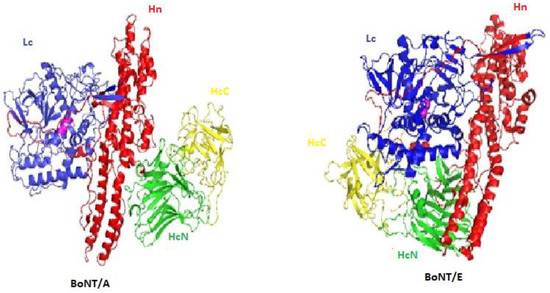
Botulinum Toxins on Human Pain
A topical collection in Toxins (ISSN 2072-6651). This collection belongs to the section "Bacterial Toxins".
Viewed by 287207
Share This Topical Collection
Editor
 Prof. Dr. Bahman Jabbari
Prof. Dr. Bahman Jabbari
 Prof. Dr. Bahman Jabbari
Prof. Dr. Bahman Jabbari
E-Mail
Collection Editor
Department of Neurology, Yale University School of Medicine, New Haven, CT 06519, USA
Interests: Botulinum toxin, Botulinum neurotoxin, Neuropathic pain , neuralgia, pain, pain medicine
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Animal studies have shown analgesic and anti-inflammatory effects for botulinum neurotoxins (BoNTs) via a variety of mechanisms. Over the past 20 years, a number of controlled studies have provided evidence for the efficacy of botulinum neurotoxins in alleviating the human pain. The list of pain disorders in which treatment with BoNTs therapy have produced favorable results is long and include pain from cervical dystonia, chronic migraine, post-herpetic, post-traumatic, and trigeminal neuralgias, chronic lateral epicondylitis, plantar faciitis, piriformis syndrome, pain associated with total knee arthroplasty, allodynia of diabetic neuropathy, pelvic pain, painful knee osteoarthritis, lower back pain, post-operative pain in children with cerebral palsy after adductor release surgery, anterior knee pain with vastus lateralis imbalance, post-operative pain after mastectomy, anal sphincter spasms, and pain after hemorrhoidectomy. This Topical Collection of Toxins is dedicated to the effects of BoNT therapy in human pain disorders.
Prof. Dr. Bahman Jabbari
Collection Editor
Manuscript Submission Information
Manuscripts for the topical collection can be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. All papers will be peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on this website. The topical collection considers regular research articles, short communications and review articles. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page.
Please visit the Instructions for Authors page before submitting a manuscript. The article processing charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Keywords
- Botulinum toxin
- Botulinum neurotoxin
- Neuropathic pain
- neuralgia, pain
- pain medicine
Related Special Issue
Published Papers (33 papers)
Open AccessArticle
The Therapeutic Effect of Botulinum Toxin Type A on Trigeminal Neuralgia: Are There Any Differences between Type 1 versus Type 2 Trigeminal Neuralgia?
by
Yan Tereshko, Mariarosaria Valente, Enrico Belgrado, Chiara Dalla Torre, Simone Dal Bello, Giovanni Merlino, Gian Luigi Gigli and Christian Lettieri
Cited by 1 | Viewed by 1278
Abstract
Background: Botulinum toxin type A is an effective treatment for trigeminal neuralgia. Moreover, its efficacy in type 2 trigeminal neuralgia and comparative studies between type 1 and type 2 trigeminal neuralgia (TN) still need to be improved. Methods: We treated 40 TN patients
[...] Read more.
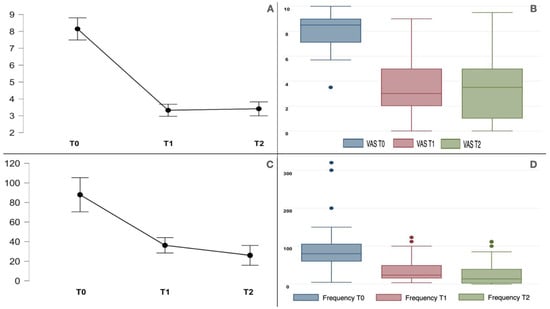
Background: Botulinum toxin type A is an effective treatment for trigeminal neuralgia. Moreover, its efficacy in type 2 trigeminal neuralgia and comparative studies between type 1 and type 2 trigeminal neuralgia (TN) still need to be improved. Methods: We treated 40 TN patients with onabotulinumtoxinA; 18 had type 1 TN, and 22 had type 2 TN. We compared the baseline pain score with the Visual Analogue Scale (VAS) and paroxysm frequency (number per week) at the baseline with those obtained at 1-month and 3-month follow-ups. Nonetheless, we compared the baseline Penn Facial Pain Scale with the scores obtained at the 1-month follow-up. Results: BoNT/A effectively reduced pain intensity and frequency at the 1-month and 3-month follow-ups. Moreover, the type 1 TN and type 2 TN groups had baseline pain scores of 7.8 ± 1.65 and 8.4 ± 1.1, respectively. Pain significantly improved (
p < 0.001) in both groups to 3.1 ± 2.3 (type 1 TN) and 3.5 ± 2.3 (type 2 TN) at the 1-month follow-up and to 3.2 ± 2.5 (type 1 TN) and 3.6 ± 2.5 (type 2 TN) at the 3-month follow-up. There was no difference between the two groups (
p 0.345). The baseline paroxysm frequencies (number per week) were 86.7 ± 69.3 and 88.9 ± 62.2 for the type 1 and type 2 TN groups, respectively; they were significantly reduced in both groups at the 1-month and 3-month follow-ups without significant differences between the two groups (
p 0.902). The Pain Facial Pain Scale improved at the 1-month follow-up, and no significant differences were found between the two groups. There was a strong correlation between background pain and paroxysm pain intensity (r 0.8,
p < 0.001). Conclusions: Botulinum toxin type A effectively reduced the pain, paroxysm frequency, and PFPS scores of type 1 and type 2 trigeminal neuralgia patients without statistically significant differences. Facial asymmetry was the only adverse event.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
Botulinum Toxin Type A for the Treatment of Auriculotemporal Neuralgia—A Case Series
by
Yan Tereshko, Enrico Belgrado, Christian Lettieri, Gian Luigi Gigli and Mariarosaria Valente
Cited by 4 | Viewed by 2092
Abstract
Auriculotemporal neuralgia is a rare pain disorder in which anesthetic nerve blockade is usually effective but not always resolutive. Botulinum toxin type A has proven to be effective in treating neuropathic pain, and patients with auriculotemporal neuralgia could also benefit from this treatment.
[...] Read more.
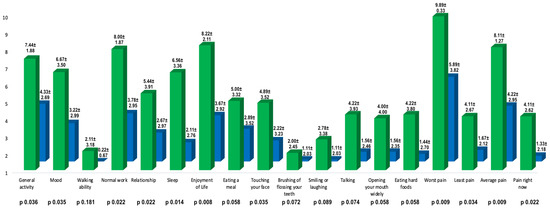
Auriculotemporal neuralgia is a rare pain disorder in which anesthetic nerve blockade is usually effective but not always resolutive. Botulinum toxin type A has proven to be effective in treating neuropathic pain, and patients with auriculotemporal neuralgia could also benefit from this treatment. We described nine patients with auriculotemporal neuralgia treated with botulinum toxin type A in the territory of auriculotemporal nerve innervation. We compared the basal NRS and Penn facial pain scale scores with those obtained 1 month after BoNT/A injections. Both Penn facial pain scale (96.67 ± 24.61 vs. 45.11 ± 36.70,
p 0.004; mean reduction 52.57 ± 36.50) and NRS scores (8.11 ± 1.27 vs. 4.22 ± 2.95,
p 0.009; mean reduction 3.89 ± 2.52) improved significantly at one month after treatment. The mean duration of the effect of BoNT/A on pain was 95.00 ± 53.03 days and no adverse effects were reported.
Full article
►▼
Show Figures
Open AccessArticle
OnabotulinumtoxinA Add-On to Monoclonal Anti-CGRP Antibodies in Treatment-Refractory Chronic Migraine
by
Andreas A. Argyriou, Emmanouil V. Dermitzakis, Georgia Xiromerisiou and Michail Vikelis
Cited by 17 | Viewed by 4423
Abstract
We sought to assess the effectiveness of combining dual therapy with onabotulinumtoxinA (BTX) add-on to anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies (anti-CGRP MAbs) in treatment-refractory patients with chronic migraine (CM). We retrospectively reviewed the medical files of 19 treatment-refractory patients with CM who
[...] Read more.
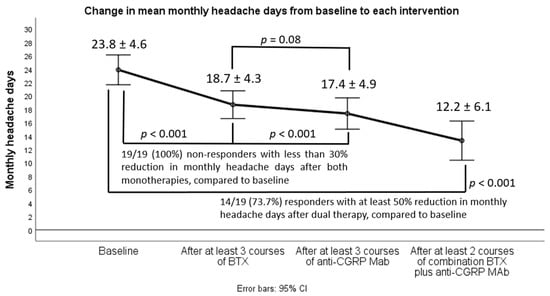
We sought to assess the effectiveness of combining dual therapy with onabotulinumtoxinA (BTX) add-on to anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies (anti-CGRP MAbs) in treatment-refractory patients with chronic migraine (CM). We retrospectively reviewed the medical files of 19 treatment-refractory patients with CM who had failed to two oral migraine preventatives, at least three consecutive BTX cycles (less than 30% response rate), at least three consecutive sessions with either fremanezumab or erenumab (less than 30% response rate), and were eventually switched to dual therapy with BTX add-on to any of the already-given anti-CGRP MAbs. We then assessed from baseline to each monotherapy or dual intervention predefined efficacy follow-up the changes in the following efficacy outcomes: (i) monthly headache days (MHD), (ii) monthly days with moderate/severe peak headache intensity, and (iii) monthly days with intake of any acute headache medication. Response (50% reduction in MHD) rates, safety, and tolerability were also determined. In the majority of cases (
n = 14), dual targeting proved effective and was associated with clinically meaningful improvement in all efficacy variables; 50% response rates (also disability and QOL outcomes) coupled with favorable safety/tolerability. Our results advocate in favor of the view that dual therapy is effective and should be considered in difficult-to-treat CM patients who have failed all available monotherapies.
Full article
►▼
Show Figures
Open AccessCase Report
Subcutaneous BoNT/A Injection for Intractable Pain and Disability in Complex Regional Pain Syndrome: A Case Report
by
Yan Tereshko, Chiara Dalla Torre, Christian Lettieri, Enrico Belgrado, Gian Luigi Gigli and Mariarosaria Valente
Cited by 4 | Viewed by 1902
Abstract
We treated a 51-year-old woman with refractory Complex Regional Pain Syndrome type I (CRPS-I) involving her left hand and forearm with subcutaneous injections of BoNT/A. The injections were performed every 3 months, with a total of six treatments. Each treatment was able to
[...] Read more.
We treated a 51-year-old woman with refractory Complex Regional Pain Syndrome type I (CRPS-I) involving her left hand and forearm with subcutaneous injections of BoNT/A. The injections were performed every 3 months, with a total of six treatments. Each treatment was able to effectively improve pain and motor impairment; however, the duration of the effect was limited to only a few months. BoNT/A could improve patients’ quality of life with CRPS; however, extensive clinical studies are needed to determine its role in clinical practice.
Full article
►▼
Show Figures
Open AccessReview
Variability of Botulinum Toxins: Challenges and Opportunities for the Future
by
Christine Rasetti-Escargueil, Emmanuel Lemichez and Michel R. Popoff
Cited by 13 | Viewed by 7160
Abstract
Botulinum neurotoxins (BoNTs) are the most potent known toxins, and are therefore classified as extremely harmful biological weapons. However, BoNTs are therapeutic drugs that are widely used and have an increasing number of applications. BoNTs show a high diversity and are divided into
[...] Read more.
Botulinum neurotoxins (BoNTs) are the most potent known toxins, and are therefore classified as extremely harmful biological weapons. However, BoNTs are therapeutic drugs that are widely used and have an increasing number of applications. BoNTs show a high diversity and are divided into multiple types and subtypes. Better understanding of the activity at the molecular and clinical levels of the natural BoNT variants as well as the development of BoNT-based chimeric molecules opens the door to novel medical applications such as silencing the sensory neurons at targeted areas and dermal restoration. This short review is focused on BoNTs’ variability and the opportunities or challenges posed for future clinical applications.
Full article
►▼
Show Figures
Open AccessArticle
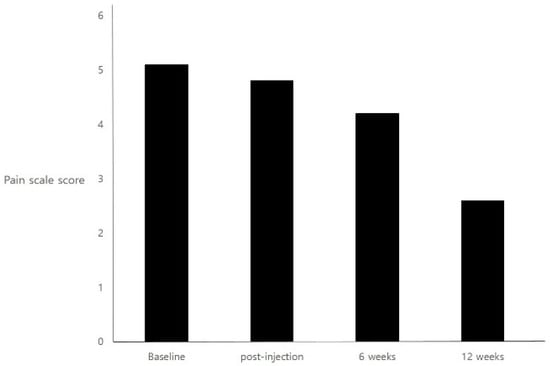
Safety and Efficacy of PrabotulinumtoxinA (Nabota®) Injection for Cervical and Shoulder Girdle Myofascial Pain Syndrome: A Pilot Study
by
Da-ye Kim and Jae Min Kim
Cited by 11 | Viewed by 5200
Abstract
Myofascial pain syndrome is a common painful condition encountered in the general population. Previous studies evaluating the efficacy of botulinum toxin for the treatment of myofascial pain syndrome are limited, with variable results. This prospective study investigated the efficacy and safety of direct
[...] Read more.
Myofascial pain syndrome is a common painful condition encountered in the general population. Previous studies evaluating the efficacy of botulinum toxin for the treatment of myofascial pain syndrome are limited, with variable results. This prospective study investigated the efficacy and safety of direct injection of Prabotulinumtoxin A (Nabota
®) into painful muscle groups for cervical and shoulder girdle myofascial pain. Twelve patients with chronic myofascial pain syndrome of the neck and shoulder underwent an injection of Prabotulinumtoxin A. Painful muscles containing trigger points were injected in the mid-belly. Pain scores and quality of life measurements were assessed at baseline, as well as 6 weeks and 12 weeks post-injection. Safety and tolerability were also assessed. This trial is registered under clinical research information service (CRIS) number KCT0001634. Patients injected with Prabotulinumtoxin A showed a significant improvement in pain at 12 weeks (
p < 0.001). At 6 weeks, the pain had not significantly improved compared with baseline (
p = 0.063). However, at that time, 41.7% of patients were characterized as Prabotulinumtoxin A responders, with a 30% reduction in pain rating score compared to baseline. In the Neck Disability Index scores, the patients demonstrated significant improvement at both 6 weeks and 12 weeks. No serious adverse effects occurred during the study. Prabotulinumtoxin A injection into chronically painful muscles associated with cervical and shoulder girdle myofascial pain syndrome resulted in an improvement in pain scores and quality of life lasting at least 12 weeks. Additionally, the injections were well tolerated. As these are preliminary findings in a pilot study, future studies should carefully consider using randomized, controlled, prospective trials.
Full article
►▼
Show Figures
Open AccessArticle
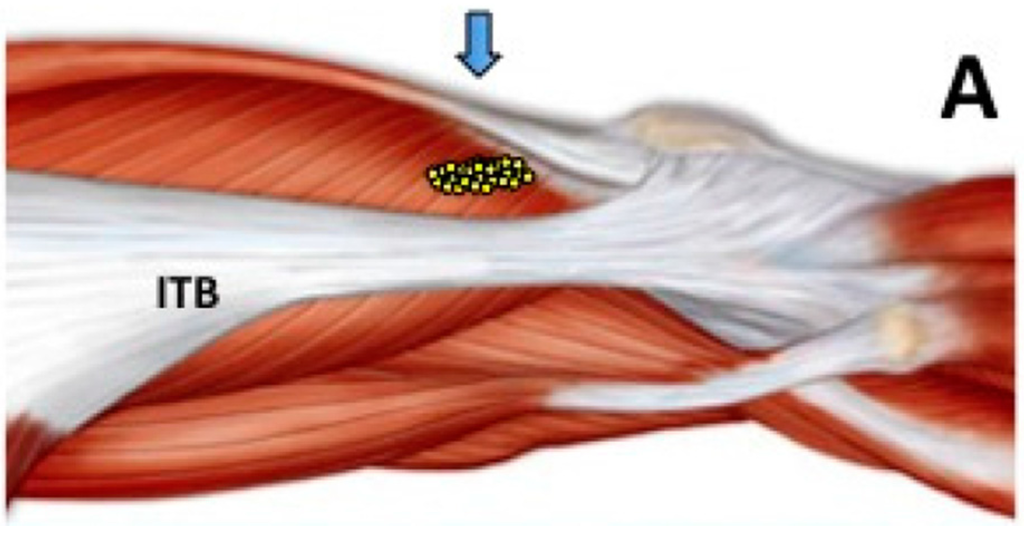
The Anatomical Basis of Paradoxical Masseteric Bulging after Botulinum Neurotoxin Type A Injection
by
Hyung-Jin Lee, In-Won Kang, Kyle K. Seo, You-Jin Choi, Seong-Taek Kim, Kyung-Seok Hu and Hee-Jin Kim
Cited by 29 | Viewed by 16601
Abstract
The aim of this study was to determine the detailed anatomical structures of the superficial part of the masseter and to elucidate the boundaries and locations of the deep tendon structure within the superficial part of the masseter. Forty-four hemifaces from Korean and
[...] Read more.
The aim of this study was to determine the detailed anatomical structures of the superficial part of the masseter and to elucidate the boundaries and locations of the deep tendon structure within the superficial part of the masseter. Forty-four hemifaces from Korean and Thai embalmed cadavers were used in this study. The deep tendon structure was located deep in the lower third of the superficial part of the masseter. It was observed in all specimens and was designated as a deep inferior tendon (DIT). The relationship between the masseter and DIT could be classified into three types according to the coverage pattern: Type A, in which areas IV and V were covered by the DIT (27%, 12/44); Type B, in which areas V and VI were covered by the DIT (23%, 10/44); and Type C, in which areas IV, V, and VI were covered by the DIT (50%, 22/44). The superficial part of the masseter consists of not only the muscle belly but also the deep tendon structure. Based on the results obtained in this morphological study, we recommend performing layer-by-layer retrograde injections into the superficial and deep muscle bellies of the masseter.
Full article
►▼
Show Figures
Open AccessArticle
Abobotulinum Toxin A in the Treatment of Chronic Low Back Pain
by
Duarte Machado, Aditya Kumar and Bahman Jabbari
Cited by 11 | Viewed by 5332
Abstract
Chronic low back pain is a debilitating condition with a complex and multifactorial pathophysiology. Botulinum neurotoxins (BoNTs) have strong analgesic effects, as shown in both animal models of pain and in human beings. A randomized, double-blind, placebo-controlled, parallel format study to investigate the
[...] Read more.
Chronic low back pain is a debilitating condition with a complex and multifactorial pathophysiology. Botulinum neurotoxins (BoNTs) have strong analgesic effects, as shown in both animal models of pain and in human beings. A randomized, double-blind, placebo-controlled, parallel format study to investigate the efficacy of abobotulinum toxin A (aboA) in chronic low back pain was conducted. The study cohort consisted of 18 patients who received 100 units of aboA into each of the five lumbar extensor spinae muscles unilaterally or bilaterally (total dose 500 to 1000 units), and 19 who received normal saline of the same volume. The level of pain and quality of life were assessed using the visual analogue scale (VAS) and three questionnaires including the Oswestry Low Back Pain Disability Questionnaire (OLBPDQ). Patients’ perception of improvement was recorded via patient global impression of change (PGIC). The primary outcome measure, the proportion of responders with VAS of <4 at 6 weeks, was not met, but the data was significantly in favor of aboA at 4 weeks (
p = 0.008). The total Oswestry score representing quality of life improved in the aboA group compared to the placebo group (
p = 0.0448). Moreover, significantly more patients reported their low back pain as “much improved” in the abobotulinum toxin A group (0.0293).
Full article
Open AccessArticle
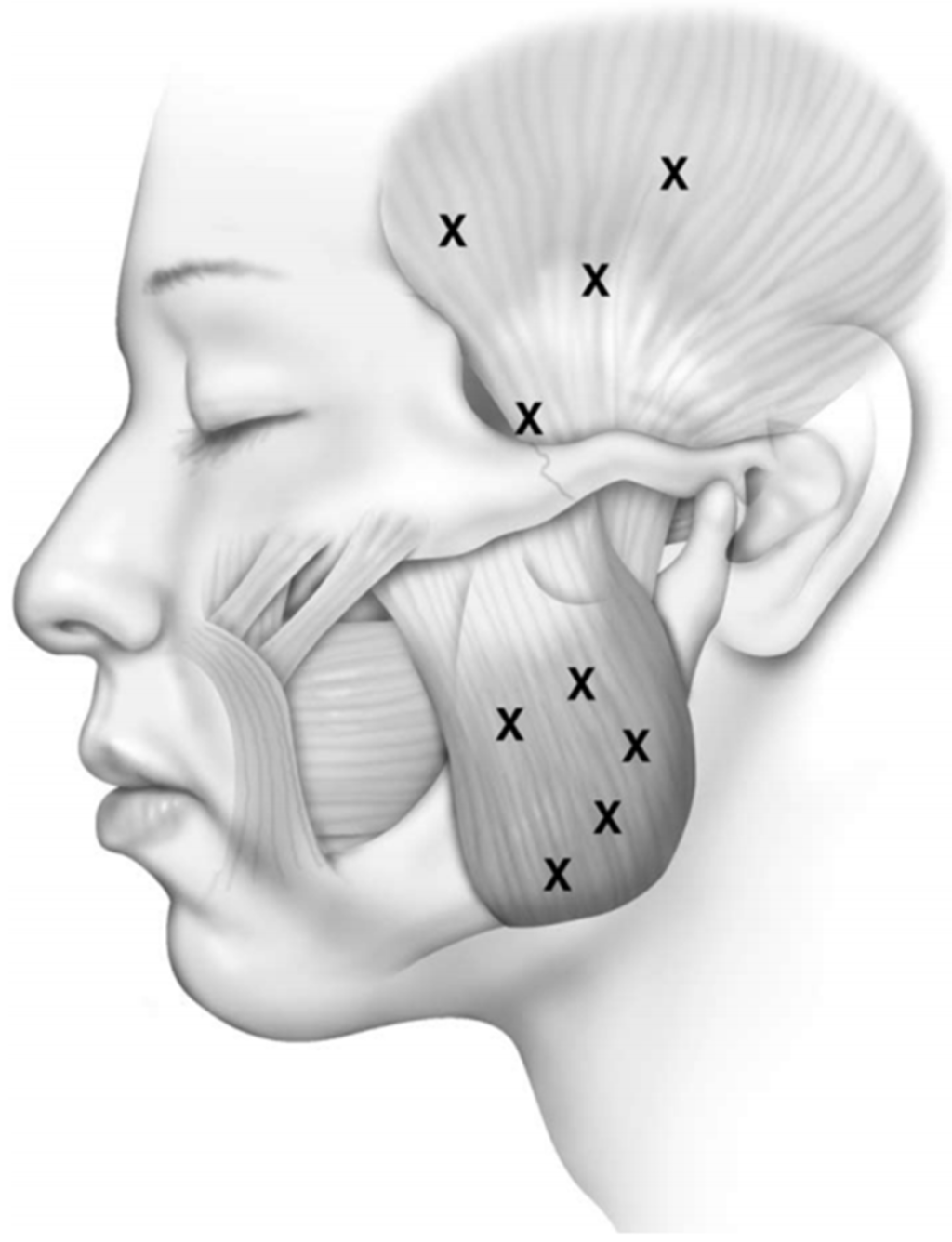
Effective Botulinum Toxin Injection Guide for Treatment of Temporal Headache
by
You-Jin Choi, Won-Jae Lee, Hyung-Jin Lee, Kang-Woo Lee, Hee-Jin Kim and Kyung-Seok Hu
Cited by 17 | Viewed by 11586
Abstract
This study involved an extensive analysis of published research on the morphology of the temporalis muscle in order to provide an anatomical guideline on how to distinguish the temporalis muscle and temporalis tendon by observing the surface of the patient’s face. Twenty-one hemifaces
[...] Read more.
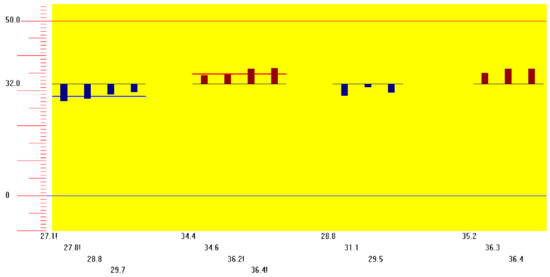
This study involved an extensive analysis of published research on the morphology of the temporalis muscle in order to provide an anatomical guideline on how to distinguish the temporalis muscle and temporalis tendon by observing the surface of the patient’s face. Twenty-one hemifaces of cadavers were used in this study. The temporalis muscles were dissected clearly for morphological analysis between the temporalis muscle and tendon. The posterior border of the temporalis tendon was classified into three types: in Type I the posterior border of the temporalis tendon is located in front of reference line L2 (4.8%, 1/21), in Type II it is located between reference lines L2 and L3 (85.7%, 18/21), and in Type III it is located between reference lines L3 and L4 (9.5%, 2/21). The vertical distances between the horizontal line passing through the jugale (LH) and the temporalis tendon along each of reference lines L0, L1, L2, L3, and L4 were 29.7 ± 6.8 mm, 45.0 ± 8.8 mm, 37.7 ± 11.1 mm, 42.5 ± 7.5 mm, and 32.1 ± 0.4 mm, respectively. BoNT-A should be injected into the temporalis muscle at least 45 mm vertically above the zygomatic arch. This will ensure that the muscle region is targeted and so produce the greatest clinical effect with the minimum concentration of BoNT-A.
Full article
►▼
Show Figures
Open AccessArticle
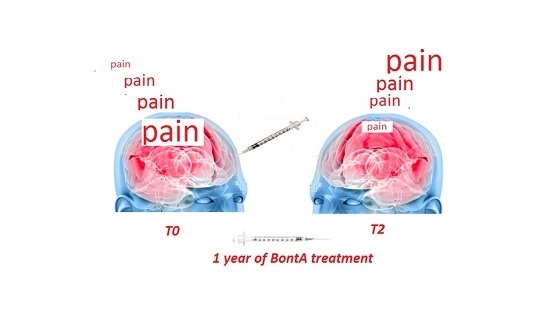
Effects of OnabotulintoxinA on Habituation of Laser Evoked Responses in Chronic Migraine
by
Marina De Tommaso, Marianna Delussi, Katia Ricci, Anna Montemurno, Irene Carbone and Eleonora Vecchio
Cited by 22 | Viewed by 5068
Abstract
Onabotulintoxin A (BontA) is an efficacious preventive treatment for chronic migraine, though the specific mechanism of action is still under discussion. The study aims: (1) To evaluate pain processing modifications in chronic migraine patients (CM) under single BontA administration in pericranial muscles, by
[...] Read more.
Onabotulintoxin A (BontA) is an efficacious preventive treatment for chronic migraine, though the specific mechanism of action is still under discussion. The study aims: (1) To evaluate pain processing modifications in chronic migraine patients (CM) under single BontA administration in pericranial muscles, by means of CO
2 Laser Evoked Potentials (LEPs) obtained by the stimulation of the skin over the right frontal and trapezius injection sites and hand dorsum, in a double blind placebo controlled crossover design. (2) To correlate main LEPs findings with clinical outcome after one year of BontA treatment. Twenty refractory CM patients were included in the analysis. The LEPs were recorded in basal conditions and seven days after BontA (PREEMPT protocol) and saline solution injection. The N1, N2 and P2 amplitude and latencies and N2P2 habituation index were evaluated and correlated with the percent change of headache frequency after one year of toxin treatment. After seven days of BontA treatment, a normalization of the trigeminal habituation index was observed, which was correlated with the clinical outcome after one year of BontA therapy. Patients displaying trigeminal LEPs facilitation at T0 time showed a more efficient therapeutic outcome. Neurotoxin may exert a modulating effect on trigeminal nociception, normalizing central neurotransmission.
Full article
►▼
Show Figures
Open AccessArticle
Incobotulinum Toxin-A Improves Post-Surgical and Post-Radiation Pain in Cancer Patients
by
Rezvan Rostami, Shivam Om Mittal, Reza Radmand and Bahman Jabbari
Cited by 24 | Viewed by 5944
Abstract
Cancer patients who undergo surgery or radiation can develop persistent focal pain at the site of radiation or surgery. Twelve patients who had surgery or radiation for local cancer and failed at least two analgesic medications for pain control were prospectively enrolled in
[...] Read more.
Cancer patients who undergo surgery or radiation can develop persistent focal pain at the site of radiation or surgery. Twelve patients who had surgery or radiation for local cancer and failed at least two analgesic medications for pain control were prospectively enrolled in a research protocol. Patients were injected up to 100 units of incobotulinum toxin A (IncoA) intramuscularly or subcutaneously depending on the type and location of pain (muscle cramp or neuropathic pain). Two patients passed away, one dropped out due to a skin reaction and another patient could not return for the follow up due to his poor general condition. All remaining 8 subjects (Age 31–70, 4 female) demonstrated significant improvement of Visual Analog Scale (VAS) (3 to 9 degrees, average 3.9 degrees) and reported significant satisfaction in Patients’ Global Impression of Change scale (PGIC) (7 out of 8 reported the pain as much improved). Three of the 8 patients reported significant improvement of quality of life.
Full article
Open AccessCase Report
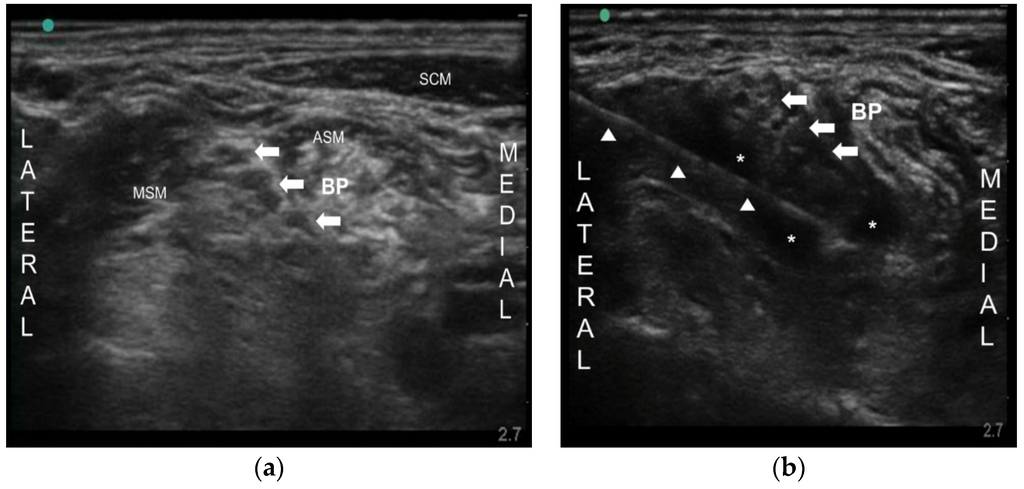
Ultrasound-Guided Nerve Block with Botulinum Toxin Type A for Intractable Neuropathic Pain
by
Young Eun Moon, Jung Hyun Choi, Hue Jung Park, Ji Hye Park and Ji Hyun Kim
Cited by 19 | Viewed by 10114
Abstract
Neuropathic pain includes postherpetic neuralgia (PHN), painful diabetic neuropathy (PDN), and trigeminal neuralgia, and so on. Although various drugs have been tried to treat neuropathic pain, the effectiveness of the drugs sometimes may be limited for chronic intractable neuropathic pain, especially when they
[...] Read more.
Neuropathic pain includes postherpetic neuralgia (PHN), painful diabetic neuropathy (PDN), and trigeminal neuralgia, and so on. Although various drugs have been tried to treat neuropathic pain, the effectiveness of the drugs sometimes may be limited for chronic intractable neuropathic pain, especially when they cannot be used at an adequate dose, due to undesirable severe side effects and the underlying disease itself. Botulinum toxin type A (BoNT-A) has been known for its analgesic effect in various pain conditions. Nevertheless, there are no data of nerve block in PHN and PDN. Here, we report two patients successfully treated with ultrasound-guided peripheral nerve block using BoNT-A for intractable PHN and PDN. One patient had PHN on the left upper extremity and the other patient had PDN on a lower extremity. Due to side effects of drugs, escalation of the drug dose could not be made. We injected 50 Botox units (BOTOX
®, Allergan Inc., Irvine, CA, USA) into brachial plexus and lumbar plexus, respectively, under ultrasound. Their pain was significantly decreased for about 4–5 months. Ultrasound-guided nerve block with BoNT-A may be an effective analgesic modality in a chronic intractable neuropathic pain especially when conventional treatment failed to achieve adequate pain relief.
Full article
►▼
Show Figures
Open AccessArticle
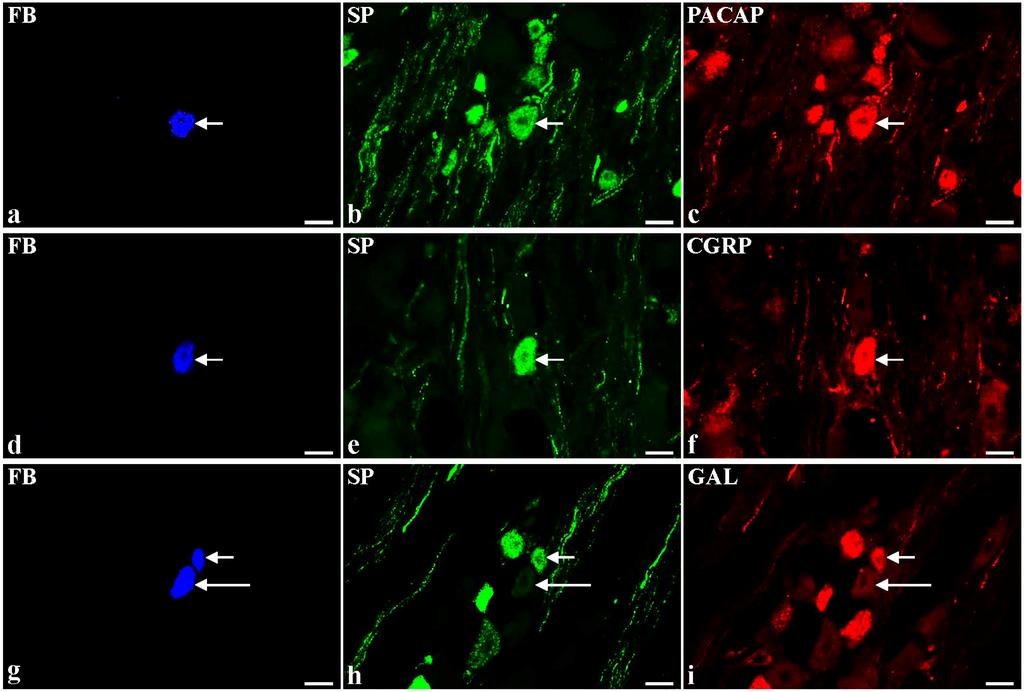
Botulinum Toxin Type A Induces Changes in the Chemical Coding of Substance P-Immunoreactive Dorsal Root Ganglia Sensory Neurons Supplying the Porcine Urinary Bladder
by
Agnieszka Bossowska, Ewa Lepiarczyk, Urszula Mazur, Paweł Janikiewicz and Włodzimierz Markiewicz
Cited by 15 | Viewed by 6088
Abstract
Botulinum toxin (BTX) is a potent neurotoxin which blocks acetylcholine release from nerve terminals, and therefore leads to cessation of somatic motor and/or parasympathetic transmission. Recently it has been found that BTX also interferes with sensory transmission, thus, the present study was aimed
[...] Read more.
Botulinum toxin (BTX) is a potent neurotoxin which blocks acetylcholine release from nerve terminals, and therefore leads to cessation of somatic motor and/or parasympathetic transmission. Recently it has been found that BTX also interferes with sensory transmission, thus, the present study was aimed at investigating the neurochemical characterization of substance P-immunoreactive (SP-IR) bladder-projecting sensory neurons (BPSN) after the toxin treatment. Investigated neurons were visualized with retrograde tracing method and their chemical profile was disclosed with double-labelling immunohistochemistry using antibodies against SP, calcitonin gene-related peptide (CGRP), pituitary adenylate cyclase activating polypeptide (PACAP), neuronal nitric oxide synthase (nNOS), galanin (GAL), calbindin (CB), and somatostatin (SOM). In the control group (
n = 6), 45% of the total population of BPSN were SP-IR. Nearly half of these neurons co-expressed PACAP or CGRP (45% and 35%, respectively), while co-localization of SP with GAL, nNOS, SOM or CB was found less frequently (3.7%, 1.8%, 1.2%, and 0.7%, respectively). In BTX-treated pigs (
n = 6), toxin-injections caused a decrease in the number of SP-IR cells containing CGRP, SOM or CB (16.2%, 0.5%, and 0%, respectively) and a distinct increase in these nerve cells immunopositive to GAL (27.2%). The present study demonstrates that BTX significantly modifies the chemical phenotypes of SP-IR BPSN.
Full article
►▼
Show Figures
Open AccessReview
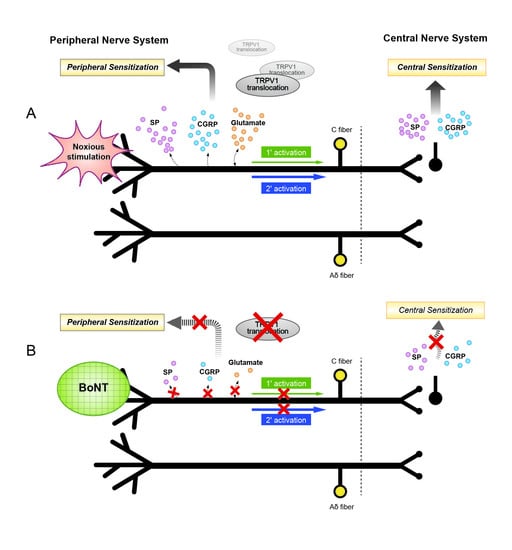
Current Status and Future Directions of Botulinum Neurotoxins for Targeting Pain Processing
by
Sabine Pellett, Tony L. Yaksh and Roshni Ramachandran
Cited by 52 | Viewed by 10006
Abstract
Current evidence suggests that botulinum neurotoxins (BoNTs) A1 and B1, given locally into peripheral tissues such as skin, muscles, and joints, alter nociceptive processing otherwise initiated by inflammation or nerve injury in animal models and humans. Recent data indicate that such locally delivered
[...] Read more.
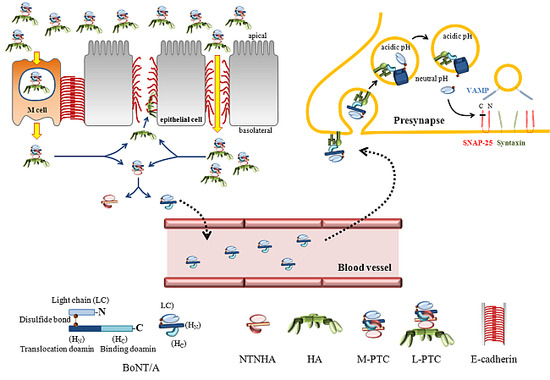
Current evidence suggests that botulinum neurotoxins (BoNTs) A1 and B1, given locally into peripheral tissues such as skin, muscles, and joints, alter nociceptive processing otherwise initiated by inflammation or nerve injury in animal models and humans. Recent data indicate that such locally delivered BoNTs exert not only local action on sensory afferent terminals but undergo transport to central afferent cell bodies (dorsal root ganglia) and spinal dorsal horn terminals, where they cleave SNAREs and block transmitter release. Increasing evidence supports the possibility of a trans-synaptic movement to alter postsynaptic function in neuronal and possibly non-neuronal (glial) cells. The vast majority of these studies have been conducted on BoNT/A1 and BoNT/B1, the only two pharmaceutically developed variants. However, now over 40 different subtypes of botulinum neurotoxins (BoNTs) have been identified. By combining our existing and rapidly growing understanding of BoNT/A1 and /B1 in altering nociceptive processing with explorations of the specific characteristics of the various toxins from this family, we may be able to discover or design novel, effective, and long-lasting pain therapeutics. This review will focus on our current understanding of the molecular mechanisms whereby BoNTs alter pain processing, and future directions in the development of these agents as pain therapeutics.
Full article
►▼
Show Figures
Open AccessArticle
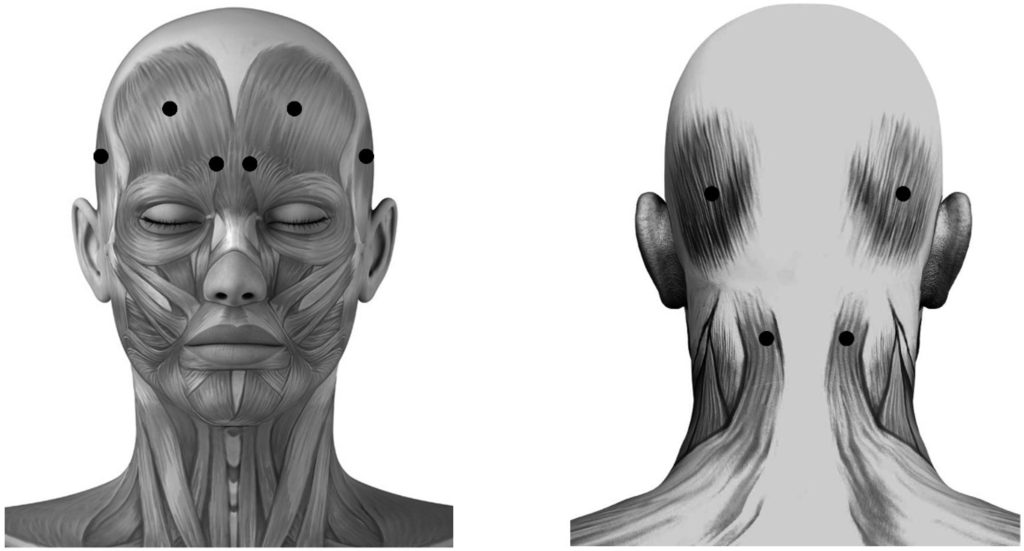
Acupoint Injection of Onabotulinumtoxin A for Migraines
by
Min Hou, Jun-Fan Xie, Xiang-Pan Kong, Yi Zhang, Yu-Feng Shao, Can Wang, Wen-Ting Ren, Guang-Fu Cui, Le Xin and Yi-Ping Hou
Cited by 16 | Viewed by 8631
Abstract
Onabotulinumtoxin A (BoNTA) has been reported to be effective in the therapy for migraines. Acupuncture has been used worldwide for the treatment of migraine attacks. Injection of a small amount of drug at acupuncture points is an innovation as compared to traditional acupuncture.
[...] Read more.
Onabotulinumtoxin A (BoNTA) has been reported to be effective in the therapy for migraines. Acupuncture has been used worldwide for the treatment of migraine attacks. Injection of a small amount of drug at acupuncture points is an innovation as compared to traditional acupuncture. The purpose of this study was to evaluate and compare the effectiveness of fixed (muscle)-site and acupoint-site injections of BoNTA for migraine therapy in a randomized, double-blinded, placebo-controlled clinical trial extending over four months. Subjects with both episodic and chronic migraines respectively received a placebo (
n = 19) or BoNTA (2.5 U each site, 25 U per subject) injection at fixed-sites (
n = 41) including occipitofrontalis, corrugator supercilii, temporalis and trapeziue, or at acupoint-sites (
n = 42) including Yintang (EX-HN3), Taiyang (EX-HN5), Baihui (GV20), Shuaigu (GB8), Fengchi (GB20) and Tianzhu (BL10). The variations between baseline and BoNTA post-injection for four months were calculated monthly as outcome measures. BoNTA injections at fixed-sites and acupoint-sites significantly reduced the migraine attack frequency, intensity, duration and associated symptoms for four months compared with placebo (
p < 0.01). The efficacy of BoNTA for migraines in the acupoint-site group (93% improvement) was more significant than that in the fixed-site group (85% improvement) (
p < 0.01). BoNTA administration for migraines is effective, and at acupoint-sites shows more efficacy than at fixed-sites. Further blinded studies are necessary to establish the efficacy of a low dose toxin (25 U) introduced with this methodology in chronic and episodic migraines.
Full article
►▼
Show Figures
Open AccessArticle
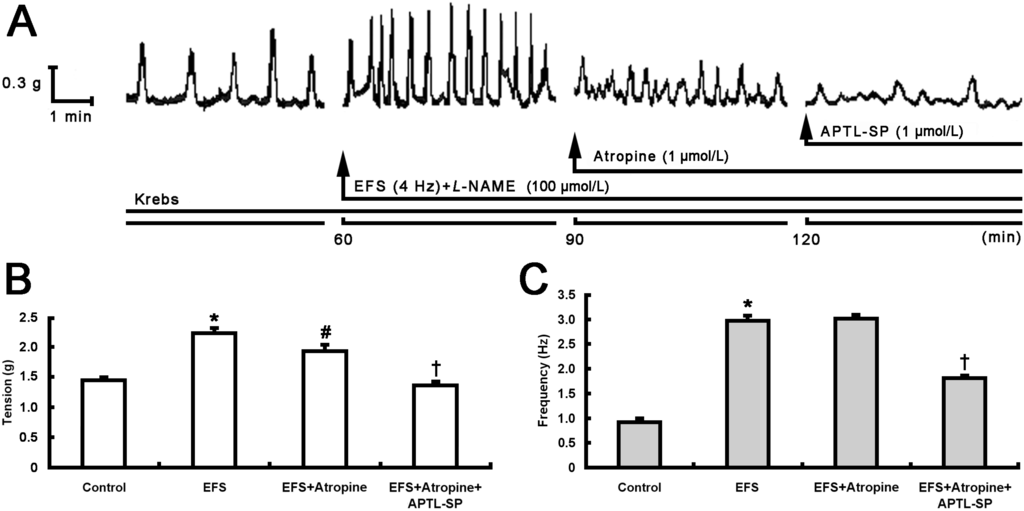
The Inhibitory Effect of Botulinum Toxin Type A on Rat Pyloric Smooth Muscle Contractile Response to Substance P In Vitro
by
Yu-Feng Shao, Jun-Fan Xie, Yin-Xiang Ren, Can Wang, Xiang-Pan Kong, Xiao-Jian Zong, Lin-Lan Fan and Yi-Ping Hou
Cited by 8 | Viewed by 7733
Abstract
A decrease in pyloric myoelectrical activity and pyloric substance P (SP) content following intrasphincteric injection of botulinum toxin type A (BTX-A) in free move rats have been demonstrated in our previous studies. The aim of the present study was to investigate the inhibitory
[...] Read more.
A decrease in pyloric myoelectrical activity and pyloric substance P (SP) content following intrasphincteric injection of botulinum toxin type A (BTX-A) in free move rats have been demonstrated in our previous studies. The aim of the present study was to investigate the inhibitory effect of BTX-A on rat pyloric muscle contractile response to SP
in vitro and the distributions of SP and neurokinin 1 receptor (NK1R) immunoreactive (IR) cells and fibers within pylorus. After treatment with atropine, BTX-A (10 U/mL), similar to [D-Arg
1, D-Phe
5, D-Trp
7,9, Leu
11]-SP (APTL-SP, 1 μmol/L) which is an NK1R antagonist, decreased electric field stimulation (EFS)-induced contractile tension and frequency, whereas, subsequent administration of APTL-SP did not act on contractility. Incubation with BTX-A at 4 and 10 U/mL for 4 h respectively decreased SP (1 μmol/L)-induced contractions by 26.64% ± 5.12% and 74.92% ± 3.62%. SP-IR fibers and NK1R-IR cells both located within pylorus including mucosa and circular muscle layer. However, fewer SP-fibers were observed in pylorus treated with BTX-A (10 U/mL). In conclusion, BTX-A inhibits SP release from enteric terminals in pylorus and EFS-induced contractile responses when muscarinic cholinergic receptors are blocked by atropine. In addition, BTX-A concentration- and time-dependently directly inhibits SP-induced pyloric smooth muscle contractility.
Full article
►▼
Show Figures
Open AccessReview
Botulinum Toxin Type A as a Therapeutic Agent against Headache and Related Disorders
by
Siro Luvisetto, Parisa Gazerani, Carlo Cianchetti and Flaminia Pavone
Cited by 53 | Viewed by 8902
Abstract
Botulinum neurotoxin A (BoNT/A) is a toxin produced by the naturally-occurring
Clostridium botulinum that causes botulism. The potential of BoNT/A as a useful medical intervention was discovered by scientists developing a vaccine to protect against botulism. They found that, when injected into a
[...] Read more.
Botulinum neurotoxin A (BoNT/A) is a toxin produced by the naturally-occurring
Clostridium botulinum that causes botulism. The potential of BoNT/A as a useful medical intervention was discovered by scientists developing a vaccine to protect against botulism. They found that, when injected into a muscle, BoNT/A causes a flaccid paralysis. Following this discovery, BoNT/A has been used for many years in the treatment of conditions of pathological muscle hyperactivity, like dystonias and spasticities. In parallel, the toxin has become a “glamour” drug due to its power to ward off facial wrinkles, particularly frontal, due to the activity of the mimic muscles. After the discovery that the drug also appeared to have a preventive effect on headache, scientists spent many efforts to study the potentially-therapeutic action of BoNT/A against pain. BoNT/A is effective at reducing pain in a number of disease states, including cervical dystonia, neuropathic pain, lower back pain, spasticity, myofascial pain and bladder pain. In 2010, regulatory approval for the treatment of chronic migraine with BoNT/A was given, notwithstanding the fact that the mechanism of action is still not completely elucidated. In the present review, we summarize experimental evidence that may help to clarify the mechanisms of action of BoNT/A in relation to the alleviation of headache pain, with particular emphasis on preclinical studies, both in animals and humans. Moreover, we summarize the latest clinical trials that show evidence on headache conditions that may obtain benefits from therapy with BoNT/A.
Full article
Open AccessReview
The Role of Botulinum Toxin Type A in the Clinical Management of Refractory Anterior Knee Pain
by
Barbara J. Singer, Benjamin I. Silbert, Peter L. Silbert and Kevin P. Singer
Cited by 7 | Viewed by 7479
Abstract
Anterior knee pain is a highly prevalent condition affecting largely young to middle aged adults. Symptoms can recur in more than two thirds of cases, often resulting in activity limitation and reduced participation in employment and recreational pursuits. Persistent anterior knee pain is
[...] Read more.
Anterior knee pain is a highly prevalent condition affecting largely young to middle aged adults. Symptoms can recur in more than two thirds of cases, often resulting in activity limitation and reduced participation in employment and recreational pursuits. Persistent anterior knee pain is difficult to treat and many individuals eventually consider a surgical intervention. Evidence for long term benefit of most conservative treatments or surgical approaches is currently lacking. Injection of Botulinum toxin type A to the distal region of vastus lateralis muscle causes a short term functional “denervation” which moderates the influence of vastus lateralis muscle on the knee extensor mechanism and increases the relative contribution of the vastus medialis muscle. Initial data suggest that, compared with other interventions for anterior knee pain, Botulinum toxin type A injection, in combination with an active exercise programme, can lead to sustained relief of symptoms, reduced health care utilisation and increased activity participation. The procedure is less invasive than surgical intervention, relatively easy to perform, and is time- and cost-effective. Further studies, including larger randomized placebo-controlled trials, are required to confirm the effectiveness of Botulinum toxin type A injection for anterior knee pain and to elaborate the possible mechanisms underpinning pain and symptom relief.
Full article
►▼
Show Figures
Open AccessReview
Botulinum Toxin for Neuropathic Pain: A Review of the Literature
by
Hyun-Mi Oh and Myung Eun Chung
Cited by 105 | Viewed by 15119
Abstract
Botulinum neurotoxin (BoNT), derived from
Clostridium botulinum,
has been used therapeutically for focal dystonia, spasticity, and chronic migraine. Its spectrum as a potential treatment for neuropathic pain has grown. Recent opinions on the mechanism behind the antinociceptive effects of BoNT suggest that
[...] Read more.
Botulinum neurotoxin (BoNT), derived from
Clostridium botulinum,
has been used therapeutically for focal dystonia, spasticity, and chronic migraine. Its spectrum as a potential treatment for neuropathic pain has grown. Recent opinions on the mechanism behind the antinociceptive effects of BoNT suggest that it inhibits the release of peripheral neurotransmitters and inflammatory mediators from sensory nerves. There is some evidence showing the axonal transport of BoNT, but it remains controversial. The aim of this review is to summarize the experimental and clinical evidence of the antinociceptive effects, mechanisms, and therapeutic applications of BoNT for neuropathic pain conditions, including postherpetic neuralgia, complex regional pain syndrome, and trigeminal neuralgia. The PubMed and OvidSP databases were searched from 1966 to May 2015. We assessed levels of evidence according to the American Academy of Neurology guidelines. Recent studies have suggested that BoNT injection is an effective treatment for postherpetic neuralgia and is likely efficient for trigeminal neuralgia and post-traumatic neuralgia. BoNT could also be effective as a treatment for diabetic neuropathy. It has not been proven to be an effective treatment for occipital neuralgia or complex regional pain syndrome.
Full article
►▼
Show Figures
Open AccessReview
Ultrasound-Guided Injection of Botulinum Toxin Type A for Piriformis Muscle Syndrome: A Case Report and Review of the Literature
by
Andrea Santamato, Maria Francesca Micello, Giovanni Valeno, Raffaele Beatrice, Nicoletta Cinone, Alessio Baricich, Alessandro Picelli, Francesco Panza, Giancarlo Logroscino, Pietro Fiore and Maurizio Ranieri
Cited by 30 | Viewed by 12015
Abstract
Piriformis muscle syndrome (PMS) is caused by prolonged or excessive contraction of the piriformis muscle associated with pain in the buttocks, hips, and lower limbs because of the close proximity to the sciatic nerve. Botulinum toxin type A (BoNT-A) reduces muscle hypertonia as
[...] Read more.
Piriformis muscle syndrome (PMS) is caused by prolonged or excessive contraction of the piriformis muscle associated with pain in the buttocks, hips, and lower limbs because of the close proximity to the sciatic nerve. Botulinum toxin type A (BoNT-A) reduces muscle hypertonia as well as muscle contracture and pain inhibiting substance P release and other inflammatory factors. BoNT-A injection technique is important considering the difficult access of the needle for deep location, the small size of the muscle, and the proximity to neurovascular structures. Ultrasound guidance is easy to use and painless and several studies describe its use during BoNT-A administration in PMS. In the present review article, we briefly updated current knowledge regarding the BoNT therapy of PMS, describing also a case report in which this syndrome was treated with an ultrasound-guided injection of incobotulinumtoxin A. Pain reduction with an increase of hip articular range of motion in this patient with PMS confirmed the effectiveness of BoNT-A injection for the management of this syndrome.
Full article
►▼
Show Figures
Open AccessArticle
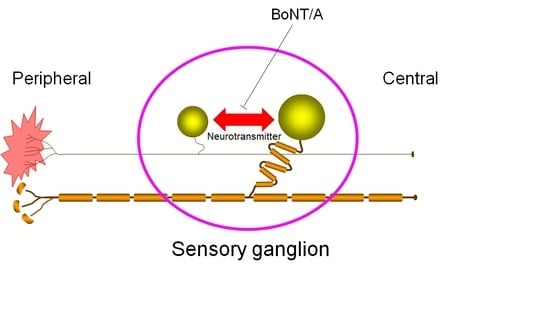
Cross-Excitation in Peripheral Sensory Ganglia Associated with Pain Transmission
by
Katsuhiro Omoto, Kotaro Maruhama, Ryuji Terayama, Yumiko Yamamoto, Osamu Matsushita, Tomosada Sugimoto, Keiji Oguma and Yoshizo Matsuka
Cited by 11 | Viewed by 5836
Abstract
Despite the absence of synaptic contacts, cross-excitation of neurons in sensory ganglia during signal transmission is considered to be chemically mediated and appears increased in chronic pain states. In this study, we modulated neurotransmitter release in sensory neurons by direct application of type
[...] Read more.
Despite the absence of synaptic contacts, cross-excitation of neurons in sensory ganglia during signal transmission is considered to be chemically mediated and appears increased in chronic pain states. In this study, we modulated neurotransmitter release in sensory neurons by direct application of type A botulinum neurotoxin (BoNT/A) to sensory ganglia in an animal model of neuropathic pain and evaluated the effect of this treatment on nocifensive. Unilateral sciatic nerve entrapment (SNE) reduced the ipsilateral hindpaw withdrawal threshold to mechanical stimulation and reduced hindpaw withdrawal latency to thermal stimulation. Direct application of BoNT/A to the ipsilateral L4 dorsal root ganglion (DRG) was localized in the cell bodies of the DRG and reversed the SNE-induced decreases in withdrawal thresholds within 2 days of BoNT/A administration. Results from this study suggest that neurotransmitter release within sensory ganglia is involved in the regulation of pain-related signal transmission.
Full article
►▼
Show Figures
Open AccessArticle
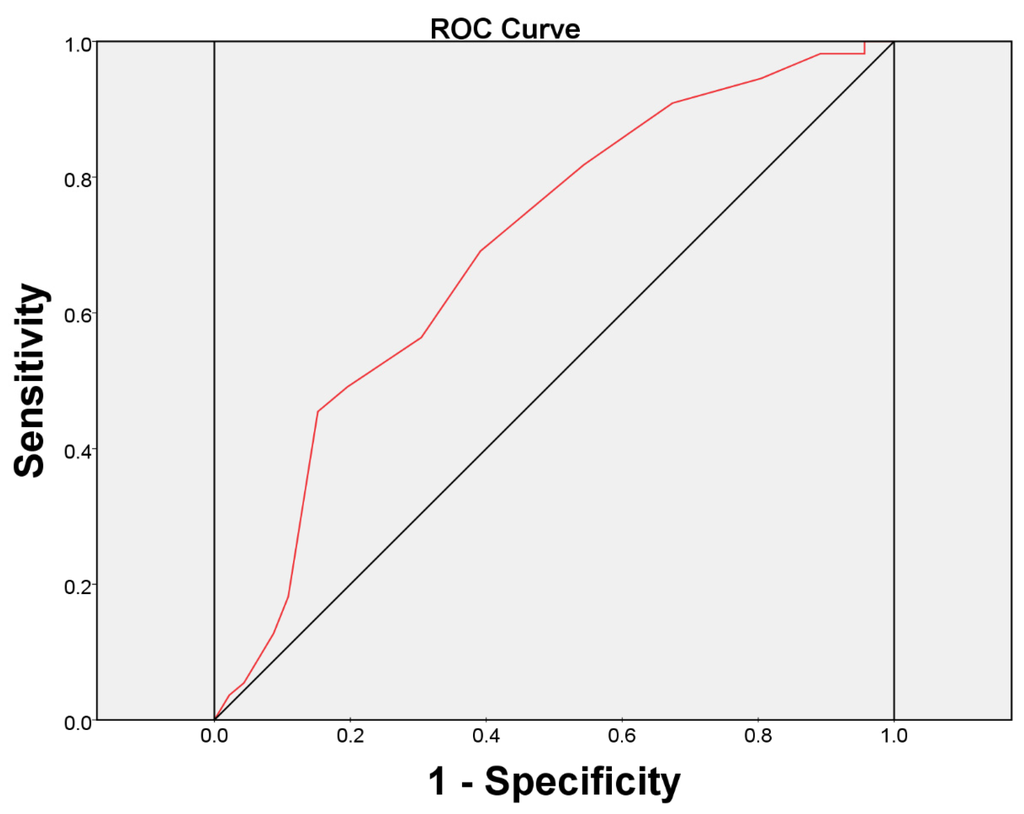
O’Leary-Sant Symptom Index Predicts the Treatment Outcome for OnabotulinumtoxinA Injections for Refractory Interstitial Cystitis/Bladder Pain Syndrome
by
Yuh-Chen Kuo and Hann-Chorng Kuo
Cited by 10 | Viewed by 5966
Abstract
Although intravesical injection of onabotulinumtoxinA (BoNT-A) has been proved promising in treating patients with interstitial cystitis/bladder pain syndrome (IC/BPS), what kind of patients that may benefit from this treatment remains unclear. This study investigated the predictors for a successful treatment outcome. Patients with
[...] Read more.
Although intravesical injection of onabotulinumtoxinA (BoNT-A) has been proved promising in treating patients with interstitial cystitis/bladder pain syndrome (IC/BPS), what kind of patients that may benefit from this treatment remains unclear. This study investigated the predictors for a successful treatment outcome. Patients with IC/BPS who failed conventional treatments were enrolled to receive intravesical injection of 100 U of BoNT-A immediately followed by hydrodistention. Variables such as O’Leary-Sant symptom and problem indexes (ICSI and ICPI), pain visual analogue scale (VAS), functional bladder capacity (FBC), voiding diary, and urodynamic parameters were measured at baseline and six months after treatment. A global response assessment (GRA) ≥ 2 at six months was defined as successful. There were101 patients enrolled. Significant improvements were observed in mean ICSI, ICPI, OSS (ICSI + ICPI), pain VAS, FBC, frequency, nocturia and GRA at six months after BoNT-A injections (all
p < 0.05). The successful rate at six months was 46/101 (45.54%). Multivariate logistic regression revealed the baseline ICSI (odds ratio = 0.770, 95% confidence interval = 0.601–0.989) was the only predictor for a treatment outcome. ICSI ≥ 12 was the most predictive cutoff value for a treatment failure, with a ROC area of 0.70 (sensitivity = 69.1%, specificity = 60.9%).
Full article
►▼
Show Figures
Open AccessReview
Temporomandibular Myofacial Pain Treated with Botulinum Toxin Injection
by
Niv Mor, Christropher Tang and Andrew Blitzer
Cited by 42 | Viewed by 17192
Abstract
This article reviews the diagnoses and treatment of temporomandibular disorders (TMD) and outlines of the role of botulinum toxin (BoNT) in the treatment of myofacial TMD. This manuscript includes a brief history of the use of BoNT in the treatment of pain, the
[...] Read more.
This article reviews the diagnoses and treatment of temporomandibular disorders (TMD) and outlines of the role of botulinum toxin (BoNT) in the treatment of myofacial TMD. This manuscript includes a brief history of the use of BoNT in the treatment of pain, the mechanism of action of BoNT, and the techniques for injections, adverse effects and contraindications when using BoNT to treat mayofacial pain caused by TMD.
Full article
►▼
Show Figures
Open AccessReview
Treatment of Chronic Migraine with OnabotulinumtoxinA: Mode of Action, Efficacy and Safety
by
Délia Szok, Anett Csáti, László Vécsei and János Tajti
Cited by 22 | Viewed by 7056
Abstract
Background: Chronic migraine is a common, highly disabling, underdiagnosed and undertreated entity of migraine. It affects 0.9%–2.2% of the general adult population. The present paper overviews the preclinical and clinical data regarding the therapeutic effect of onabotulinumtoxinA in chronic migraineurs. Methods: A literature
[...] Read more.
Background: Chronic migraine is a common, highly disabling, underdiagnosed and undertreated entity of migraine. It affects 0.9%–2.2% of the general adult population. The present paper overviews the preclinical and clinical data regarding the therapeutic effect of onabotulinumtoxinA in chronic migraineurs. Methods: A literature search was conducted in the database of PubMed up to 20 May 2015 for articles related to the pathomechanism of chronic migraine, the mode of action, and the efficacy, safety and tolerability of onabotulinumtoxinA for the preventive treatment of chronic migraine. Results: The pathomechanism of chronic migraine has not been fully elucidated. The mode of action of onabotulinumtoxinA in the treatment of chronic migraine is suggested to be related to the inhibition of the release of calcitonin gene-related peptide and substance P in the trigeminovascular system. Randomized clinical trials demonstrated that long-term onabotulinumtoxinA fixed-site and fixed-dose (155–195 U) intramuscular injection therapy was effective and well tolerated for the prophylactic treatment of chronic migraine. Conclusions: Chronic migraine is a highly devastating entity of migraine. Its exact pathomechanism is unrevealed. Two-third of chronic migraineurs do not receive proper preventive medication. Recent clinical studies revealed that onabotulinumtoxinA was an efficacious and safe treatment for chronic migraine.
Full article
►▼
Show Figures
Open AccessArticle
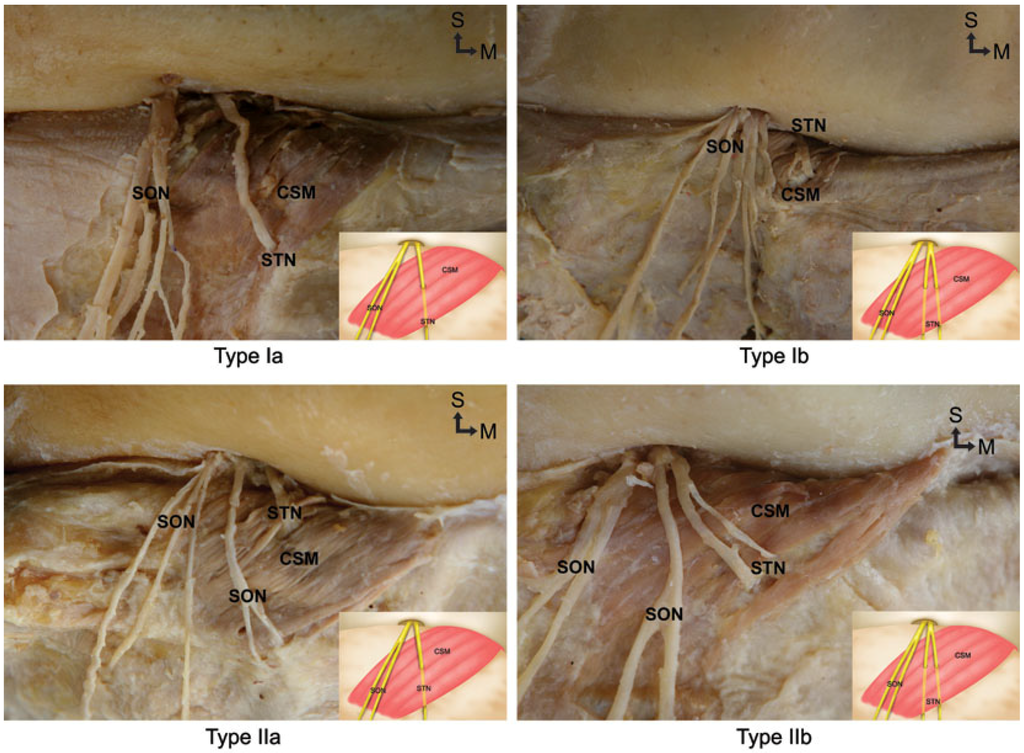
Topographic Relationship between the Supratrochlear Nerve and Corrugator Supercilii Muscle—Can This Anatomical Knowledge Improve the Response to Botulinum Toxin Injections in Chronic Migraine?
by
Hyung-Jin Lee, Kwang-Seok Choi, Sung-Yoon Won, Prawit Apinuntrum, Kyung-Seok Hu, Seong-Taek Kim, Tanvaa Tansatit and Hee-Jin Kim
Cited by 17 | Viewed by 17820
Abstract
Chronic migraine has been related to the entrapment of the supratrochlear nerve within the corrugator supercilii muscle. Recently, research has shown that people who have undergone botulinum neurotoxin A injection in frontal regions reported disappearance or alleviation of their migraines. There have been
[...] Read more.
Chronic migraine has been related to the entrapment of the supratrochlear nerve within the corrugator supercilii muscle. Recently, research has shown that people who have undergone botulinum neurotoxin A injection in frontal regions reported disappearance or alleviation of their migraines. There have been numerous anatomical studies conducted on Caucasians revealing possible anatomical problems leading to migraine; on the other hand, relatively few anatomical studies have been conducted on Asians. Thus, the aim of the present study was to determine the topographic relationship between the supratrochlear nerve and corrugator supercilii muscle in the forehead that may be the cause of migraine. Fifty-eight hemifaces from Korean and Thai cadavers were used for this study. The supratrochlear nerve entered the corrugator supercilii muscle in every case. Type I, in which the supratrochlear nerve emerged separately from the supraorbital nerve at the medial one-third portion of the orbit, was observed in 69% (40/58) of cases. Type II, in which the supratrochlear nerve emerged from the orbit at the same location as the supraorbital nerve, was observed in 31% (18/58) of cases.
Full article
►▼
Show Figures
Open AccessReview
Treatment of Chronic Migraine with Focus on Botulinum Neurotoxins
by
Sara M. Schaefer, Christopher H. Gottschalk and Bahman Jabbari
Cited by 22 | Viewed by 9936
Abstract
Migraine is the most common neurological disorder, and contributes to disability and large healthcare costs in the United States and the world. The treatment of migraine until recently has focused on medications, both abortive and prophylactic, but treatment of chronic migraine has been
[...] Read more.
Migraine is the most common neurological disorder, and contributes to disability and large healthcare costs in the United States and the world. The treatment of migraine until recently has focused on medications, both abortive and prophylactic, but treatment of chronic migraine has been revolutionized with the introduction of botulinum toxin injection therapy. In this review, we explore the current understanding of migraine pathophysiology, and the evolution of the use of botulinum toxin therapy including proposed pathophysiological mechanisms through animal data. We also discuss the similarities and differences between three injection techniques.
Full article
►▼
Show Figures
Open AccessArticle
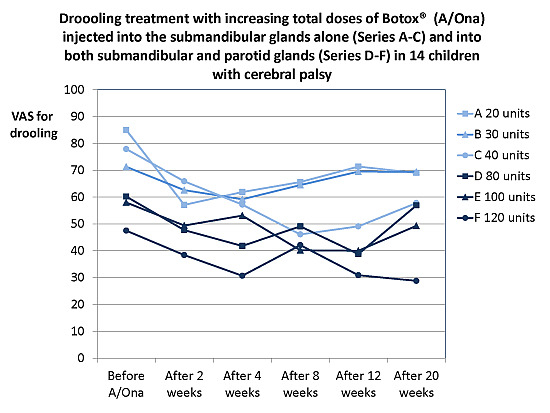
Onabotulinumtoxin A Treatment of Drooling in Children with Cerebral Palsy: A Prospective, Longitudinal Open-Label Study
by
Eigild Møller, Søren Anker Pedersen, Pablo Gustavo Vinicoff, Allan Bardow, Joan Lykkeaa, Pia Svendsen and Merete Bakke
Cited by 16 | Viewed by 6118
Abstract
The aim of this prospective open-label study was to treat disabling drooling in children with cerebral palsy (CP) with onabotulinumtoxin A (A/Ona, Botox
®) into submandibular and parotid glands and find the lowest effective dosage and least invasive method. A/Ona was injected
[...] Read more.
The aim of this prospective open-label study was to treat disabling drooling in children with cerebral palsy (CP) with onabotulinumtoxin A (A/Ona, Botox
®) into submandibular and parotid glands and find the lowest effective dosage and least invasive method. A/Ona was injected in 14 children, Mean age 9 years, SD 3 years, under ultrasonic guidance in six successive Series, with at least six months between injections. Doses and gland involvement increased from Series A to F (units (U) per submandibular/parotid gland: A, 10/0; B, 15/0; C, 20/0; D, 20/20; E, 30/20; and F, 30/30). The effect was assessed 2, 4, 8, 12, and 20 weeks after A/Ona (drooling problems (VAS), impact (0–7), treatment effect (0–5), unstimulated whole saliva (UWS) flow and composition)) and analyzed by two-way ANOVA. The effect was unchanged–moderate in A to moderate–marked in F. Changes in all parameters were significant in E and F, but with swallowing problems ≤5 weeks in 3 of 28 treatments. F had largest VAS and UWS reduction (64% and 49%). We recommend: Start with dose D A/Ona (both submandibular and parotid glands and a total of 80 U) and increase to E and eventually F (total 120 U) without sufficient response.
Full article
►▼
Show Figures
Open AccessReview
Botulinum Toxin Type A for the Treatment of Neuropathic Pain in Neuro-Rehabilitation
by
Domenico Intiso, Mario Basciani, Andrea Santamato, Marta Intiso and Filomena Di Rienzo
Cited by 38 | Viewed by 8732
Abstract
Pain is a natural protective mechanism and has a warning function signaling imminent or actual tissue damage. Neuropathic pain (NP) results from a dysfunction and derangement in the transmission and signal processing along the nervous system and it is a recognized disease in
[...] Read more.
Pain is a natural protective mechanism and has a warning function signaling imminent or actual tissue damage. Neuropathic pain (NP) results from a dysfunction and derangement in the transmission and signal processing along the nervous system and it is a recognized disease in itself. The prevalence of NP is estimated to be between 6.9% and 10% in the general population. This condition can complicate the recovery from stroke, multiple sclerosis, spinal cord lesions, and several neuropathies promoting persistent disability and poor quality of life. Subjects suffering from NP describe it as burning, itching, lancing, and numbness, but hyperalgesia and allodynia represent the most bothersome symptoms. The management of NP is a clinical challenge and several non-pharmacological and pharmacological interventions have been proposed with variable benefits. Botulinum toxin (BTX) as an adjunct to other interventions can be a useful therapeutic tool for the treatment of disabled people. Although BTX-A is predominantly used to reduce spasticity in a neuro-rehabilitation setting, it has been used in several painful conditions including disorders characterized by NP. The underlying pharmacological mechanisms that operate in reducing pain are still unclear and include blocking nociceptor transduction, the reduction of neurogenic inflammation by inhibiting neural substances and neurotransmitters, and the prevention of peripheral and central sensitization. Some neurological disorders requiring rehabilitative intervention can show neuropathic pain resistant to common analgesic treatment. This paper addresses the effect of BTX-A in treating NP that complicates frequent disorders of the central and peripheral nervous system such as spinal cord injury, post-stroke shoulder pain, and painful diabetic neuropathy, which are commonly managed in a rehabilitation setting. Furthermore, BTX-A has an effect in relief pain that may characterize less common neurological disorders including post-traumatic neuralgia, phantom limb, and complex regional pain syndrome with focal dystonia. The use of BTX-A could represent a novel therapeutic strategy in caring for neuropathic pain whenever common pharmacological tools have been ineffective. However, large and well-designed clinical trials are needed to recommend BTX-A use in the relief of neuropathic pain.
Full article
►▼
Show Figures
Open AccessReview
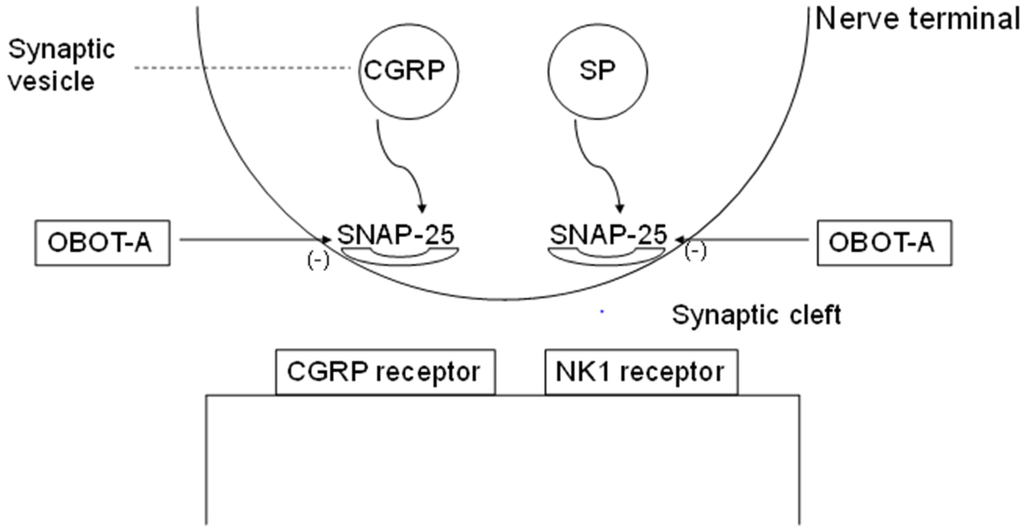
Botulinum Toxin as a Pain Killer: Players and Actions in Antinociception
by
Dong-Wan Kim, Sun-Kyung Lee and Joohong Ahnn
Cited by 40 | Viewed by 12777
Abstract
Botulinum neurotoxins (BoNTs) have been widely used to treat a variety of clinical ailments associated with pain. The inhibitory action of BoNTs on synaptic vesicle fusion blocks the releases of various pain-modulating neurotransmitters, including glutamate, substance P (SP), and calcitonin gene-related peptide (CGRP),
[...] Read more.
Botulinum neurotoxins (BoNTs) have been widely used to treat a variety of clinical ailments associated with pain. The inhibitory action of BoNTs on synaptic vesicle fusion blocks the releases of various pain-modulating neurotransmitters, including glutamate, substance P (SP), and calcitonin gene-related peptide (CGRP), as well as the addition of pain-sensing transmembrane receptors such as transient receptor potential (TRP) to neuronal plasma membrane. In addition, growing evidence suggests that the analgesic and anti-inflammatory effects of BoNTs are mediated through various molecular pathways. Recent studies have revealed that the detailed structural bases of BoNTs interact with their cellular receptors and SNAREs. In this review, we discuss the molecular and cellular mechanisms related to the efficacy of BoNTs in alleviating human pain and insights on engineering the toxins to extend therapeutic interventions related to nociception.
Full article
►▼
Show Figures
Open AccessReview
Pain Relief in Cervical Dystonia with Botulinum Toxin Treatment
by
Carlos Henrique Ferreira Camargo, Lígia Cattai and Hélio Afonso Ghizoni Teive
Cited by 46 | Viewed by 7222
Abstract
Dystonia is a neurological disorder characterized by intermittent or sustained muscle contractions that cause abnormal, usually repetitive, movements and postures. Dystonic movements can be tremulous and twisting and often follow a pattern. They are frequently associated with overflow muscle activation and may be
[...] Read more.
Dystonia is a neurological disorder characterized by intermittent or sustained muscle contractions that cause abnormal, usually repetitive, movements and postures. Dystonic movements can be tremulous and twisting and often follow a pattern. They are frequently associated with overflow muscle activation and may be triggered or worsened by voluntary action. Most voluntary muscles can be affected and, in the case of the neck muscles, the condition is referred to as cervical dystonia (CD), the most common form of dystonia. The high incidence of pain distinguishes CD from other focal dystonias and contributes significantly to patient disability and low quality of life. Different degrees of pain in the cervical region are reported by more than 60% of patients, and pain intensity is directly related to disease severity. Botulinum toxin (BoNT) is currently considered the treatment of choice for CD and can lead to an improvement in pain and dystonic symptoms in up to 90% of patients. The results for BoNT/A and BoNT/B are similar. The complex relationship between pain and dystonia has resulted in a large number of studies and more comprehensive assessments of dystonic patients. When planning the application of BoNT, pain should be a key factor in the choice of muscles and doses. In conclusion, BoNT is highly effective in controlling pain, and its analgesic effect is sustained for a long time in most CD patients.
Full article
Open AccessReview
Novel Treatment of Chronic Bladder Pain Syndrome and Other Pelvic Pain Disorders by OnabotulinumtoxinA Injection
by
Jia-Fong Jhang and Hann-Chorng Kuo
Cited by 29 | Viewed by 9719
Abstract
Chronic pelvic pain (CPP) is defined as pain in the pelvic organs and related structures of at least 6 months’ duration. The pathophysiology of CPP is uncertain, and its treatment presents challenges. Botulinum toxin A (BoNT-A), known for its antinociceptive, anti-inflammatory, and muscle
[...] Read more.
Chronic pelvic pain (CPP) is defined as pain in the pelvic organs and related structures of at least 6 months’ duration. The pathophysiology of CPP is uncertain, and its treatment presents challenges. Botulinum toxin A (BoNT-A), known for its antinociceptive, anti-inflammatory, and muscle relaxant activity, has been used recently to treat refractory CPP with promising results. In patients with interstitial cystitis/bladder pain syndrome, most studies suggest intravesical BoNT-A injection reduces bladder pain and increases bladder capacity. Repeated BoNT-A injection is also effective and reduces inflammation in the bladder. Intraprostatic BoNT-A injection could significantly improve prostate pain and urinary frequency in the patients with chronic prostatitis/chronic pelvic pain syndrome. Animal studies also suggest BoNT-A injection in the prostate decreases inflammation in the prostate. Patients with CPP due to pelvic muscle pain and spasm also benefit from localized BoNT-A injections. BoNT-A injection in the pelvic floor muscle improves dyspareunia and decreases pelvic floor pressure. Preliminary studies show intravesical BoNT-A injection is useful in inflammatory bladder diseases such as chemical cystitis, radiation cystitis, and ketamine related cystitis. Dysuria is the most common adverse effect after BoNT-A injection. Very few patients develop acute urinary retention after treatment.
Full article
►▼
Show Figures
Open AccessReview
Treatment of Gastrointestinal Sphincters Spasms with Botulinum Toxin A
by
Giuseppe Brisinda, Nicola Sivestrini, Giuseppe Bianco and Giorgio Maria
Cited by 17 | Viewed by 7954
Abstract
Botulinum toxin A inhibits neuromuscular transmission. It has become a drug with many indications. The range of clinical applications has grown to encompass several neurological and non-neurological conditions. One of the most recent achievements in the field is the observation that botulinum toxin
[...] Read more.
Botulinum toxin A inhibits neuromuscular transmission. It has become a drug with many indications. The range of clinical applications has grown to encompass several neurological and non-neurological conditions. One of the most recent achievements in the field is the observation that botulinum toxin A provides benefit in diseases of the gastrointestinal tract. Although toxin blocks cholinergic nerve endings in the autonomic nervous system, it has also been shown that it does not block non-adrenergic non-cholinergic responses mediated by nitric oxide. This has promoted further interest in using botulinum toxin A as a treatment for overactive smooth muscles and sphincters. The introduction of this therapy has made the treatment of several clinical conditions easier, in the outpatient setting, at a lower cost and without permanent complications. This review presents current data on the use of botulinum toxin A in the treatment of pathological conditions of the gastrointestinal tract.
Full article
Open AccessReview
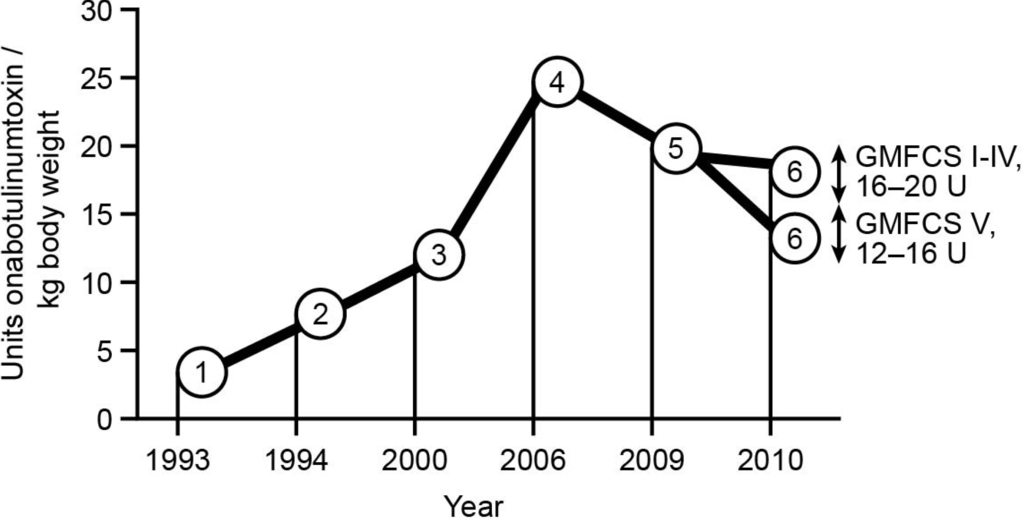
Best Clinical Practice in Botulinum Toxin Treatment for Children with Cerebral Palsy
by
Walter Strobl, Tim Theologis, Reinald Brunner, Serdar Kocer, Elke Viehweger, Ignacio Pascual-Pascual and Richard Placzek
Cited by 94 | Viewed by 15403
Abstract
Botulinum toxin A (BoNT-A) is considered a safe and effective therapy for children with cerebral palsy (CP), especially in the hands of experienced injectors and for the majority of children. Recently, some risks have been noted for children with Gross Motor Classification Scale
[...] Read more.
Botulinum toxin A (BoNT-A) is considered a safe and effective therapy for children with cerebral palsy (CP), especially in the hands of experienced injectors and for the majority of children. Recently, some risks have been noted for children with Gross Motor Classification Scale (GMFCS) of IV and the risks are substantial for level V. Recommendations for treatment with BoNT-A have been published since 1993, with continuous optimisation and development of new treatment concepts. This leads to modifications in the clinical decision making process, indications, injection techniques, assessments, and evaluations. This article summarises the state of the art of BoNT-A treatment in children with CP, based mainly on the literature and expert opinions by an international paediatric orthopaedic user group. BoNT-A is an important part of multimodal management, to support motor development and improve function when the targeted management of spasticity in specific muscle groups is clinically indicated. Individualised assessment and treatment are essential, and should be part of an integrated approach chosen to support the achievement of motor milestones. To this end, goals should be set for both the long term and for each injection cycle. The correct choice of target muscles is also important; not all spastic muscles need to be injected. A more focused approach needs to be established to improve function and motor development, and to prevent adverse compensations and contractures. Furthermore, the timeline of BoNT-A treatment extends from infancy to adulthood, and treatment should take into account the change in indications with age.
Full article
►▼
Show Figures