Low Temperature Plasma against SCaBER Cancer Cells. Plasma Process. Polym. 2014, 11, 1150. [Google Scholar] [CrossRef]
- Laroussi, M. From Killing Bacteria to Destroying Cancer Cells: Twenty Years of Plasma Medicine. Plasma Process. Polym. 2014, 11, 1138. [Google Scholar] [CrossRef]
- Köritzer, J.; Boxhammer, V.; Schäfer, A.; Shimizu, T.; Klämpfl, T.G.; Li, Y.-F.; Welz, C.; Schwenk-Zieger, F.; Morfill, G.E.; Zimmermann, J.L.; et al. Restoration of sensitivity in chemo-resistant glioma cells by cold atmospheric plasma. PLos ONE 2013, 8, e64498. [Google Scholar]
- Schlegel, J.; Koritzer, J.; Boxhammer, V. Plasma in Cancer Treatment. Clin. Plasma Med. 2013, 1, 2. [Google Scholar] [CrossRef]
- Vandamme, M.; Robert, E.; Pesnele, S.; Barbosa, E.; Dozias, S.; Sobilo, J.; Lerondel, S.; Le Pape, A.; Pouvesle, J.-M. Antitumor Effects of Plasma Treatment on U87 Glioma Xenografts: Preliminary Results. Plasma Process. Polym. 2010, 7, 264. [Google Scholar] [CrossRef]
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.; Trink, B. Cold Plasma Selectivity and the Possibility of a Paradigm Shift in Cancer Therapy. Br. J. Cancer. 2011, 105, 1295. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M.; Keidar, M. Plasma & Cancer. Plasma Process. Polym. 2014, 11, 1118. [Google Scholar]
- Fridman, G.; Shereshevsky, A.; Jost, M.M.; Brooks, A.D.; Fridman, A.; Gutsol, A.; Vasilets, V.; Friedman, G. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in melanoma skin cancer cell lines. Plasma Chem. Plasma Process. 2007, 27, 163. [Google Scholar] [CrossRef]
- Volotskova, O.; Hawley, T.S.; Stepp, M.A.; Keidar, M. Targeting the cancer cell cycle by cold atmospheric plasma. Sci Rep. UK 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Bahn, J.H.; Lee, S-H.; Kim, G.-Y.; Jun, S.-I.; Lee, K.; Baek, S.J. Induction of cell growth arrest by atmospheric non-thermal plasma in colorectal cancer cells. J. Biotechnol. 2010, 150, 530. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Mizuno, M.; Ishikawa, K.; Nakamura, K.; Kajiyama, H.; Kano, H.; Kikkawa, F.; Hori, M. Plasma activated medium selectively kills glioblastoma brain tumor cells by down-regulating a survival signaling molecule, AKT kinase. Plasma Med. 2013, 1, 265. [Google Scholar] [CrossRef]
- Barekzi, N.; Laroussi, M. Dose-dependent killing of leukemia cells by low-temperature plasma. J. Phys. D Appl. Phys. 2012, 45, 422002. [Google Scholar] [CrossRef]
- Huang, J.; Li, H.; Chen, W.; Lu, G.-H.; Wang, X.-Q.; Zhang, G.-P.; Ostrikov, K.; Wang, P.-Y.; Yang, S.-Z. Dielectric barrier discharge plasma in Ar/O2 promoting apoptosis behavior in A549 cancer cells. Appl. Phys. Lett. 2011, 99, 253701. [Google Scholar] [CrossRef]
- Kim, Y.; Ballato, J.; Foy, P.; Hawkins, T.; Wei, Y.; Li, J.; Kim, S.O. Apoptosis of lung carcinoma cells induced by a flexible optical fiber-based cold microplasma. Biosens. Bioelectron. 2011, 28, 333. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Mizuno, M.; Ishikawa, K.; Nakamura, K.; Utsumi, F.; Kajiyama, H.; Kano, H.; Maruyama, S.; Kikkawa, F.; Hori, M. Cell survival and proliferation signaling pathways are downregulated by plasma activated medium in glioblastoma brain tumor cells. Plasma Med. 2012, 2, 207. [Google Scholar] [CrossRef]
- Tanaka, H.; Mizuno, M.; Kikkawa, F.; Hori, M. Interactions between a plasma-activated medium and cancer cells. Plasma Med. 2016, 6, 101. [Google Scholar] [CrossRef]
- Mohades, S.; Laroussi, M.; Sears, J.; Barekzi, N.; Razavi, H. Evaluation of the Effects of a Plasma Activated Medium on Cancer Cells. Phys. Plasmas 2015, 22, 122001. [Google Scholar] [CrossRef]
- Mohades, S.; Barekzi, N.; Razavi, H.; Maramuthu, V.; Laroussi, M. Temporal Evaluation of Antitumor Efficiency of Plasma Activated Media. Plasma Process. Polym. 2016, 13, 1206. [Google Scholar] [CrossRef]
- Mohades, S.; Laroussi, M.; Maruthamuthu, V. Moderate Plasma Activated Media Supresses Proliferation and Migration of MDCK Epithelial Cells. J. Phys. D Appl. Phys. 2017, 50, 185205. [Google Scholar] [CrossRef]
- Graves, D. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 263001. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive Species in Non-equilibrium Atmospheric Pressure Plasma: Generation, Transport, and Biological Effects. Phys. Rep. 2016, 630, 1. [Google Scholar] [CrossRef]
- Laroussi, M.; Lu, X.; Keidar, M. Perspective: The Physics, Diagnostics, and Applications of Atmospheric Pressure Low Temperature Plasma Sources Used in Plasma Medicine. J. Appl. Phys. 2017, 122, 020901. [Google Scholar] [CrossRef]
- Zhao, S.; Xiong, Z.; Mao, X.; Meng, D.; Lei, Q.; Li, Y.; Deng, P.; Chen, M.; Tu, M.; Lu, X.; et al. Atmospheric Pressure Room Temperature Plasma Jets Facilitate Oxidative and Nitrative Stress and Lead to Endoplasmic Reticulum Stress Dependent Apoptosis in HepG2 Cells. PLOS ONE 2013, 8, e73665. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zou, F.; Zhao, S.; Lu, X.; He, G.; Xiong, Z.; Xiong, Q.; Zhao, Q.; Deng, P.; Huang, J.; et al. On the Mechanism of Plasma Inducing Cell Apoptosis. IEEE Trans. Plasma Sci. 2010, 38, 2451. [Google Scholar] [CrossRef]
- Yan, X.; Xiong, Z.; Zou, F.; Zhao, S.; Lu, X.; Yang, G.; He, G.; Ostrikov, K. Plasma-Induced Death of HepG2 Cancer Cells: Intracellular Effects of Reactive Species. Plasma Process. Polym. 2012, 9, 59. [Google Scholar] [CrossRef]
- Ishaq, M.; Evans, M.; Ostrikov, K. Effects of Atmospheric gas Plasmas on Cancer Cell Signaling. Int. J. Cancer 2014, 134, 1517. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, M.; Kumar, S.; Varinli, H.; Han, Z.J.; Rider, A.E.; Evans, M.; Murphy, A.B.; Ostrokov, K. Atmospheric Gas Plasma-Induced ROS Production Activates TNS-ASK1 Pathway for the Induction of Melanoma Cancer Cell Apoptosis. Mol. Biol. Cells 2014, 25, 1523. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-S.; Szili, E.J.; Gaur, N.; Hong, S.-H.; Futura, H.; Kurita, H.; Mizuno, A.; Hatta, A.; Short, R.D. How to assess the plasma delivery of RONS into tissue fluid and tissue. J. Phys. D Appl. Phys. 2016, 49, 304005. [Google Scholar] [CrossRef]
- Hong, S.-H.; Szili, E.J.; Toby, A.; Jenkins, A.; Short, R.D. Ionized gas (plasma) delivery of reactive oxygen species (ROS) into artificial cells. J. Phys. D Appl. Phys. 2014, 47, 362001. [Google Scholar] [CrossRef]
- Szili, E.J.; Bradley, J.W.; Short, R.D. A ‘tissue model’ to study the plasma delivery of reactive oxygen species. J. Phys. D Appl. Phys. 2014, 47, 152002. [Google Scholar] [CrossRef]
- Gaur, N.; Szili, E.J.; Oh, J.; Hong, S.; Michelmore, A.; Graves, D.B.; Hatta, A.; Short, R.D. Combined effect of protein and oxygen on reactive oxygen and nitrogen species in the plasma treatment of tissue. Appl. Phys. Lett. 2015, 107, 103703. [Google Scholar] [CrossRef]
- He, T.; Liu, D.; Xu, H.; Liu, Z.; Xu, D.; Li, D.; Li, Q.; Rong, M.; Kong, M. A ‘tissue model’ to study the barrier effects of living tissues on the reactive species generated by surface air discharge. J. Phys. D Appl. Phys. 2016, 49, 205204. [Google Scholar] [CrossRef]
- Duan, J.; Lu, X.; He, G. On the penetration depth of reactive oxygen and nitrogen species generated by a plasma jet through real biological tissue. Phys. Plasmas 2017, 24, 073506. [Google Scholar] [CrossRef]
- Begum, A.; Laroussi, M.; Pervez, M.R. Atmospheric Pressure helium/air plasma Jet: Breakdown Processes and Propagation Phenomenon. AIP Adv. 2013, 3, 062117. [Google Scholar] [CrossRef]
- Sobota, A.; Guaitella, O.; Garcia-Caurel, E. Experimentally obtained values of electric field of an atmospheric pressure plasma jet impinging on a dielectric surface. J. Phys. D Appl. Phys. 2013, 46, 372001. [Google Scholar] [CrossRef]
- Stretenovic, G.B.; Krstic, I.B.; Kovacevic, V.V.; Obradovic, A.M.; Kuraica, M.M. Spatio-temporally resolved electric field measurements in helium plasma jet. J. Phys. D Appl. Phys. 2014, 47, 102001. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, S.; Cheng, W.; Lu, X. Electric field measurements in an atmospheric-pressure microplasma jet using Stark polarization emission spectroscopy of helium atom. Eur. Phys. J. Spec. Top. 2017, 226, 2979. [Google Scholar] [CrossRef]
- Pervez, M.R.; Begum, A.; Laroussi, M. Plasma Based Sterilization: Overview and the Stepwise Inactivation Process of Microbial by Non-thermal Atmospheric Pressure Plasma Jet. Int. J. Eng. Technol. 2014, 14, 7. [Google Scholar]
- Kogelschatz, U. Dielectric-Barrier Discharges: Their History, Discharge Physics, and Industrial Applications. Plasma Chem. Plasma Proc. 2003, 23, 1. [Google Scholar] [CrossRef]
- Brandenburg, R. Dielectric barrier discharges: progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci. Technol. 2017, 26, 053001. [Google Scholar] [CrossRef]
- Laroussi, M.; Lu, X. Room Temperature Atmospheric Pressure Plasma Plume for Biomedical Applications. Appl. Phys. Lett. 2005, 87, 113902. [Google Scholar] [CrossRef]
- Lu, X.; Laroussi, M.; Puech, V. On Atmospheric Pressure Non-equilibrium Plasma Jets and Plasma Bullets. Plasma Sources Sci. Technol. 2012, 21, 034005. [Google Scholar] [CrossRef]
- Weltmann, K.-D.; Kindel, E.; von Woedtke, T.; Hähnel, M.; Stieber, M.; Brandenburg, R. Atmospheric-pressure plasma sources: Prospective tools for plasma medicine. Pure Appl. Chem. 2010, 82, 1223. [Google Scholar] [CrossRef]
- Teschke, M.; Kedzierski, J.; Finantu-Dinu, E.G.; Korzec, D.; Engemann, J. High Speed Photographs of a Dielectric Barrier Atmospheric Pressure Plasma Jet. IEEE Trans. Plasma Sci. 2005, 33, 310. [Google Scholar] [CrossRef]
- Lu, X.; Laroussi, M. Dynamics of an Atmospheric Pressure Plasma Plume Generated by Submicrosecond Voltage Pulses. J. Appl. Phys. 2006, 100, 063302. [Google Scholar] [CrossRef]
- Mericam-Bourdet, N.; Laroussi, M.; Begum, A.; Karakas, E. Experimental Investigations of Plasma Bullets. J. Phys. D Appl. Phys. 2009, 42, 055207. [Google Scholar] [CrossRef]
- Sands, B.L.; Ganguly, B.N.; Tachibana, K. A Streamer-like Atmospheric Pressure Plasma Jet. Appl. Phys. Lett. 2008, 92, 151503. [Google Scholar] [CrossRef] [Green Version]
- Karakas, E.; Koklu, M.; Laroussi, M. Correlation between helium mole fraction and plasma bullet propagation in low temperature plasma jets. J. Phys. D Appl. Phys. 2010, 43, 155202. [Google Scholar] [CrossRef]
- Boeuf, J.-P.; Yang, L.; Pitchford, L. Dynamics of guided streamer (plasma bullet) in a helium jet in air at atmospheric pressure. J. Phys. D Appl. Phys. 2013, 46, 015201. [Google Scholar] [CrossRef]
- Naidis, G. Modeling of streamer propagation in atmospheric-pressure helium plasma jets. J. Phys. D Appl. Phys. 2010, 43, 402001. [Google Scholar] [CrossRef]
- Yousfi, M.; Eichwald, O.; Merbahi, N.; Jomma, N. Analysis of ionization wave dynamics in low-temperature plasma jets from fluid modeling supported by experimental investigations. Plasma Sources Sci. Technol. 2012, 21, 045003. [Google Scholar] [CrossRef]
- Breden, D.; Miki, K.; Raja, L.L. Self-consistent 2D modeling of cold atmospheric-pressure plasma jets/bullets. Plasma Sources Sci. Technol. 2012, 21, 034011. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.; Laroussi, M.; Ostrikov, K. Guided Ionization Waves: Theory and Experiments. Physics Reports 2014, 540, 123. [Google Scholar] [CrossRef]
- Laroussi, M. Low temperature plasma jet for biomedical applications: A review. IEEE Trans. Plasma Sci. 2015, 43, 703. [Google Scholar] [CrossRef]
- Szili, E.J.; Gaur, N.; Hong, S.-H.; Kurita, H.; Oh, J.-S.; Ito, M.; Mizuno, A.; Hatta, A.; Cowin, A.J.; Graves, D.B.; et al. The assessment of cold atmospheric plasma treatment of DNA in synthetic models of tissue fluid, tissue and cells. J. Phys. D Appl. Phys. 2017, 50, 274001. [Google Scholar] [CrossRef]
- Ray, A.; Ranieri, P.; Karamchand, L.; Yee, B.; Foster, J.; Kopleman, R. Real-Time Monitoring of Intracellular Chemical Changes in Response to Plasma Irradiation. Plasma Med. 2017, 7, 7. [Google Scholar] [CrossRef]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laroussi, M. Plasma Medicine: A Brief Introduction. Plasma 2018, 1, 47-60. https://doi.org/10.3390/plasma1010005
Laroussi M. Plasma Medicine: A Brief Introduction. Plasma. 2018; 1(1):47-60. https://doi.org/10.3390/plasma1010005
Chicago/Turabian StyleLaroussi, Mounir. 2018. "Plasma Medicine: A Brief Introduction" Plasma 1, no. 1: 47-60. https://doi.org/10.3390/plasma1010005




