Diazonium Salts: Versatile Molecular Glues for Sticking Conductive Polymers to Flexible Electrodes
Abstract
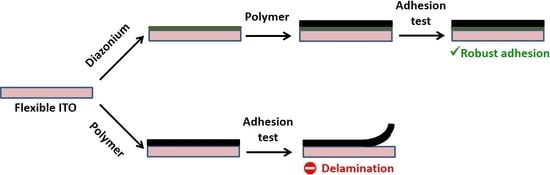
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Diazonium Modification of Flexible ITO
2.3. Preparation and Electrochemical Characterization of Polypyrrole Films on Diazonium-Modified ITO
2.4. Surface Analysis
3. Results and Discussion
3.1. Brief Description of the Strategy and the Objectives of the Work
3.2. Electrochemical Modification of Flexible ITO Sheets with Diazonium Salts
3.3. Electrodeposition of Polypyrrole on ITO
3.3.1. Electrochemical Characterization of Poly(pyrrole-benzene sulfonic acid) Films on Aryl-Modified ITO
3.3.2. XPS Characterization of PPy Top Coats and Reference Materials
3.4. Practical Adhesion Aspects of Polypyrrole Thin Layers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MacDiarmid, A.G. Polyaniline and polypyrrole: Where are we headed? Synth. Met. 1997, 84, 27–34. [Google Scholar] [CrossRef]
- George, P.M.; Lyckman, A.W.; LaVan, D.A.; Hegde, A.; Leung, Y.; Avasare, R.; Testa, C.; Alexander, P.M.; Langer, R.; Sur, M. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials 2005, 26, 3511–3519. [Google Scholar] [CrossRef] [PubMed]
- Bharti, M.; Singh, A.; Samanta, S.; Debnath, A.K.; Aswal, D.K.; Muthe, K.P.; Gadkari, S.C. Flexo-green Polypyrrole—Silver nanocomposite films for thermoelectric power generation. Energy Convers. Manag. 2017, 144, 143–152. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Zhou, L.; Huang, X.; Yu, C. Polypyrrole-coated zinc ferrite hollow spheres with improved cycling stability for lithium-ion batteries. Small 2016, 12, 3732–3737. [Google Scholar] [CrossRef] [PubMed]
- Omastová, M.; Trchová, M.; Kovárová, J.; Stejskal, J. Synthesis and structural study of polypyrroles prepared in the presence of surfactants. Synth. Met. 2003, 138, 447–455. [Google Scholar] [CrossRef]
- Sadki, S.; Schottland, P.; Brodie, N.; Sabouraud, G. The mechanisms of pyrrole electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar]
- Cruz-Silva, R.; Amaro, E.; Escamilla, A.; Nicho, M.E.; Sepulveda-Guzman, S.; Arizmendi, L.; Romero-Garcia, J.; Castillon-Barraza, F.F.; Farias, M.H. Biocatalytic synthesis of polypyrrole powder colloids and films using horseradish peroxidase. J. Colloid Interface Sci. 2008, 328, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Cabet, E.; Lilienbaum, A.; Hamadi, S.; Abderrabba, M.; Chehimi, M.M. Polypyrrole/Ag/mesoporous silica nanocomposite particles: Design by photopolymerization in aqueous medium and antibacterial activity. J. Taiwan Inst. Chem. Eng. 2017, 80, 1022–1030. [Google Scholar] [CrossRef]
- Hamouma, O.; Oukil, D.; Omastová, M.; Chehimi, M.M. Flexible paper@carbonnanotube@polypyrrole composites: The combined pivotal roles of diazonium chemistry and sonochemicalpolymerization. Colloids Surf. Physicochem. Eng. Asp. 2018, 538, 350–360. [Google Scholar] [CrossRef]
- Omastová, M.; Mičušík, M. Polypyrrole coating of inorganic and organic materials by chemical oxidative polymerization. Chem. Pap. 2012, 66, 392–414. [Google Scholar] [CrossRef]
- Baibarac, M.; Gómez-Romero, P. Nanocomposites based on conducting polymers and carbon nanotubes: From fancy materials to functional applications. J. Nanosci. Nanotechnol. 2006, 6, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Sung, H.; Han, S.; Paik, W. Polypyrrolefilm formation by solution-surface electropolymerization: Influence of solvents and doped anions. J. Phys. Chem. 1994, 98, 1250–1252. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Aeiyach, S.; Delamar, M.; Lacaze, P.C. Electropolymerization of pyrrole on iron electrodes: Influence of solvent and electrolyte on the nature of the deposits. J. Electroanal. Chem. Interfacial Electrochem. 1990, 284, 351–369. [Google Scholar] [CrossRef]
- Kaplin, D.A.; Qutubuddin, S. Electrochemically synthesized polypyrrolefilms: Effects of polymerization potential and electrolyte type. Polymer 1995, 36, 1275–1286. [Google Scholar] [CrossRef]
- Raso, M.A.; González-Tejera, M.J.; Carrillo, I.; Sanchez De La Blanca, E.; García, M.V.; Redondo, M.I. Electrochemical nucleation and growth of poly-N-methylpyrrole on copper. Thin Solid Films 2015, 19, 2387–2392. [Google Scholar] [CrossRef]
- Sharifirad, M.; Omrani, A.; Rostami, A.A.; Khoshroo, M. Electrodeposition and characterization of polypyrrolefilms on copper. J. Electroanal. Chem. 2010, 645, 149–158. [Google Scholar] [CrossRef]
- Alfaro-López, H.M.; Aguilar-Hernandez, J.R.; Garcia-Borquez, A.; Hernandez-Perez, M.A.; Contreras-Puente, G.S. Electropolymerization of polypyrrole films in aqueous solution with side-coupler agent to hydrophobic groups. Interface Control. Org. Thin Films 2009, 129, 73–78. [Google Scholar]
- Wang, Y.; Northwood, D.O. An investigation into the nucleation and growth of an electropolymerized polypyrrole coating on a 316L stainless steel surface. Thin Solid Films 2008, 516, 7427–7432. [Google Scholar] [CrossRef]
- Castro-Beltran, A.; Dominguez, C.; Bahena-Uribe, D.; Sepulveda-Guzmana, S.; Cruz-Silva, R. Effect of non-electroactive additives on the early stage pyrrole electropolymerization on indium tin oxide electrodes. Thin Solid Films 2014, 566, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Salmi, Z.; Joshi, N.; Jha, P.; Kumar, A.; Lecoq, H.; Lau, S.; Chehimi, M.M.; Aswal, D.K.; Gupta, S.K. Photo-induced synthesis of polypyrrole-silver nanocomposite films on N-(3-trimethoxysilylpropyl)pyrrole-modified biaxially oriented polyethylene terephthalate flexible substrates. RSC Adv. 2013, 3, 5506–5523. [Google Scholar] [CrossRef]
- Bouktit, B.; Salmi, Z.; Decorse, P.; Lecocq, H.; Jouini, M.; Aswal, D.; Singh, A.; Chehimi, M.M. Polypyrrole/Ag nanocomposite films on diazonium salt modified indium tin oxide substrate. J. Colloid Sci. Biotechnol. 2013, 2, 200–210. [Google Scholar] [CrossRef]
- Lo, M.; Diaw, A.K.D.; Gningue-Sall, D.; Aaron, J.-J.; Oturan, M.A.; Chehimi, M.M. The role of diazonium interface chemistry in the design of high performance polypyrrole-coated flexible ITO sensing electrodes. Electrochem. Commun. 2017, 77, 14–17. [Google Scholar] [CrossRef]
- Patterson, N.; Ignaszak, A. Modification of glassy carbon with polypyrrole through an aminophenyl linker to create supercapacitive materials using bipolar electrochemistry. Electrochem. Commun. 2018, 93, 10–14. [Google Scholar] [CrossRef]
- Mevellec, V.; Rousse, S.; Tessier, L.; Chancolon, J.; Mayne-L’Hermite, M.; Deniau, G.; Viel, P.; Palacin, S. Grafting polymers on surfaces: A new powerful and versatile diazonium salt-based one-step process in aqueous media. Chem. Mater. 2007, 19, 6323–6330. [Google Scholar] [CrossRef]
- Viel, P.; Le, X.T.; Huc, V.; Bar, J.; Benedetto, A.; Le Goff, A.; Filoramo, A.; Alamarguy, D.; Noël, S.; Baraton, L.; et al. Covalent grafting onto self-adhesive surfaces based on aryldiazonium salt seed layers. J. Mater. Chem. 2008, 18, 5913–5920. [Google Scholar] [CrossRef] [Green Version]
- Alageel, O.; Abdallah, M.N.; Luo, Z.Y.; Del-Rio-Highsmith, J.; Cerruti, M.; Tamimi, F. Bonding metals to poly(methyl methacrylate) using aryldiazonium salts. Dent. Mater. 2015, 31, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.F.; Rattana, A.; Abel, M.-L. Interfacial chemistry of adhesives on hydrated aluminium and hydrated aluminium treated with an organosilane. Surf. Interface Anal. 2004, 36, 1449–1468. [Google Scholar] [CrossRef]
- Jesson, D.A.; Abel, M.-L.; Hay, J.N.; Smith, P.A.; Watts, J.F. Organic-inorganic hybrid nanoparticles: Surface characteristics and interactions with a polyester resin. Langmuir 2006, 22, 5144–5151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tashiro, H.; Nakaya, M.; Hotta, A. Enhancement of the gas barrier property of polymers by DLC coating with organosilane interlayer. Diam. Relat. Mater. 2013, 35, 7–13. [Google Scholar] [CrossRef]
- Bélanger, D.; Pinson, J. Electrografting: A powerful method for surface modification. Chem. Soc. Rev. 2011, 40, 3995–4048. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Firestone, M.A.; Auciello, O.; Carlisle, J.A. Surface functionalization of ultrananocrystalline diamond films by electrochemical reduction of aryldiazonium salts. Langmuir 2004, 20, 11450–11456. [Google Scholar] [CrossRef] [PubMed]
- Mirkhalaf, F.; Graves, J.E. Nanostructured electrocatalysts immobilised on electrode surfaces and organic film templates. Chem. Pap. 2012, 66, 472–483. [Google Scholar] [CrossRef]
- Bakas, I.; Yilmaz, G.; Ait-Touchente, Z.; Lamouri, A.; Lang, P.; Battaglini, N.; Carbonnier, B.; Chehimi, M.M.; Yagci, Y. Diazonium salts for surface-confined visible light radical photopolymerization. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3506–3515. [Google Scholar] [CrossRef]
- Stockhausen, V.; Nguyen, V.Q.; Martin, P.; Lacroix, J.-C. Botom-up electrochemical fabrication of conjugated ultrathin layers with tailored switchable properties. ACS Appl. Mater. Interfaces 2017, 9, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fantin, M.; Park, S.; Gottlieb, E.; Fu, L.; Matyjaszewski, K. Electrochemically mediated reversible addition−fragmentation chain-transfer polymerization. Macromolecules 2017, 50, 7872–7879. [Google Scholar] [CrossRef] [PubMed]
- Gui, A.L.; Luais, E.; Peterson, J.R.; Godding, J.J. Zwitterionic phenyl layers: Finally, stable, anti-biofouling coatings that do not passivate electrodes. ACS Appl. Mater. Interfaces 2013, 5, 4827–4835. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Zhang, Y.; Jiang, C.; Qi, M.; Liu, G. Advances on aryldiazonium salt chemistry based interfacial fabrication for sensing applications. ACS Appl. Mater. Interfaces 2017, 9, 5031–5049. [Google Scholar] [CrossRef] [PubMed]
- Guselnikova, O.; Postnikov, P.; Elashnikova, R.; Trusova, M.; Kalachyova, Y.; Libansky, M.; Barek, J.; Kolska, Z.; Švorčík, V.; Lyutakov, O. Surface modification of Au and Ag plasmonic thin films via diazonium chemistry: Evaluation of structure and properties. Colloids Surf. A Physicochem. Eng. Asp. 2017, 516, 274–285. [Google Scholar] [CrossRef]
- Jlassi, K.; Chandran, S.; Poothanari, M.A.; Benna-Zayani, M.; Thomas, S.; Chehimi, M.M. Clay/polyaniline hybrid through diazonium chemistry: Conductive nanofiller with unusual effects on interfacial properties of epoxy nanocomposites. Langmuir 2016, 32, 3514–3524. [Google Scholar] [CrossRef] [PubMed]
- Sandomierski, M.; Strzemiecka, B.; Chehimi, M.M.; Voelkel, A. Reactive diazonium-modified silica fillers for high-performance polymers. Langmuir 2016, 32, 11646–11654. [Google Scholar] [CrossRef] [PubMed]
- Jacques, A.; Chehimi, M.M.; Poleunis, C.; Delcorte, A.; Delhalle, J.; Mekhalif, Z. Grafting of 4-pyrrolyphenyldiazonium in situ generated on NiTi, an adhesion promoter for pyrrole electropolymerisation. Electrochim. Acta 2016, 211, 879–890. [Google Scholar] [CrossRef]
- Kullapere, M.; Mirkhalaf, F.; Tammeveski, K. Electrochemical behaviour of glassy carbon electrodes modified with aryl groups. Electrochim. Acta 2010, 56, 166–173. [Google Scholar] [CrossRef]
- Jacques, A.; Devillers, S.; Delhalle, J.; Mekhalif, Z. Electrografting of in situ generated pyrrole derivative diazonium salt for the surface modification of nickel. Electrochim. Acta 2013, 109, 781–789. [Google Scholar] [CrossRef]
- Shul, G.; Weissmann, M.; Bélanger, D. Electrochemical characterization of glassy carbon electrode modified with 1,10-phenanthroline groups by two pathways: Reduction of the corresponding diazonium ions and reduction of phenanthroline. Electrochim. Acta 2015, 162, 146–155. [Google Scholar] [CrossRef]
- Seck, S.M.; Charvet, S.; Fall, M.; Baudrin, E.; Geneste, F.; Lejeune, M.; Benlahsen, M. Functionalization of amorphous nitrogenated carbon thin film electrodes for improved detection of cadmium vs. copper cations. J. Electroanal. Chem. 2015, 738, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Moraes Silva, S.; Fan, S.; Wu, Y.; Tanzirul Alam, M.; Liu, G.; Gooding, J.J. Aryldiazonium salt derived mixed organic layers: From surface chemistry to their applications. J. Electroanal. Chem. 2017, 785, 265–278. [Google Scholar] [CrossRef]
- Samanta, S.; Bakas, I.; Singh, A.; Aswal, D.K.; Chehimi, M.M. In situ diazonium-modified flexible ITO-coated PEN substrates for the deposition of adherent silver–polypyrrole nanocomposite films. Langmuir 2014, 30, 9397–9406. [Google Scholar] [CrossRef] [PubMed]
- Baranton, S.; Belanger, D. Electrochemical derivatization of carbon surface by reduction of in situ generated diazonium cations. J. Phys. Chem. B 2005, 109, 24401–24410. [Google Scholar] [CrossRef] [PubMed]
- Downard, A.J.; Prince, M.J. Barrier properties of organic monolayers on glassy carbon electrodes. Langmuir 2001, 17, 5581–5586. [Google Scholar] [CrossRef]
- Wu, J.-S.; Gu, D.-W.; Huang, D.; Shen, L.-J. Chemical in situ polymerization of polypyrrole nanoparticles on the hydrophilic/hydrophobic surface of SiO2 substrates. Synth. React. Inorg. Met. Org. Nano Met. Chem. 2012, 42, 1064–1070. [Google Scholar] [CrossRef]
- Wang, P.C.; Huang, Z.; MacDiarmid, A.G. Critical dependency of the conductivity of polypyrrole and polyaniline films on the hydrophobicity/hydrophilicity of the substrate surface. Synth. Met. 1999, 101, 852–853. [Google Scholar] [CrossRef]
- Perruchot, C.; Chehimi, M.M.; Delamar, M.; Cabet-Deliry, E.; Miksa, B.; Slomkowski, S.; Khan, M.A.; Armes, S.P. Chemical deposition and characterization of thin polypyrrole films on glass plates: Role of organosilane treatment. Colloid Polym. Sci. 2000, 278, 1139–1154. [Google Scholar] [CrossRef]
- Azioune, A.; Chehimi, M.M.; Miksa, B.; Basinska, T.; Slomkowski, S. Hydrophobic protein−polypyrrole interactions: The role of van der Waals and Lewis acid−base forces as determined by contact angle measurements. Langmuir 2002, 18, 1150–1156. [Google Scholar] [CrossRef]
- AïtAtmane, Y.; Sicard, L.; Lamouri, A.; Pinson, J.; Sicard, M.; Masson, C.; Nowak, S.; Decorse, P.; Piquemal, J.-Y.; Galtayries, A.; et al. Functionalization of aluminum nanoparticles using a combination of aryl diazonium salt chemistry and iniferter method. J. Phys. Chem. C 2013, 117, 26000–26006. [Google Scholar] [CrossRef]
- Jacques, A.; Devillers, S.; Arrotin, B.; Delhalle, J.; Mekhalif, Z. Polyelectrolyte multilayers deposition on Nitinol modified by in situ generated diazonium in gentle conditions. J. Electrochem. Soc. 2014, 161, G55–G62. [Google Scholar] [CrossRef]










| Surface | Irel (%) | Rct (kΩ/cm2) | θ (°) |
|---|---|---|---|
| ITO-SO3H | 3.90 | 68.8 | 48.7 ± 0.5 |
| ITO-CO2H | 11.2 | 47.3 | 70.7 ± 0.4 |
| ITO-CN | 15.7 | 44.0 | 81.2 ± 0.3 |
| ITO-NH-Ph | 23.6 | 9.32 | 103.1 ± 0.3 |
| ITO-NH2 | 46.6 | 8.97 | 116 ± 0.4 |
| ITO-N(CH3)2 | 48.6 | 7.15 | 136.7 ± 0.1 |
| Electrode Material | Eox (V) | Ered (V) | Jpox (µA/cm2) |
|---|---|---|---|
| ITO-N(CH3)2 | 0.25 | −0.53 | 972 |
| ITO-NH2 | 0.27 | −0.52 | 960 |
| ITO-NH-Ph | 0.2 | −0.5 | 936 |
| ITO-CN | 0.25 | −0.58 | 208 |
| ITO-CO2H | 0.41 | −0.59 | 908 |
| ITO-SO3H | 0.24 | −0.43 | 228 |
| Materials | C | N | S | O | In | Sn | In4d/In3d |
|---|---|---|---|---|---|---|---|
| ITO | 32.5 | - | - | 39.1 | 25.4 | 3.06 | 0.155 |
| ITO-SO3H | 45.2 | 0.77 | 1.00 | 30.0 | 20.5 | 2.43 | 0.167 |
| ITO-SO3H-PPy | 73.9 | 9.29 | 1.38 | 15.4 | - | - | |
| ITO-COOH | 58.6 | 1.68 | - | 25.1 | 11.3 | 3.34 | 0.174 |
| ITO-COOH-PPy | 78.7 | 10.5 | 1.13 | 9.71 | - | - | |
| ITO-CN | 71.4 | 4.91 | - | 16.8 | 5.88 | 1.04 | 0.179 |
| ITO-CN-PPy | 75.2 | 9.23 | 2.12 | 13.5 | - | - | |
| ITO-NH-Ph | 49.2 | 1.97 | - | 29.6 | 16.2 | 3.05 | 0.171 |
| ITO-NH-Ph-PPy | 75.5 | 9.83 | 1.71 | 12.9 | - | - | |
| ITO-NH2 | 42.9 | 1.31 | - | 33.1 | 16.2 | 6.55 | 0.159 |
| ITO-NH2-PPy | 77.3 | 12.2 | 2.22 | 8.22 | - | - | |
| ITO-N(CH3)2 | 52.7 | 1.13 | - | 28.2 | 15.5 | 2.48 | 0.181 |
| ITO-N(CH3)2-PPy | 76.5 | 6.94 | 1.18 | 15.4 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, M.; Pires, R.; Diaw, K.; Gningue-Sall, D.; Oturan, M.A.; Aaron, J.-J.; Chehimi, M.M. Diazonium Salts: Versatile Molecular Glues for Sticking Conductive Polymers to Flexible Electrodes. Surfaces 2018, 1, 43-58. https://doi.org/10.3390/surfaces1010005
Lo M, Pires R, Diaw K, Gningue-Sall D, Oturan MA, Aaron J-J, Chehimi MM. Diazonium Salts: Versatile Molecular Glues for Sticking Conductive Polymers to Flexible Electrodes. Surfaces. 2018; 1(1):43-58. https://doi.org/10.3390/surfaces1010005
Chicago/Turabian StyleLo, Momath, Rémi Pires, Karim Diaw, Diariatou Gningue-Sall, Mehmet A. Oturan, Jean-Jacques Aaron, and Mohamed M. Chehimi. 2018. "Diazonium Salts: Versatile Molecular Glues for Sticking Conductive Polymers to Flexible Electrodes" Surfaces 1, no. 1: 43-58. https://doi.org/10.3390/surfaces1010005