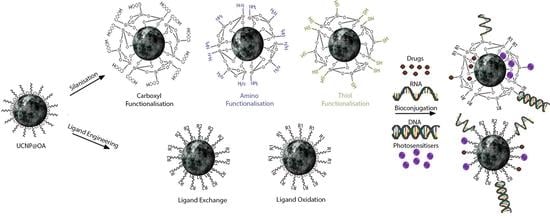
Ligand engineering comprises two methods of surface modification. The first being the oxidation of ligands present on UCNPs surfaces (e.g., oleate) via an oxidiser such as the Lemieux-von Rudloff reagent or ozone.
Figure 1 shows a typical ligand oxidation process, which could result in terminal carboxylates, aldehydes or epoxides [
5,
17,
40]. The oxidative alteration of ligands is not a commonly used approach to UCNPs surface modification due to the poor dispersal in aqueous media of resultant UCNPs and the limited variability in functional moieties [
4,
5,
29]. Although ligand oxidation exhibits no influence on morphology or size of UCNPs [
41,
42], work conducted by Naccache, R. et al. (2009) [
43] determined that prolonged oxidation (>2 h) of oleate ligands on NaGdF
4: Yb
3+, Ho
3+ UCNPs resulted in an MnO
2 precipitate that was challenging to remove, reduced the hydrophilicity of the UCNPs in water and significantly weakened the luminescent emission of the particles. Interestingly, Deng, R. et al. (2011) [
42] found that the addition of glutathione (GSH) in low amounts to MnO
2-modified NaYF
4: Yb
3+, Tm
3+ UCNPs led to a substantial recovery of emission from the particles. It is asserted that the enhancement of the optical properties was a result of GSH-mediated reduction of MnO
2 to Mn
2+. Therefore, in the case of prolonged oxidation, it may be possible to remove the MnO
2 and recover emission of the particles if necessary.
Various hydrophilic ligands have been introduced onto the surfaces of UCNPs via ligand exchange, including ligands such as citrate [
3,
5,
29], hexanedioic acid [
3,
5,
29], polyethylene glycol (PEG) derivatives [
5,
10,
29,
31], 6-aminohexanoic acid [
3,
5,
46], polyacrylic acid (PAA) derivatives [
10,
43,
47], and phosphate derivatives [
10,
31,
48]. Newly affixed ligands tend to require a certain length in order to be able to provide functionality that can be accessible for subsequent modification but this can be advantageously used in the design of nanoparticle stability. For instance, 1,10-decanedicarboxylic acid (DDA) has been demonstrated to closely crosslink NaYF
4: Yb
3+, Er
3+ UCNPs and Fe
3O
4 NPs via terminal carboxylic acid moieties. Due to the short length of the DDA ligand compared to the oleate ligand, oleate concentration could be optimized so as to functionalise the UCNPs with both oleate and DDA in an advantageous manner via the balance of the ligand exchange progress dynamically. The length of the oleate ligands prevent undesired interactions with anchored carboxylic acid groups present on the shorter DDA ligands by means of steric hindrance, thereby preventing substitution of the crosslinker [
49]. Preferably, the newly affixed ligands should be multidentate as the type and number of co-ordination sites on the hydrophobic and hydrophilic ligands strongly influence the efficacy of the exchange. For example, the higher co-ordination displayed by carboxylates makes them a favoured choice over amine-containing ligands, particularly when coating positively-charged UCNPs (i.e., NaYF
4: Yb
3+, Er
3+) [
50].
2.1.1. Carboxyl Moiety Modified UCNPs via Ligand Engineering
The exchange of ligands present on as-synthesised UCNPs with ligands exhibiting a carboxylic acid functional moiety would be the most effective method of modification under ligand engineering to impart such functionality. Due to the hydrophilic properties exhibited by carboxyls as well as the ease of coupling between carboxylic moieties and amine residues commonly found on biomolecules, it is a prevalent functionality for bio-sensing applications [
41,
51,
52,
53]. Furthermore, an additional benefit gained by carboxyl functionalisation is the capability to store particles for lengthier periods at an alkaline pH due to deprotonation of the terminal hydroxyl present on the carboxylic acids. The presence of a formal negative charge gives rise to a larger negative zeta potential and, thus, greater colloidal stability of the sample, substantially reducing agglomeration of the nanoparticles over time [
17].
Table 1 summarised the surface modified UCNPs via ligand engineering with carboxyl moieties and their corresponding applications.
Two representative works published by Kumar and Zhang (2009–2010) [
51,
52] established the realisation of DNA-based bio-sensors via the modification of NaYF
4: Yb
3+, Tm
3+ UCNP surfaces with diethylenetriaminepentaacetic acid (DTPA). A ligand exchange with oleate allowed for modification of DTPA onto the UCNPs surface whereby exposed carboxyl groups were then coupled to terminus –NH
2 groups on ss-DNA via carbodiimide chemistry without the addition of NHS derivatives. Whilst the bio-sensors are similar in concept, they are slightly different in practice. The first sensor [
51] relies on ss-DNA that is present on the surface of the UCNPs to convert to a hairpin conformation following interactions with free Hg
2+ ions in solution. The change in conformation not only entraps the free Hg
2+ ions but an intercalating dye (SYBR Green I) that is also present in solution. When the nanoparticles are excited, their emission is absorbed by SYBRG (~477 nm) followed by SYBRG emission between 490–520 nm thus indicating entrapment of Hg
2+ and the realisation of a DNA-based mercuric ion bio-sensor. The secondary sensor [
52] works via the conjugation of complimentary ss-DNA in solution to the ss-DNA present on the UCNPs surface. If complimentary ss-DNA successfully links to the UCNP, the intercalating dye (SYBRG) present in the solution gets trapped between the cross-linked DNA, so when the UCNPs are excited their emission is absorbed by the dye and a subsequent emission at 490–520 nm is seen. This signals the presence of complimentary DNA, which aims to be correlated to identify diseases, mutagens or pathogens by association.
Using the same carboxy group exposed on the UCNPs surface, further work conducted by Wang and Zhang (2014) [
53] determines a ligase-assisted DNA-based bio-sensor by addition of surface carboxyls through ligand exchange of oleate-capped NaYF
4: Yb
3+, Tm
3+ with poly(acrylic) acid. Amine-terminated ss-DNA was conjugated to the UCNPs via carbodiimide and NHS chemistry, followed by introduction of an intercalating dye (SYBRG), complementary ss-DNA target ss-DNA into solution. The ss-DNA present on the UCNPs as well as the complementary ss-DNA in solution are matching segments to the target ss-DNA, where a successful match leads to DNA hybridisation followed by ligation, dehybridisation of target DNA and a subsequent hairpin formation between the segments (
Figure 3). Ligation of the DNA and subsequent hairpinning would not occur if the segments of ss-DNA were mismatched to the target ss-DNA in solution. As a consequence, this mechanism of action allows for both accurate selectivity and sensitivity regarding determination of target ss-DNA presence. Furthermore, system temperatures during the experiment were manipulated to achieve desired results; this is due to hybridisation occurring at low temperatures and dehybridisation occurring at high temperature, so alteration of temperatures allowed for the acceleration of these processes. Advantageously, the addition of PAA to the surface of the UCNPs allowed for polymeric encapsulation of the nanoparticle cores, which is ideal for the protection from the surrounding aqueous environment, thus prolonging the storage life of the particles.
Although not a typical approach, the oxidation of ligands present on the surface of UCNPs as-synthesised can also be a means of achieving bio-sensing capabilities. Research conducted by Chen et al. (2008) [
41] demonstrated the biosensing application of various lanthanide-doped UCNPs (NaYF
4: Yb
3+ with activator variations of Er
3+, Ho
3+ or Tm
3+) coated with azelaic acid (AA) ligands. The oleate-capped UCNPs were directly oxidised via addition of the Lemieux-von Rudloff reagent, yielding an exposed terminal carboxylic acid group on the azelaic acid ligands. Addition of the carboxyl moiety imparted both hydrophilicity and bio-conjugation functionality, thus allowing the subsequent cross-linking of streptavidin to the terminal carboxyls via carbodiimide chemistry involving NHS. The streptavidin was further conjugated to capture-DNA that was added to solution with complimentary reporter-DNA attached to a TAMRA quencher. Given that the complimentary reporter-DNA successfully conjugates to the capture-DNA present on the UCNPs, the quencher would then be located in close proximity to the UCNP. This allows for luminescent resonance energy transfer (LRET) to occur as phosphorescence emitted by the UCNP is absorbed by the quencher due to the absorption spectrum of the quencher overlapping the green emission (~544 nm) of Er
3+.
The use of carboxyl moieties is not limited to biosensor applications but can also be used for multi-modal theranostic purposes as well, with one possible example being the use of chitosan coated UCNPs for their use in photo-dynamic therapy as demonstrated by Cui et al. [
54]. Amphiphilic chitosan (specifically
N-succinyl-
N′-octyl chitosan) was coated onto NaYF
4: Yb
3+, Er
3+ UCNPs whereby the hydrophobic photosensitiser ZnPc was loaded into the chitosan. However, due to the amphiphilic nature of the chitosan (SOC), it can be either coated via layer-by-layer attraction (hydrophobic interactions with surface residing oleate alkyl chains) due to exposed octyl groups on SOC or through a ligand exchange via terminal carboxyl groups. Either process would not change the overall mechanism of the application but the latter would be an example of carboxyls being used exclusively for their co-ordination abilities (in the same way oleate coordinates to UCNPs). ZnPc was loaded internally into the SOC via hydrophobic interactions whereby excitation of the UCNPs leads to 660 nm visible light emission that activates the release of reactive oxygen species (ROS) from activated ZnPc molecules. The researchers determined a loading capacity of 10.8%, a marked improvement from a comparison where ZnPc loading in mesoporous silica showed only ~0.1% loading capacity [
57], most likely due to the hydrophilic nature of the silica. Furthermore, induced cell apoptosis was determined in MCF-7 cells as a result of successful ROS release, thereby indicating the successful realisation of an UCNP-based photodynamic therapy system.
Similarly, Liebherr et al. (2012) [
55] developed colloidally-stable NaYF
4: Yb
3+, Er
3+ UCNPs via ligand exchange of oleate with maleimide-polyethylene glycol (MA-PEG) that exhibits terminal carboxyl groups. The carboxyl groups were used to coordinate the MA-PEG to the UCNPs, thus resulting in exposed thiol-sensitive maleimide groups that can be used for bioconjugation. Coupling of FITC-labelled
γ-globulin to the UCNPs was accomplished through addition of tris(2-carboxyethyl)phosphine (TCEP), a reduction agent that reduces existing cysteine disulphides present in the
γ-globulin, allowing for thioether bond formation with the exposed maleimide groups. Confirmation of successful coupling was proven via the measuring of fluorescence of the labeled
γ-globulins on both activate and inactive maleimide-functionalised UCNPs whereby active UCNPs demonstrated greater relative red emission while dispersed in water than inactive UCNPs. The reported UCNPs can be utilised as a platform in various bioconjugation applications involving thiols, such as biosensing, bioimaging, and therapeutic delivery.
The aforementioned examples represent not only the various ways to modify the surface of UCNPs with terminal carboxyls but for the most part, typical applications involving the carboxyl moiety being used for the addition of hydrophilic properties, biomolecule cross-linking capabilities and/or co-ordination capabilities. However, surface moieties can exhibit complex multi-functional properties that can give rise to effective utilisation in various applications. Jia et al. (2013) [
56] developed surface modified NaYF
4: Yb
3+, Er
3+ UCNPs with poly(acrylic) acid for their use in a pH-triggered controlled drug delivery system that demonstrates various functionalities of terminal carboxyl groups in practice. At a neutral pH, the anti-cancer drug doxorubicin (DOX) was bound to terminal carboxyl groups residing on the poly(acrylic) acid surface via electrostatic interactions following deprotonation of the carboxyls. As DOX exhibits a positive charge, it becomes tightly bound to the negatively charged carboxyls, leading to the conjugation of DOX molecules surrounding the surface of the UCNPs. Moreover, alteration of pH conditions can be used to manipulate desired operations; the loading rate of DOX was determined to be high in weakly alkaline environments (approx. 1% at pH 2, and 32% at pH 7) whilst the rate of release was determined to be high in acidic environments. Further assessment by the group testified to successful drug delivery within in vitro HeLa cells by cytotoxicity assays, where cell viability decreased by 50%. In this case, the carboxyl moieties demonstrate multi-functionality, that being: (1) the capacity to conjugate the anti-cancer drug DOX; (2) the control of the rates of both loading and release via manipulation of environmental pH; (3) imparting hydrophilic properties to the UCNPs so as to increase biocompatibility through surface residing carboxyls; and (4) coordinating poly(acrylic) acid to the UCNPs through internal carboxylate interactions.
Finally, NaGdF
4: Yb
3+, Er
3+ UCNPs were modified to achieve multifunctional upconverting liposomal nanocarriers for their use in anti-cancer drug delivery treatments by Huang et al. (2016) [
26] The surface of the UCNPs was modified with citrate ligands which exhibit tri-carboxyl moieties that demonstrate multiple functionalities in practice (as shown in
Figure 4). Firstly, deprotonation of the carboxyls leads to subsequent coordination of the citrate ligands to the surface of the UCNPs via a terminal carboxylate moiety. The two remaining surface carboxyl groups then are used to (1) impart hydrophilic properties to the UCNPs; (2) provide a hydrophilic environment for the loading of the anticancer drug DOX; and (3) conjugate as well as stabilise DOX molecules through ionic interactions (carboxylates exhibit a net negative charge whilst DOX exhibits a net positive charge). As DOX has an absorption range overlapping with part of the UCNP emission range (515 nm–570 nm), UV–VIS can be used to quantify the loading capacity of the UCNPs. Following encapsulation of the UCNPs in a liposome made of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and cholesterol in a ratio of 2:1, DOX was loaded into Lipo@DOX and UCNP@DOX@Lipo samples. It was determined that the loading efficiency of Lipo@DOX was 98% whilst UCNP@DOX@Lipo demonstrated a loading efficiency of 72%, the group asserted that this is most likely due to the decreased internal volume of the liposome from UCNPs occupying internal space. Passive drug release of the UCNP@DOX@Lipo particles was tested in both solutions of phosphate buffered saline (PBS) (imitates in vitro conditions) and 50% foetal bovine serum (FBS) in PBS (imitates in vivo conditions), with passive leaking only accounting for 6% loss of DOX in PBS over a 12 h period at 37 °C. As the overall passive release was minimal, PBS can be considered a viable storage solution whilst passive release of 39% in 50% FBS + PBS solution indicates the influence of external factors, such as serum protein and lipid bilayer destabilisation interactions that further the complexity of application in vivo.
2.1.2. Amino Moiety Modified UCNPs via Ligand Engineering
Much like carboxyl moieties, amino groups are favoured due to the hydrophilic properties they impart onto UCNPs and their ability to also be cross-linked via the well-established carbodiimide coupling protocol. Conversely, they exhibit positive charges at physiological pH which may be desirable for certain applications. The summary of the surface modified UCNPs via ligand engineering process with amino moieties is exhibited in
Table 2.
An exchange between oleate and
o-phosphorylethanolamine (PEA), which exhibits both terminal amino and phosphate groups, was carried out on NaYF
4: Yb
3+, Tm
3+ UCNPs in research conducted by Fedoryshin et al. (2014) [
25]. The exchange led to the affixing of primary amines to the surface of the particles for their use in a remotely triggered anti-cancer drug delivery system (as displayed in
Figure 5). As phosphate exhibits both greater coordination sites and binding affinity than carboxylates, the exchange favours the replacement of oleate carboxylate groups with terminal phosphates [
10]. Conjugation of the photocleavable prodrug ONB-5-FU to the UCNPs via carbodiimide chemistry involved the addition of NHS, resulting in the formation of an amide bond. Due to ONB-5-FU exhibiting an overlapping UV absorption range with that of UV emission range of the UCNPs, excitation of UCNPs resulted in 364 nm UV light emission that efficiently photocleaved the prodrug. This allowed for detachment and consequent release of 5-fluorouracil, where cleavage is proposed to occur at the C-N bond that couples ONB and 5-FU. It was determined that the rate of release could be tuned with laser power output (10, 30 and 80 mW) and that as high as 77% of the prodrug was effectively released via remote triggering through NIR excitation in comparison to traditional direct UV excitation. Laser excitation at 80 mW resulted in an observed initial rate constant (k
o) of 130 μM min
−1 while, conversely, at 10 mW, a k
o of 18 μM min
−1 was observed.
Similarly, work presented by Doughan et al. (2014) [
58] utilises PEA to realise a biosensing application through solid-phase covalent immobilisation of UCNPs (
Figure 6). A significant problem presented to the applications of UCNPs in aqueous mediums is the agglomeration of particles in solution, which can be overcome through the use of UCNPs in solid-phase at interfaces. Standard as-synthesised NaYF
4: Yb
3+, Tm
3+ UCNPs were transitioned to a hydrophilic phase via a ligand exchange with oleate ligands present on the UCNPs and PEA. The phosphate moiety of the PEA coordinates to the surface of the UCNPs whilst the terminal amines extend outward, providing hydrophilicity and conjugation capabilities.
Glass slides, which were used as the interface medium, were functionalised with surface silanol groups by washing with NH
4OH and HCl in the presence of H
2O
2. Subsequently they were amine functionalised via (3-aminopropyl)trimethoxysilane (APTMS) followed by reductive amination with sodium cyanoborohyride (NaBH
3CN). The resulting aldehyde-functionalised glass slides provided an interface for which the PEA-modified UCNPs could now be coupled and immobilised to through covalent bonding. Incubation of the glass slides over 2 h followed drop spotting of UCNPs with NaBH
3CN on desired locations so as to allow reductive amination to occur and the formation of a subsequent alkyl amine bond between the underside of the UCNPs and slide (as shown in
Figure 6).
The surface residing amino groups on the UCNPs were functionalised with thrombin after residual aldehyde groups on the slide were inactivated with ethanolamine in the presence of NaBH3CN. Amine-modified thrombin specific aptamer-1 was drop cast onto active UCNP spots after the surface amines were reacted with glutaraldehyde. As quantum dots (QDs) exhibit a spectral overlap with UCNPs, displaying an absorbance over their emission range (~330–490 nm), they can be used as effective energy acceptors in an LRET-based energy donor-acceptor system of UCNPs and QDs. As a result, QDs were functionalised with thrombin specific aptamer-2 and subsequently incubated for 6 h with UCNPs so as to allow the aptamers to attach. If the aptamers have successfully attached, the UCNPs will undergo a LRET process with the QDs when excited at 980 nm. Confirmation and quantification can be achieved via the consequent QD emission at 614 nm and peak ratio, whereby it was determined that the assay showed strong selectivity for thrombin through control testing. In contrast to 0.1 μM thrombin, a 1 μM solution of BSA displayed a signal that was 80% lower while also demonstrating a signal that was 90% lower for the non-specific adsorption of QDs onto the surface of the interface.
An endeavour to produce carbohydrate-coated UCNPs for lectin recognition was undertaken by Bogdan et al. (2010) [
59]. After NaGdF
4: Yb
3+, Er
3+ UCNPS were synthesised with oleate ligands present on the surface, they subsequently underwent a ligand-exchange with poly(amido)amine (PMAM). As PMAM exhibits four terminal amino moieties, one moiety can exchange and co-ordinate to the UCNP surface while the remaining three groups provide both hydrophilicity and further bioconjugation capabilities. Synthetic multivalent carbohydrates demonstrate efficient inhibition of protein-carbohydrate interactions due to strong binding affinities. Therefore, by cross-linking synthetic carbohydrates to the UCNPs, the UCNPs gain the ability to interact and detect bacteria or virus infections. The synthetic carbohydrate p-isothiocyanatophenyl a-D-mannopyranoside was coupled to the UCNPs via thiourea bond formation between the terminal amino groups residing on the UCNP surface and the terminal thiocyanate on the synthetic carbohydrate. Further testing through an LRET assay led to the determination that the mannose moiety successfully conjugated to protein receptors by use of a RITC-labelled plant lectin Con A (RITC-Con A). As the RITC-Con A absorption overlaps with the emission range of the UCNPs (550 nm), it gives off a characteristic emission at 585 nm that can indicate successful attachment of the carbohydrate to protein receptors after UCNP excitation. The group demonstrated that there was a matching boost in acceptor fluorescence to UCNP green emission decline after excitation of the UCNPs at 980 nm. Furthermore, the addition of Gd
3+ to the nanocore allows for great contrast when imaging via magnetic resonance imaging (MRI) as it exhibits a considerable magnetic moment.
2.1.3. Thiol Moiety Modified UCNPs via Ligand Engineering
Equivalently to carboxyl and amino moieties, thiol moieties can be used to co-ordinate to UCNPs, impart hydrophilicity and grant the capability to further conjugate biomolecules for extended application. Although they cannot undergo carbodiimide reactions, thiols can present an alternative option for operations where carboxyl and amino cannot be applied effectively.
Table 3 summarised the surface modified UCNPs via ligand engineering with thiol moieties and their corresponding applications.
Successful development of superparamagnetic Fe
3O
4/NaYF
4: Yb
3+, Er
3+ hybrid nanoparticles was demonstrated by Shen et al. (2010) [
49] whereby 11-mercaptoundecanoic acid (MUA) was surface modified onto NaYF
4: Yb
3+, Er
3+ UCNPs via a ligand exchange with oleate ligands present on the as-synthesised particles. As MUA exhibits both terminal thiol and carboxylic moieties, it displays the ability to co-ordinate to the UCNPs via the carboxyl moiety whilst being capable of further cross-linking through residual thiol moieties. These thiol moieties play a crucial role in the cross-linking process due to the specific affinity they boast toward Fe
3O
4 NPs. Compared to UCNPs coated with 1,10-decanedicarboxylic acid (DDA) which exhibits terminal carboxyl moieties, the UCNPs@MUA were not only smaller in size than the UCNPs@DDA but also absorbed Fe
3O
4 NPs more densely. It is inferred that the MUA perhaps inhibits the growth of Fe
3O
4 on the surface of the UCNPs due to a robust interaction between Fe and S. Furthermore, the group present evidence to support effective magnetic separation ability whereby they explain that the ligand spacing between Fe
3O
4 and the UCNPs allows for the superparamagnetic properties of Fe
3O
4 to be kept intact.
Inversely, research by Dong, B. et al. (2011) [
60] establishes the use of the thiol moiety as a co-ordinating moiety to the surface of NaYF
4: Yb
3+, Er
3+ UCNPs for therapeutic photothermal applications (as displayed in
Figure 7). Notably, it has been reported that ligands which have been exchanged to UCNP surfaces via thiol coordination require additional functionalisation so as to undergo cell internalisation effectively [
5]. As-synthesised UCNPs underwent a ligand exchange with thioglycolic acid (TGA). Absence of the characteristic -SH peak at 2600 cm
−1 as determined via FTIR analysis led the group to assert that the -SH group is not only responsible for the connection to the UCNPs but the impartation of hydrophilic properties as well. The deprotonation and subsequent negative charge exhibited by the now surface residing carboxyl moieties was used to conjugate Ag
+ ions to the surface of the UCNP, forming an Ag
+ ionic shell. Addition of the Ag
+ shell to the nanoparticles granted an increase in temperature when the UCNPs were excited at 980 nm, compared to UCNPs without the Ag
+ shell present on the surface. Ag
+-coated UCNPs in solution reached an increased temperature of 303 K compared to 293 K from uncoated and irradiated UCNPs at the same power density range. Further tests led to the determination of significantly reduced cell viability of both HepG2 and BCap-37 cells, whereby after 20 min of irradiation viability reached as low as 4.62% and 5.43%, respectively.
Similarly, a novel method for the synthesis of sub-10 nm Ln
3+-:BaF
2/Ln
3+:SrF
2 UCNPs was developed by Chen et al. (2011) [
61], where Ln
3+ = La – Lu. Synthesis of Tm
3+, Yb
3+:BaF
2/Ln
3+:SrF
2 active-core/active-shell UCNPS was followed by exchange of the terminal oleate ligands with TGA for the sole purpose of aqueous dispersal. Again, the thiol moiety co-ordinates to the surface of the UCNPs whilst the terminal carboxyl moieties reside on the surface of the UCNPs can grant further bioconjugation abilities. Design of an active-core/active-shell provides NIR-NIR dual-mode luminescence due to the upconverting emission of Tm
3+ at 802 nm upon excitation of Yb
3+, as well as the NIR downconverting emissions both of Yb
3+ at 975 nm and Nd
3+ at 1054 nm. Furthermore, shell encapsulation of the core allows for protection of core ions from external interaction such as solvents and ligands that may cause vibrational deactivation.
Lastly, related work published by Kumar et al. (2009) [
62] also establishes the use of the thiol moiety as a co-ordinating moiety to the UCNP surface for a combined optical and magnetic resonance imaging application. After the synthesis of NaYF
4: Gd
3+, Eu
3+ UCNPs, as-present oleate ligands were exchanged with 3-mercaptopropionic acid (MPA), a ligand that has both a thiol and carboxyl moiety incorporated into its structure. The thiol moiety of MPA co-ordinates to the surface of the UCNPs whilst the residual carboxyl moieties provide further functionality by means of bioconjugation. Various tumour-specific antibodies were conjugated to the nanoparticles, such as anti-mesothelin, anti-claudin 4, transferrin via carbodiimide chemistry between terminal amino groups on the antibodies and the surface carboxyl groups of the UCNPs. Confocal images of Panc 1 cells confirmed substantial enhancement of cell uptake for antibody modified UCNPs compared to UCNPs without antibody modification, a result of a receptor-mediated process. Furthermore, it has been reported that corresponding receptors to the antibodies are overexpressed on the surface of these particular cells [
63] and as a result, a high amount of UCNPs can be seen targeting and binding specifically to the receptors on the surface of the cells.