Drosophila - A Model System for Developmental Biology
Share This Topical Collection
Editor
Topical Collection Information
Dear Colleagues,
It has been more than one hundred years since Thomas Hunt Morgan established Drosophila as a premier organism for biological research, and still Drosophila remains one of the most commonly-used model organisms in a variety of fields. Drosophila have been used to model development and aging, disease and homeostasis, stem cells and differentiation, neurogenesis and neurodegeneration, and many, many other processes. At times, throughout its history as a model organism, Drosophila appeared to be nearing the end of its usefulness as “better” approaches emerged, and experimental limits were reached. Despite this, new methods always appeared, better approaches turned out to have their limitations, and Drosophila research persevered. The model organisms C. elegans and Drosophila remain the major sources for rapid, genetic analysis of development and its inverse, aging. With the rise of CRISPR/Cas9, and the demand for human cell based experiments, the invertebrate models are again under pressure. This Special Issue of the Journal of Developmental Biology will provide an overview of the current standing, and most importantly highlight the directions for future invertebrate research. Contributions can be reviews, as well as research papers, covering topics of model organism research.
Dr. Nick Tolwinski
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Journal of Developmental Biology is an international peer-reviewed open access quarterly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 1800 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- Drosophila
- Development
- Aging
- C. elegans
- Signaling
- Metabolism
- Lipidomics
- Neurobiology
- Stem Cells
Published Papers (15 papers)
Open AccessFeature PaperReview
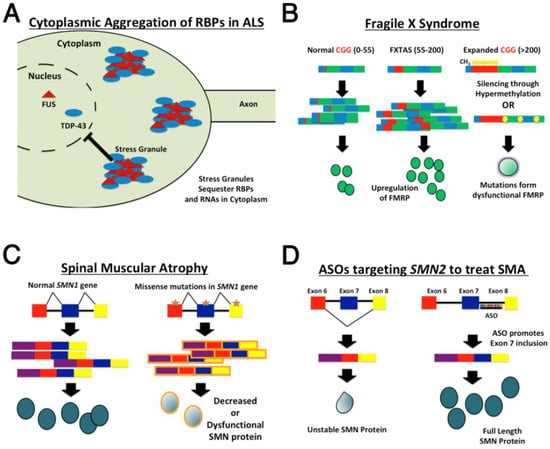
Drosophila melanogaster as a Tool for Amyotrophic Lateral Sclerosis Research
by
Krupa N. Hegde and Ajay Srivastava
Viewed by 2722
Abstract
Reliable animal model systems are an integral part of biological research. Ever since Thomas Hunt Morgan won a Nobel Prize for genetic work done using the fruit fly (
Drosophila melanogaster) as a model organism, it has played a larger and more
[...] Read more.
Reliable animal model systems are an integral part of biological research. Ever since Thomas Hunt Morgan won a Nobel Prize for genetic work done using the fruit fly (
Drosophila melanogaster) as a model organism, it has played a larger and more important role in genetic research.
Drosophila models have long been used to study neurodegenerative diseases and have aided in identifying key disease progression biological pathways. Due to the availability of a vast array of genetic manipulation tools, its relatively short lifespan, and its ability to produce many progenies,
D. melanogaster has provided the ability to conduct large-scale genetic screens to elucidate possible genetic and molecular interactions in neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s Disease, and Amyotrophic Lateral Sclerosis (ALS). With regards to ALS, many of the gene mutations that have been discovered to be linked to the disease have been modeled in
Drosophila to provide a look into a detailed model of pathogenesis. The aim of this review is to summarize key and newer developments in ALS research that have utilized
Drosophila and to provide insight into the profound use of
Drosophila as a tool for modeling this disease.
Full article
Open AccessArticle
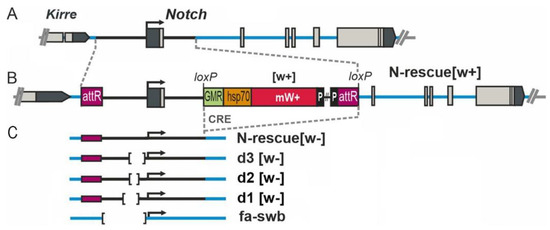
New Mutations in the 5′ Region of the Notch Gene Affect Drosophila melanogaster Oogenesis
by
Elena I. Volkova, Natalya V. Dorogova, Oleg V. Andreyenkov, Saveliy A. Tikhomirov and Sergey A. Demakov
Viewed by 2054
Abstract
The Notch pathway is an important and evolutionarily conserved signaling system involved in the development of multicellular organisms. Notch signaling plays an important role in the regulation of proliferation and differentiation of many cell types. In this study, we report new aspects of
[...] Read more.
The Notch pathway is an important and evolutionarily conserved signaling system involved in the development of multicellular organisms. Notch signaling plays an important role in the regulation of proliferation and differentiation of many cell types. In this study, we report new aspects of
Notch gene participation in oogenesis using our previously generated mutations. The mutations consist of an insertion of an auxiliary element of a transgene construct into the first intron of the gene and a series of directed deletions within the 5′ regulatory region of
Notch. We showed that some of these mutations affect
Drosophila oogenesis. This insertion, either alone or in combination with the deletion of an insulator sequence, led to lower expression of
Notch in the ovaries. As a result, the formation of egg chambers was disturbed in middle oogenesis. These abnormalities have not been described previously and imply one more function of
Notch in oogenesis. It can be assumed that
Notch is associated with not only follicular epithelium morphogenesis but also cellular mechanisms of oocyte growth.
Full article
►▼
Show Figures
Open AccessReview
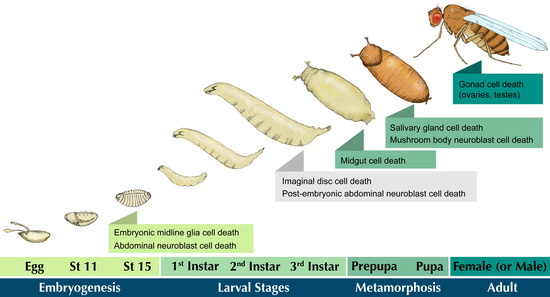
I Spy in the Developing Fly a Multitude of Ways to Die
by
Alla Yalonetskaya, Albert A. Mondragon, Johnny Elguero and Kimberly McCall
Cited by 11 | Viewed by 10399
Abstract
Cell proliferation and cell death are two opposing, yet complementary fundamental processes in development. Cell proliferation provides new cells, while developmental programmed cell death adjusts cell numbers and refines structures as an organism grows. Apoptosis is the best-characterized form of programmed cell death;
[...] Read more.
Cell proliferation and cell death are two opposing, yet complementary fundamental processes in development. Cell proliferation provides new cells, while developmental programmed cell death adjusts cell numbers and refines structures as an organism grows. Apoptosis is the best-characterized form of programmed cell death; however, there are many other non-apoptotic forms of cell death that occur throughout development.
Drosophila is an excellent model for studying these varied forms of cell death given the array of cellular, molecular, and genetic techniques available. In this review, we discuss select examples of apoptotic and non-apoptotic cell death that occur in different tissues and at different stages of
Drosophila development. For example, apoptosis occurs throughout the nervous system to achieve an appropriate number of neurons. Elsewhere in the fly, non-apoptotic modes of developmental cell death are employed, such as in the elimination of larval salivary glands and midgut during metamorphosis. These and other examples discussed here demonstrate the versatility of
Drosophila as a model organism for elucidating the diverse modes of programmed cell death.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
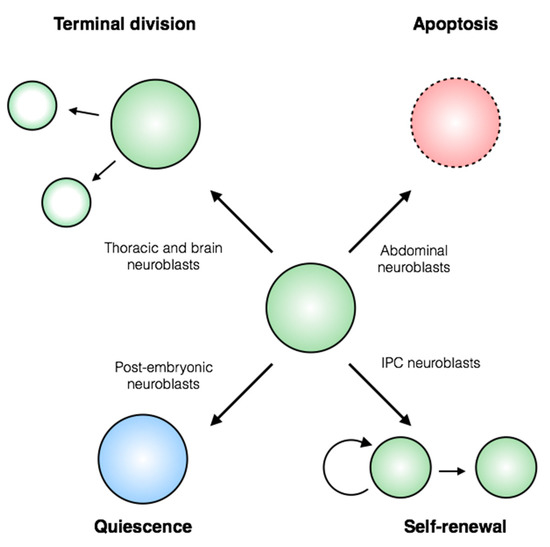
Drosophila as a Model for Developmental Biology: Stem Cell-Fate Decisions in the Developing Nervous System
by
Katherine Harding and Kristin White
Cited by 22 | Viewed by 10288
Abstract
Stem cells face a diversity of choices throughout their lives. At specific times, they may decide to initiate cell division, terminal differentiation, or apoptosis, or they may enter a quiescent non-proliferative state. Neural stem cells in the
Drosophila central nervous system do all
[...] Read more.
Stem cells face a diversity of choices throughout their lives. At specific times, they may decide to initiate cell division, terminal differentiation, or apoptosis, or they may enter a quiescent non-proliferative state. Neural stem cells in the
Drosophila central nervous system do all of these, at stereotypical times and anatomical positions during development. Distinct populations of neural stem cells offer a unique system to investigate the regulation of a particular stem cell behavior, while comparisons between populations can lead us to a broader understanding of stem cell identity.
Drosophila is a well-described and genetically tractable model for studying fundamental stem cell behavior and the mechanisms that underlie cell-fate decisions. This review will focus on recent advances in our understanding of the factors that contribute to distinct stem cell-fate decisions within the context of the
Drosophila nervous system.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
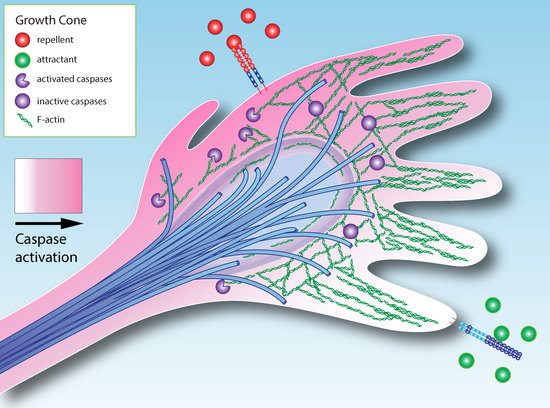
The Role of Apoptotic Signaling in Axon Guidance
by
Riley Kellermeyer, Leah M. Heydman, Grant S. Mastick and Thomas Kidd
Cited by 13 | Viewed by 6854
Abstract
Navigating growth cones are exposed to multiple signals simultaneously and have to integrate competing cues into a coherent navigational response. Integration of guidance cues is traditionally thought to occur at the level of cytoskeletal dynamics.
Drosophila studies indicate that cells exhibit a low
[...] Read more.
Navigating growth cones are exposed to multiple signals simultaneously and have to integrate competing cues into a coherent navigational response. Integration of guidance cues is traditionally thought to occur at the level of cytoskeletal dynamics.
Drosophila studies indicate that cells exhibit a low level of continuous caspase protease activation, and that axon guidance cues can activate or suppress caspase activity. We base a model for axon guidance on these observations. By analogy with other systems in which caspase signaling has non-apoptotic functions, we propose that caspase signaling can either reinforce repulsion or negate attraction in response to external guidance cues by cleaving cytoskeletal proteins. Over the course of an entire trajectory, incorrectly navigating axons may pass the threshold for apoptosis and be eliminated, whereas axons making correct decisions will survive. These observations would also explain why neurotrophic factors can act as axon guidance cues and why axon guidance systems such as Slit/Robo signaling may act as tumor suppressors in cancer.
Full article
►▼
Show Figures
Open AccessReview
Drosophila as a Model for Assessing the Function of RNA-Binding Proteins during Neurogenesis and Neurological Disease
by
Eugenia C. Olesnicky and Ethan G. Wright
Cited by 10 | Viewed by 7032
Abstract
An outstanding question in developmental neurobiology is how RNA processing events contribute to the regulation of neurogenesis. RNA processing events are increasingly recognized as playing fundamental roles in regulating multiple developmental events during neurogenesis, from the asymmetric divisions of neural stem cells, to
[...] Read more.
An outstanding question in developmental neurobiology is how RNA processing events contribute to the regulation of neurogenesis. RNA processing events are increasingly recognized as playing fundamental roles in regulating multiple developmental events during neurogenesis, from the asymmetric divisions of neural stem cells, to the generation of complex and diverse neurite morphologies. Indeed, both asymmetric cell division and neurite morphogenesis are often achieved by mechanisms that generate asymmetric protein distributions, including post-transcriptional gene regulatory mechanisms such as the transport of translationally silent messenger RNAs (mRNAs) and local translation of mRNAs within neurites. Additionally, defects in RNA splicing have emerged as a common theme in many neurodegenerative disorders, highlighting the importance of RNA processing in maintaining neuronal circuitry. RNA-binding proteins (RBPs) play an integral role in splicing and post-transcriptional gene regulation, and mutations in RBPs have been linked with multiple neurological disorders including autism, dementia, amyotrophic lateral sclerosis (ALS), spinal muscular atrophy (SMA), Fragile X syndrome (FXS), and X-linked intellectual disability disorder. Despite their widespread nature and roles in neurological disease, the molecular mechanisms and networks of regulated target RNAs have been defined for only a small number of specific RBPs. This review aims to highlight recent studies in
Drosophila that have advanced our knowledge of how RBP dysfunction contributes to neurological disease.
Full article
►▼
Show Figures
Open AccessReview
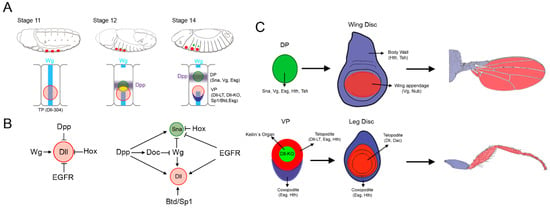
Specification and Patterning of Drosophila Appendages
by
Mireya Ruiz-Losada, David Blom-Dahl, Sergio Córdoba and Carlos Estella
Cited by 26 | Viewed by 12332
Abstract
Appendages are external projections of the body that serve the animal for locomotion, feeding, or environment exploration. The appendages of the fruit fly
Drosophila melanogaster are derived from the imaginal discs, epithelial sac-like structures specified in the embryo that grow and pattern during
[...] Read more.
Appendages are external projections of the body that serve the animal for locomotion, feeding, or environment exploration. The appendages of the fruit fly
Drosophila melanogaster are derived from the imaginal discs, epithelial sac-like structures specified in the embryo that grow and pattern during larva development. In the last decades, genetic and developmental studies in the fruit fly have provided extensive knowledge regarding the mechanisms that direct the formation of the appendages. Importantly, many of the signaling pathways and patterning genes identified and characterized in
Drosophila have similar functions during vertebrate appendage development. In this review, we will summarize the genetic and molecular mechanisms that lead to the specification of appendage primordia in the embryo and their posterior patterning during imaginal disc development. The identification of the regulatory logic underlying appendage specification in
Drosophila suggests that the evolutionary origin of the insect wing is, in part, related to the development of ventral appendages.
Full article
►▼
Show Figures
Open AccessArticle
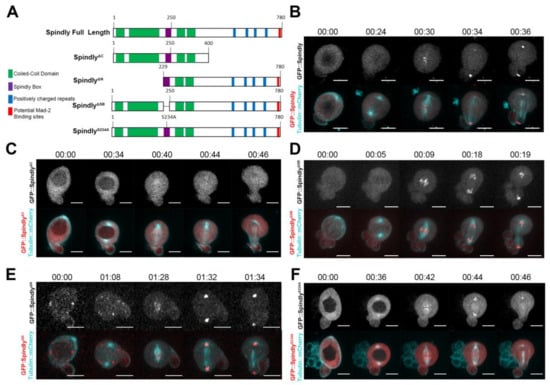
Requirement of the Dynein-Adaptor Spindly for Mitotic and Post-Mitotic Functions in Drosophila
by
Giuliana D. Clemente, Matthew R. Hannaford, Hamze Beati, Katja Kapp, Jens Januschke, Eric R. Griffis and Hans-Arno J. Müller
Cited by 7 | Viewed by 7016
Abstract
Spindly was originally identified as a specific regulator of Dynein activity at the kinetochore. In early prometaphase, Spindly recruits the Dynein/Dynactin complex, promoting the establishment of stable kinetochore-microtubule interactions and progression into anaphase. While details of Spindly function in mitosis have been worked
[...] Read more.
Spindly was originally identified as a specific regulator of Dynein activity at the kinetochore. In early prometaphase, Spindly recruits the Dynein/Dynactin complex, promoting the establishment of stable kinetochore-microtubule interactions and progression into anaphase. While details of Spindly function in mitosis have been worked out in cultured human cells and in the
C. elegans zygote, the function of Spindly within the context of an organism has not yet been addressed. Here, we present loss- and gain-of-function studies of Spindly using transgenic RNAi in
Drosophila. Knock-down of Spindly in the female germ line results in mitotic arrest during embryonic cleavage divisions. We investigated the requirements of Spindly protein domains for its localisation and function, and found that the carboxy-terminal region controls Spindly localisation in a cell-type specific manner. Overexpression of Spindly in the female germ line is embryonic lethal and results in altered egg morphology. To determine whether Spindly plays a role in post-mitotic cells, we altered Spindly protein levels in migrating cells and found that ovarian border cell migration is sensitive to the levels of Spindly protein. Our study uncovers novel functions of Spindly and a differential, functional requirement for its carboxy-terminal region in
Drosophila.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
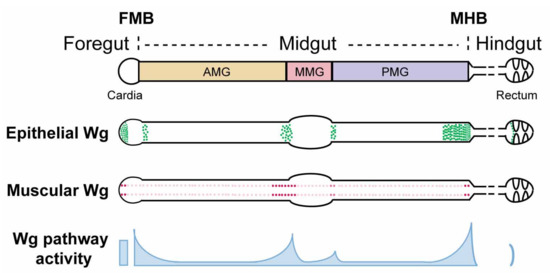
Wingless/Wnt Signaling in Intestinal Development, Homeostasis, Regeneration and Tumorigenesis: A Drosophila Perspective
by
Ai Tian, Hassina Benchabane and Yashi Ahmed
Cited by 28 | Viewed by 7062
Abstract
In mammals, the Wnt/β-catenin signal transduction pathway regulates intestinal stem cell maintenance and proliferation, whereas Wnt pathway hyperactivation, resulting primarily from the inactivation of the tumor suppressor Adenomatous polyposis coli (APC), triggers the development of the vast majority of colorectal cancers. The Drosophila
[...] Read more.
In mammals, the Wnt/β-catenin signal transduction pathway regulates intestinal stem cell maintenance and proliferation, whereas Wnt pathway hyperactivation, resulting primarily from the inactivation of the tumor suppressor Adenomatous polyposis coli (APC), triggers the development of the vast majority of colorectal cancers. The Drosophila adult gut has recently emerged as a powerful model to elucidate the mechanisms by which Wingless/Wnt signaling regulates intestinal development, homeostasis, regeneration, and tumorigenesis. Herein, we review recent insights on the roles of Wnt signaling in Drosophila intestinal physiology and pathology.
Full article
►▼
Show Figures
Open AccessReview
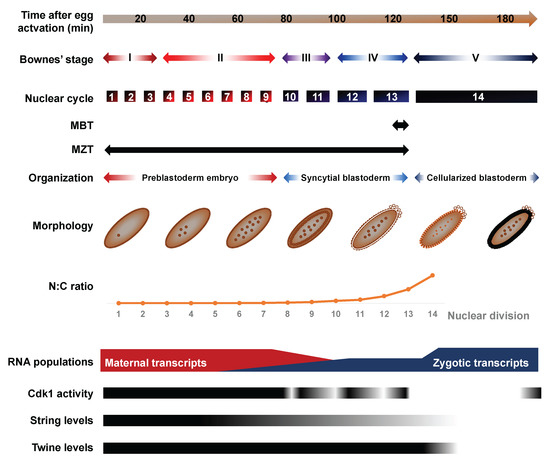
Flying the RNA Nest: Drosophila Reveals Novel Insights into the Transcriptome Dynamics of Early Development
by
Fabio Alexis Lefebvre and Éric Lécuyer
Cited by 11 | Viewed by 7006
Abstract
Early development is punctuated by a series of pervasive and fast paced transitions. These events reshape a differentiated oocyte into a totipotent embryo and allow it to gradually mount a genetic program of its own, thereby framing a new organism. Specifically, developmental transitions
[...] Read more.
Early development is punctuated by a series of pervasive and fast paced transitions. These events reshape a differentiated oocyte into a totipotent embryo and allow it to gradually mount a genetic program of its own, thereby framing a new organism. Specifically, developmental transitions that ensure the maternal to embryonic control of developmental events entail a deep remodeling of transcriptional and transcriptomic landscapes.
Drosophila provides an elegant and genetically tractable system to investigate these conserved changes at a dazzling developmental pace. Here, we review recent studies applying emerging technologies such as ribosome profiling, in situ Hi-C chromatin probing and live embryo RNA imaging to investigate the transcriptional dynamics at play during
Drosophila embryogenesis. In light of this new literature, we revisit the main models of zygotic genome activation (ZGA). We also review the contributions played by zygotic transcription in shaping embryogenesis and explore emerging concepts of processes such as transcriptional bursting and transcriptional memory.
Full article
►▼
Show Figures
Open AccessReview
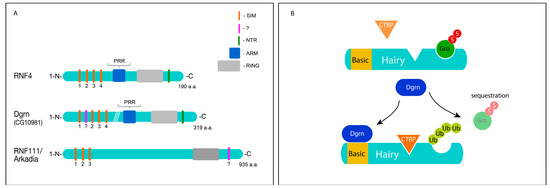
The Biology of SUMO-Targeted Ubiquitin Ligases in Drosophila Development, Immunity, and Cancer
by
Mona Abed, Eliya Bitman-Lotan and Amir Orian
Cited by 13 | Viewed by 6484
Abstract
The ubiquitin and SUMO (small ubiquitin-like modifier) pathways modify proteins that in turn regulate diverse cellular processes, embryonic development, and adult tissue physiology. These pathways were originally discovered biochemically in vitro, leading to a long-standing challenge of elucidating both the molecular cross-talk between
[...] Read more.
The ubiquitin and SUMO (small ubiquitin-like modifier) pathways modify proteins that in turn regulate diverse cellular processes, embryonic development, and adult tissue physiology. These pathways were originally discovered biochemically in vitro, leading to a long-standing challenge of elucidating both the molecular cross-talk between these pathways and their biological importance. Recent discoveries in
Drosophila established that ubiquitin and SUMO pathways are interconnected via evolutionally conserved SUMO-targeted ubiquitin ligase (STUbL) proteins. STUbL are RING ubiquitin ligases that recognize SUMOylated substrates and catalyze their ubiquitination, and include Degringolade (Dgrn) in
Drosophila and RNF4 and RNF111 in humans. STUbL are essential for early development of both the fly and mouse embryos. In the fly embryo, Dgrn regulates early cell cycle progression, sex determination, zygotic gene transcription, segmentation, and neurogenesis, among other processes. In the fly adult, Dgrn is required for systemic immune response to pathogens and intestinal stem cell regeneration upon infection. These functions of Dgrn are highly conserved in humans, where RNF4-dependent ubiquitination potentiates key oncoproteins, thereby accelerating tumorigenesis. Here, we review the lessons learned to date in
Drosophila and highlight their relevance to cancer biology.
Full article
►▼
Show Figures
Open AccessFeature PaperCommentary
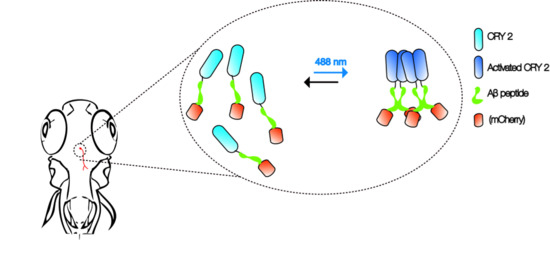
Modeling Alzheimer’s and Other Age Related Human Diseases in Embryonic Systems
by
Chu Hsien Lim and Ajay S. Mathuru
Cited by 24 | Viewed by 5790
Abstract
Modeling human disease in animals is an important strategy to discover potential methods of intervention. We suggest that there is much to be gained by employing a multi-model approach that takes advantage of different animal systems used in the laboratory simultaneously. We use
[...] Read more.
Modeling human disease in animals is an important strategy to discover potential methods of intervention. We suggest that there is much to be gained by employing a multi-model approach that takes advantage of different animal systems used in the laboratory simultaneously. We use the example of modeling Alzheimer’s disease in
Drosophila melanogaster,
Caenorhabditis elegans, and
Danio rerio to illustrate how such an approach can be employed to investigate the pathophysiology of the disease.
Full article
►▼
Show Figures
Open AccessReview
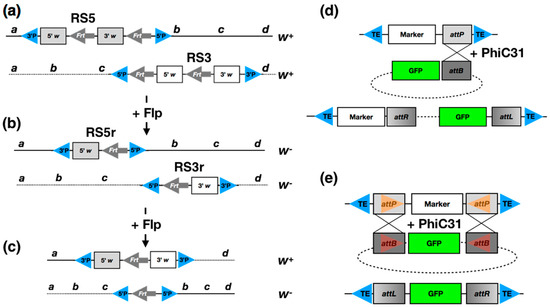
Engineering the Drosophila Genome for Developmental Biology
by
Dagmara Korona, Stefan A. Koestler and Steven Russell
Cited by 11 | Viewed by 7867
Abstract
The recent development of transposon and CRISPR-Cas9-based tools for manipulating the fly genome in vivo promises tremendous progress in our ability to study developmental processes. Tools for introducing tags into genes at their endogenous genomic loci facilitate imaging or biochemistry approaches at the
[...] Read more.
The recent development of transposon and CRISPR-Cas9-based tools for manipulating the fly genome in vivo promises tremendous progress in our ability to study developmental processes. Tools for introducing tags into genes at their endogenous genomic loci facilitate imaging or biochemistry approaches at the cellular or subcellular levels. Similarly, the ability to make specific alterations to the genome sequence allows much more precise genetic control to address questions of gene function.
Full article
►▼
Show Figures
Open AccessReview
Drosophila as a Model to Study the Link between Metabolism and Cancer
by
Héctor Herranz and Stephen M. Cohen
Cited by 18 | Viewed by 6308
Abstract
Cellular metabolism has recently been recognized as a hallmark of cancer. Investigating the origin and effects of the reprogrammed metabolism of tumor cells, and identifying its genetic mediators, will improve our understanding of how these changes contribute to disease progression and may suggest
[...] Read more.
Cellular metabolism has recently been recognized as a hallmark of cancer. Investigating the origin and effects of the reprogrammed metabolism of tumor cells, and identifying its genetic mediators, will improve our understanding of how these changes contribute to disease progression and may suggest new approaches to therapy.
Drosophila melanogaster is emerging as a valuable model to study multiple aspects of tumor formation and malignant transformation. In this review, we discuss the use of
Drosophila as model to study how changes in cellular metabolism, as well as metabolic disease, contribute to cancer.
Full article
Open AccessEditorial
Introduction: Drosophila—A Model System for Developmental Biology
by
Nicholas S. Tolwinski
Cited by 39 | Viewed by 15577
Abstract
Drosophila melanogaster, known colloquially as the fruit fly, remains one of the most commonly used model organisms for biomedical science.[...]
Full article