Phenolic Compounds in Brassica Vegetables
Abstract
:1. Introduction
2. Phenolic Compounds
| Species | Group | Common name | Organ |
|---|---|---|---|
| Brassica oleracea | acephala | Kale, collards | Leaves |
| capitata capitata | Cabbage | Terminal leaf buds (heads) | |
| capitata sabauda | Savoy cabbage | Terminal leaf buds (heads) | |
| costata | Tronchuda cabbage | Loose heads | |
| gemmifera | Brussels sprouts | Vegetative buds | |
| botrytis botrytis | Cauliflower | Inflorescences | |
| botrytis italica | Broccoli | Inflorescences | |
| gongylodes | Kohlrabi | Stem | |
| albogabra | Chinese kale | Leaves | |
| Brassica rapa | chinensis | Pak choi, bok choy | Leaves |
| dichotoma | Brown sarson, toria | Seeds | |
| narinosa | Chinese flat cabbage, wutacai | Leaves | |
| nipposinica | Mibuna, mizuna | Leaves | |
| oleifera | Turnip rape, rapeseed | Seeds | |
| pekinensis | Chinese cabbage, pe-tsai | Leaves | |
| perviridis | Komatsuna, Tendergreen | Leaves | |
| parachinensis | Choy sum | Leaves | |
| rapa | Turnip, turnip greens, turnip tops | Roots, leaves and shoots | |
| ruvo | Broccoleto | Shoots | |
| trilochularis | Yellow sarson | Seeds | |
| Brassica napus | pabularia | Leaf rape, nabicol | Leaves |
| napobrassica | Swede, rutabaga | Roots | |
| Brassica juncea | rugosa | Mustard greens | Leaves |
| capitata | Head mustard | Heads | |
| crispifolia | Cut leaf mustard | Leaves |
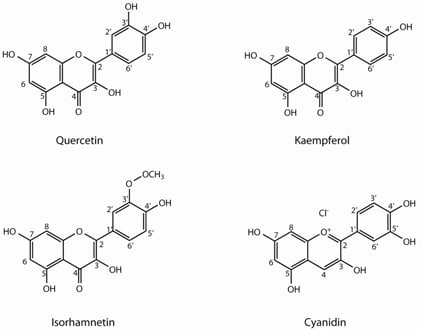
2.1. Flavonoids

2.2. Hydroxycinnamic acids

2.3. Biological activities
2.4. Bioavailability
3. Phenolic Compounds in Brassica Vegetables
3.1. Phenolic composition in Brassica oleracea crops
| Brassica oleracea | Brassica rapa | Brassica napus | Brassica juncea | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Phenolic compound | Tronchuda cabbage1 | Cauliflower2 | Kale3 | Broccoli4 | White cabbage5 | Turnip greens/tops6 | Pak choi7 | Leaf rape8 | Leaf mustard9 |
| Quercetin (Q) derivatives | |||||||||
| Q-3-O-sophorotrioside-7-O-sophoroside | x | X | |||||||
| Q-3-O-sophorotrioside-7-glucoside | x | X | x | x | |||||
| Q-3-O-sophoroside-7-O-glucoside | x | x | x | X | x | x | |||
| Q-3,7-di-O-glucoside | x | X | x | x | x | x | |||
| Q-3-O-sophoroside | x | X | x | x | x | ||||
| Q-7-O-glucoside | x | ||||||||
| Q-3-O-glucoside | x | X | x | x | |||||
| Q-3-O-(caffeoyl)-sophorotrioside-7-O-glucoside | X | x | |||||||
| Q- 3-O-(sinapoyl)-sophorotrioside-7-O-glucoside | X | x | |||||||
| Q-3-O-(feruloyl)-sophorotrioside-7-O-glucoside | X | x | |||||||
| Q-3-O-(p-coumaroyl)-sophorotrioside-7-O-glucoside | X | ||||||||
| Q-3-O-(caffeoyl)-sophoroside-7-O-glucoside | x | X | x | x | x | x | |||
| Q-3-O-(methoxycaffeoyl)-sophoroside-7-O-glucoside | x | x | x | ||||||
| Q-3-O-(sinapoyl)-sophoroside-7-O-glucoside | x | x | x | x | x | ||||
| Q-3-O-(feruloyl)-sophoroside-7-O-glucoside | x | ||||||||
| Q-3-O-(p-coumaroyl)-sophoroside-7-O-glucoside | X | ||||||||
| Q-3-O-(feruloyl)-sophoroside | x | X | x | x | x | ||||
| Kaempferol (K) derivatives | |||||||||
| K-3-O-tetraglucoside-7-O-sophoroside | x | ||||||||
| K-3-O-sophorotrioside-7-O-sophoroside | x | x | x | X | x | x | |||
| K-3-O-sohorotrioside-7-O-glucoside | x | x | X | x | x | ||||
| K-3-O-sophoroside-7-O-diglucoside | x | x | X | x | |||||
| K-3-O-sophoroside-7-O-glucoside | x | x | x | X | x | x | x | x | |
| K-3,7-di-O-glucoside | x | X | x | x | x | x | |||
| K-3-O-sophoroside | x | X | x | x | x | ||||
| K-7-O-glucoside | x | x | x | x | x | x | |||
| K-3-O-glucoside | x | X | x | ||||||
| K-3-O-(caffeoyl)-sophorotrioside-7-O-sophoroside | X | ||||||||
| K-3-O-(methoxycaffeoyl)-sophorotrioside-7-O-sophoroside | X | ||||||||
| K-O-(sinapoyl)-sophorotrioside-7-O-sophoroside | X | ||||||||
| K-O-(feruloyl)-sophorotrioside-7-O-sophoroside | X | ||||||||
| K-3-O-(p-coumaroyl)-sophorotrioside-7-O-sophoroside | X | ||||||||
| K-3-O-(caffeoyl)-sophorotrioside-7-O-glucoside | X | x | |||||||
| K-3-O-(methoxycaffeoyl)-sophorotrioside-7-O-glucoside | X | ||||||||
| K-O-(sinapoyl)-sophorotrioside-7-O-glucoside | X | ||||||||
| K-O-(feruloyl)-sophorotrioside-7-O-glucoside | X | x | |||||||
| K-3-O-(caffeoyl)sophoroside-7-O-glucoside | x | x | x | X | x | x | x | x | x |
| K-3-O-(methoxycaffeoyl)sophoroside-7-O-glucoside | x | x | x | x | x | x | x | ||
| K-3-O-(sinapoyl)-sophoroside-7-O-glucoside | x | x | x | x | x | x | x | x | |
| K-3-O-(feruloyl)-sophoroside-7-O-glucoside | x | x | x | x | x | x | x | ||
| K-3-O-(p-coumaroyl)-sophoroside-7-O-glucoside | x | x | x | x | x | ||||
| K-3-O-(methoxycaffeoyl)-sophoroside | x | X | x | x | x | ||||
| K-3-O-(sinapoyl)-sophoroside | x | x | x | x | x | ||||
| K-3-O-(feruloyl)-sophorotrioside | x | ||||||||
| K-3-O-(feruloyl)-sophoroside | x | x | x | x | x | ||||
| K-3-O-(p-coumaroyl)-sophoroside | x | x | x | ||||||
| Isorhamnetin (I) derivatives | |||||||||
| I-3-sophorotrioside-7-sophoroside | |||||||||
| I-3,7-di-O-glucoside | x | x | x | ||||||
| I-3-glucoside | x | x | x | ||||||
| Hydroxycinnamic acids | |||||||||
| 3-caffeoyl quinic acid | x | x | x | x | x | x | x | ||
| 5-caffeoyl quinic acid | x | ||||||||
| 3-p-coumaroyl quinic acid | x | x | x | x | x | x | |||
| 4-p-coumaroyl quinic acid | x | x | |||||||
| 4-caffeoyl quinic acid | x | x | |||||||
| Sinapylglucoside | x | x | x | x | |||||
| Ferulic acid | x | x | x | x | x | ||||
| 4-feruloyl quinic acid | x | x | x | ||||||
| Sinapic acid | x | x | x | x | x | x | x | ||
| 1,2-disinapoylgentiobiose | x | x | x | X | x | x | x | x | x |
| 1-sinapoyl-2-feruloylgentiobiose | x | x | x | X | x | x | x | x | x |
| 1,2,2’-trisinapoylgentiobiose | x | x | x | X | x | x | x | x | x |
| 1,2’-disinapoyl-2-feruloylgentiobiose | x | x | x | X | x | x | x | x | x |
| Crop | Compound | Reference |
|---|---|---|
| Moricandia arvensis | 3,4’-di-O-β-D-glucopyranoside-7-O-α-L-rhamnopyranoside | [80] |
| β-D-glucopyranosyl 4-O- β-D-glucopyranosylcaffeate | ||
| methyl 3-O- β-D-glucopyranosyl-5-hydroxycinnamate | ||
| β-D-glucopyranosyl 4-O-β-D-glucopyranosylbenzoate | ||
| β-D-glucopyranosyl 4-hydroxybenzoate | ||
| methyl 4-O-β-D-glucopyranosylcaffeate | ||
| 1-O-caffeoyl-β-D-glucopyranoside | ||
| 2-phenylethyl-β-D-glucopyranoside | ||
| Bunias orientalis | Kaempferol monosinapoyl di-O-glycoside | [82] |
| Kaempferol monomalonyl/monosinapoyl di-O-glycoside | ||
| Kaempferol di-O-glucoside and tri-O-glucoside | ||
| demethylated sinapic acid | ||
| Diplotaxis erucoides/Eruca sativa | Kaempferol di-O-glycoside | [82] |
| Isorhamnetin mono-O-,di-O-, and tri-O-glycosides | ||
| Quercetin monosinapoyl tri-O-glycoside | ||
| Quercetin tri-O-glycoside | ||
| Quercetin tetra-O-glycoside | ||
| Quercetin monosinapoyl di-O-glycoside | ||
| Quercetin di-O-, tri-O-, and tetra-O-glycosides | ||
| Diplotaxis tenuifolia | Quercetin-3,3‘,4‘-triglucoside | [83] |
| Quercetin-3,4‘-di-glucoside-3‘-(6-methoxycaffeoyl-glucoside) | ||
| Quercetin-3,4‘-di-glucoside-3‘-(6-caffeoyl-glucoside) | ||
| Quercetin-3,4‘-di-glucoside-3‘-(6-sinapoyl-glucoside) | ||
| Quercetin-3,4‘-di-glucoside-3‘-(6-feruloyl-glucoside) | ||
| Quercetin-3,4‘-di-glucoside-3‘-(6-p-coumaroyl-glucoside) | ||
| Quercetin-3-(2-methoxycaffeoyl-glucoside)-3‘-(6-sinapoyl-glucoside)-4‘-glucoside | ||
| Quercetin-3-(2-caffeoyl-glucoside)-3‘-(6-sinapoyl-glucoside)-4‘-glucoside | ||
| Quercetin-3-(2-sinapoyl-glucoside)-3‘-(6-sinapoyl-glucoside)-4‘-glucoside Quercetin-3-(2-feruloyl-glucoside)-3‘-(6-sinapoyl-glucoside)-4‘-glucoside | ||
| Quercetin-3-(2-feruloyl-glucoside)-3‘-(6-feruloyl-glucoside)-4‘-glucoside | ||
| Kaempferol-3,4‘-di-glucoside | ||
| Isorhamnetin-3,4‘-di-glucoside | ||
| Eruca vesicaria | Quercetin-3-glucoside | [83] |
| Kaempferol-3-glucoside | ||
| Kaempferol-3,4‘-di-glucoside | ||
| Kaempferol-3-(2-sinapoyl-glucoside)-4‘-glucoside | ||
| Isorhamnetin-3-glucoside | ||
| Isorhamnetin-3,4‘-di-glucoside | ||
| Nasturtium officinale | Quercetin 3-O-triglucoside-7-O-rhamnoside | [50] |
| Quercetin 3-O-gentiobioside-7-O-rhamnoside | ||
| Quercetin 3-O-sophoroside-7-O-rhamnoside | ||
| Quercetin 3-O-sophoroside-7-O-(caffeoyl)-rhamnoside | ||
| Quercetin 3-O-triglucoside-7-O-(caffeoyl)-rhamnoside | ||
| Quercetin 3-O-triglucoside-7-O-(sinapoyl)-rhamnoside | ||
| Quercetin 3-O-triglucoside-7-O-(feruloyl)-rhamnoside | ||
| Kaempferol 3-O-triglucoside | ||
| Kaempferol 3-O-sophoroside | ||
| Kaempferol 3-O-gentiobioside | ||
| Kaempferol 3-O-gentiobioside-7-O-rhamnoside | ||
| Kaempferol 3-O-triglucoside-7-O-rhamnoside | ||
| Kaempferol 3-O-triglucoside-7-O-(caffeoyl)-rhamnoside | ||
| Kaempferol 3-O-triglucoside-7-O-(sinapoyl)-rhamnoside | ||
| Kaempferol 3-O-triglucoside-7-O-(sinapoyl)-rhamnoside | ||
| Kaempferol 3-O-sophoroside-7-O-rhamnoside | ||
| Kaempferol 3-O-sophoroside-7-O-(caffeoyl)-rhamnoside |
3.2. Phenolic composition in Brassica rapa crops
3.3. Phenolic composition in Brassica napus crops
3.4. Phenolic composition in Brassica juncea crops
3.5. Phenolic composition in other cruciferous crops
4. Variation on Phenolic Content in Brassica Vegetables
4.1. Influence of fertilization and cropping systems on phenolic content
4.2. Influence of processing and cooking on phenolic composition
4.3. Influence of storage on phenolic content
5. Future Perspectives
6. References
- Podsedek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. Lwt-Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Health-Affecting Compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009, 8, 31–43. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Phenols, polyphenols and tannins: An overview. In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Crozier, A., Clifford, M., Ashihara, H., Eds.; Blackwell: Oxford, UK, 2006; pp. 1–24. [Google Scholar]
- Pereira, D.M.; Valentao, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Arts, I.C.W. Flavonols, flavones and flavanols - nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1081–1093. [Google Scholar] [CrossRef]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef]
- Sadilova, E.; Stintzing, F.C.; Carle, R. Anthocyanins, colour and antioxidant properties of eggplant (Solanum melongena L.) and violet pepper (Capsicum annuum L.) peel extracts. Z. Naturforsch. C 2006, 61, 527–535. [Google Scholar]
- McDougall, G. J.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red cabbage - stability to simulated gastrointestinal digestion. Phytochemistry 2007, 68, 1285–1294. [Google Scholar] [CrossRef]
- Moreno, D.A.; Perez-Balibrea, S.; Ferreres, F.; Gil-Izquierdo, A.; Garcia-Viguera, C. Acylated anthocyanins in broccoli sprouts. Food Chem. 2010, 123, 358–363. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Tatsuzawa, F.; Saito, N.; Shinoda, K.; Shigihara, A.; Honda, T. Acylated cyanidin 3-sambubioside-5-glucosides in three garden plants of the Cruciferae. Phytochemistry 2006, 67, 1287–1295. [Google Scholar] [CrossRef]
- Lo Scalzo, R.; Genna, A.; Branca, F.; Chedin, M.; Chassaigne, H. Anthocyanin composition of cauliflower (Brassica oleracea L. var. botrytis) and cabbage (B. oleracea L. var. capitata) and its stability in relation to thermal treatments. Food Chem. 2008, 107, 136–144. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomas-Barberan, F.A.; Ferreres, F. Characterisation of flavonols in broccoli (Brassica oleracea L. var. italica) by liquid chromatography-UV diode-array detection-electrospray ionisation mass spectrometry. J. Chromatogr. 2004, 1054, 181–193. [Google Scholar] [CrossRef]
- Olsen, H.; Aaby, K.; Borge, G.I.A. Characterization and Quantification of Flavonoids and Hydroxycinnamic Acids in Curly Kale (Brassica oleracea L. convar. acephala var. sabellica) by HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2009, 57, 2816–2825. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. Phenolic Component Profiles of Mustard Greens, Yu Choy, and 15 Other Brassica Vegetables. J. Agric. Food Chem. 2010, 58, 6850–6857. [Google Scholar] [CrossRef]
- Price, K.R.; Casuscelli, F.; Colquhoun, I.J.; Rhodes, M.J.C. Hydroxycinnamic acid esters from broccoli florets. Phytochemistry 1997, 45, 1683–1687. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomas-Barberan, F.A.; Garcia-Viguera, C. Potential bioactive compounds in health promotion from broccoli cultivars grown in Spain. J. Sci. Food Agric. 2002, 82, 1293–1297. [Google Scholar] [CrossRef]
- de Pascual-Teresa, S.; Moreno, D.A.; Garcia-Viguera, C. Flavanols and Anthocyanins in Cardiovascular Health: A Review of Current Evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef] [Green Version]
- Plumb, G.W.; Price, K.R.; Rhodes, M.J.C.; Williamson, G. Antioxidant properties of the major polyphenolic compounds in broccoli. Free Radical Res. 1997, 27, 429–435. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Chu, Y.H.; Chang, C.L.; Hsu, H.F. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food Agric. 2000, 80, 561–566. [Google Scholar] [CrossRef]
- Podsedek, A.; Sosnowska, D.; Redzynia, M.; Anders, B. Antioxidant capacity and content of Brassica oleracea dietary antioxidants. Int. J. Food Sci. Technol. 2006, 41, 49–58. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Skandrani, I.; Limem, I.; Neffati, A.; Boubaker, J.; Ben Sghaier, M.; Bhouri, W.; Bouhlel, I.; Kilani, S.; Ghedira, K.; Chekir-Ghedira, L. Assessment of phenolic content, free-radical-scavenging capacity genotoxic and anti-genotoxic effect of aqueous extract prepared from Moricandia arvensis leaves. Food Chem. Toxicol. 2010, 48, 710–715. [Google Scholar] [CrossRef]
- Ackland, M.L.; Van de Waarsenburg, S.; Jones, R. Synergistic antiproliferative action of the flavonols quercetin and kaempferol in cultured human cancer cell lines. In Vivo 2005, 19, 69–76. [Google Scholar]
- Fresco, P.; Borges, F.; Marques, M.P.M.; Diniz, C. The Anticancer Properties of Dietary Polyphenols and its Relation with Apoptosis. Curr. Pharm. Des. 2010, 16, 114–134. [Google Scholar] [CrossRef]
- Shen, S.S.; Callaghan, D.; Juzwik, C.; Xiong, H.Q.; Huang, P.L.; Zhang, W.D. ABCG2 reduces ROS-mediated toxicity and inflammation: A potential role in Alzheimer's disease. J. Neurochem. 2010, 114, 1590–1604. [Google Scholar] [CrossRef]
- Mullen, W.; Marks, S.C.; Crozier, A. Evaluation of phenolic compounds in commercial fruit juices and fruit drinks. J. Agric. Food Chem. 2007, 55, 3148–3157. [Google Scholar] [CrossRef]
- Llorach, R.; Espin, J.C.; Tomas-Barberan, F.A.; Ferreres, F. Valorization of cauliflower (Brassica oleracea L. var. botrytis) by-products as a source of antioxidant phenolics. J. Agric. Food Chem. 2003, 51, 2181–2187. [Google Scholar] [CrossRef]
- Kim, J.D.; Liu, L.P.; Guo, W.M.; Meydani, M. Chemical structure of flavonols in relation to modulation of angiogenesis and immune-endothelial cell adhesion. J. Nutr. Biochem. 2006, 17, 165–176. [Google Scholar] [CrossRef]
- Jung, M.; Park, M. Acetylcholinesterase inhibition by flavonoids from agrimonia pilosa. Molecules 2007, 12, 2130–2139. [Google Scholar] [CrossRef]
- Braca, A.; Fico, G.; Morelli, I.; De Simone, F.; Tomè, F.; De Tommasi, N. Antioxidant and free radical scavenging activity of flavonol glycosides from different Aconitum species. J. Ethnopharmacol. 2003, 86, 63–67. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Hayirlioglu-Ayaz, S.; Alpay-Karaoglu, S.; Gruz, J.; Valentova, K.; Ulrichova, J.; Strnad, M. Phenolic acid contents of kale (Brassica oleraceae L. var. acephala DC.) extracts and their antioxidant and antibacterial activities. Food Chem. 2008, 107, 19–25. [Google Scholar] [CrossRef]
- Yokozawa, T.; Kim, H.Y.; Cho, E.J.; Choi, J.S.; Chung, H.Y. Antioxidant Effects of isorhamnetin 3,7-Di-O-β-d-glucopyranoside isolated from mustard leaf (Brassica juncea) in Rats with Streptozotocin-Induced Diabetes. J. Agric. Food. Chem. 2002, 50, 5490–5495. [Google Scholar] [CrossRef]
- Garcia-Lafuente, A.; Guillamon, E.; Villares, A.; Rostagno, M.A.; Martinez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflammation Res. 2009, 58, 537–552. [Google Scholar] [CrossRef]
- Zum Felde, T.; Baumert, A.; Strack, D.; Becker, H.C.; Mollers, C. Genetic variation for sinapate ester content in winter rapeseed (Brassica napus L.) and development of NIRS calibration equations. Plant Breed. 2007, 126, 291–296. [Google Scholar]
- Auger, B.; Marnet, N.; Gautier, V.; Maia-Grondard, A.; Leprince, F.; Renard, M.; Guyot, S.; Nesi, N.; Routaboul, J.M. A Detailed survey of seed coat flavonoids in developing seeds of Brassica napus L. J. Agric. Food Chem. 2010, 58, 6246–6256. [Google Scholar]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar]
- Zhao, Z.H.; Moghadasian, M.H. Bioavailability of hydroxycinnamates: A brief review of in vivo and in vitro studies. Phytochem. Rev. 2010, 9, 133–145. [Google Scholar] [CrossRef]
- D'Archivio, M.; Filesi, C.; Vari, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Murota, K.; Terao, J. Antioxidative flavonoid quercetin: Implication of its intestinal absorption and metabolism. Arch. Biochem. Biophys. 2003, 417, 12–17. [Google Scholar] [CrossRef]
- Silberberg, M.; Morand, C.; Mathevon, T.; Besson, C.; Manach, C.; Scalbert, A.; Remesy, C. The bioavailability of polyphenols is highly governed by the capacity of the intestine and of the liver to secrete conjugated metabolites. Eur. J. Nutr. 2006, 45, 88–96. [Google Scholar] [CrossRef]
- Wittemer, S.M.; Ploch, M.; Windeck, T.; Muller, S.C.; Drewelow, B.; Derendorf, H.; Veit, M. Bioavailability and pharmacokinetics of caffeoylquinic acids and flavonoids after oral administration of Artichoke leaf extracts in humans. Phytomedicine 2005, 12, 28–38. [Google Scholar] [CrossRef]
- Moreno, D.A.; Carvajal, M.; Lopez-Berenguer, C.; Garcia-Viguera, C. Chemical and biological characterisation of nutraceutical compounds of broccoli. J. Pharm. Biomed. Anal. 2006, 41, 1508–1522. [Google Scholar] [CrossRef]
- Bahorun, T.; Luximon-Ramma, A.; Crozier, A.; Aruoma, O.I. Total phenol, flavonoid, proanthocyanidin and vitamin C levels and antioxidant activities of Mauritian vegetables. J. Sci. Food Agric. 2004, 84, 1553–1561. [Google Scholar] [CrossRef]
- Harbaum, B.; Hubbermann, E.M.; Wolff, C.; Herges, R.; Zhu, Z.; Schwarz, K. Identification of flavonolds and hydroxycinnamic acids in pak choi varieties (Brassica campestris L. ssp chinensis var. communis) by HPLC-ESI-MSn and NMR and their quantification by HPLC-DAD. J. Agric. Food Chem. 2007, 55, 8251–8260. [Google Scholar] [CrossRef]
- Ferreres, F.; Valentao, P.; Llorach, R.; Pinheiro, C.; Cardoso, U.; Pereira, J.A.; Sousa, C.; Seabra, R.M.; Andrade, P.B. Phenolic compounds in external leaves of tronchuda cabbage (Brassica oleracea L. var. costata DC). J. Agric. Food Chem. 2005, 53, 2901–2907. [Google Scholar]
- Llorach, R.; Gil-Izquierdo, A.; Ferreres, F.; Tomas-Barberan, F.A. HPLC-DAD-MS/MS ESI characterization of unusual highly glycosylated acylated flavonoids from cauliflower (Brassica oleracea L. var. botrytis) agroindustrial byproducts. J. Agric. Food Chem. 2003, 51, 3895–3899. [Google Scholar] [CrossRef]
- Martinez-Sanchez, A.; Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F. A comparative study of flavonoid compounds, vitamin C, and antioxidant properties of baby leaf Brassicaceae species. J. Agric. Food Chem. 2008, 56, 2330–2340. [Google Scholar] [CrossRef]
- Nielsen, J.K.; Norbaek, R.; Olsen, C.E. Kaempferol tetraglucosides from cabbage leaves. Phytochemistry 1998, 49, 2171–2176. [Google Scholar] [CrossRef]
- Price, K.R.; Casuscelli, F.; Colquhoun, I.J.; Rhodes, M.J.C. Composition and content of flavonol glycosides in broccoli florets (Brassica oleracea) and their fate during cooking. J. Sci. Food Agric. 1998, 77, 468–472. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Galardi, C.; Corti, G.; Agnelli, A.; Vincieri, F.F.; Heimler, D. Flavonoids in leaves of black cabbage (Brassica oleracea var. acephala DC. subvar. viridis cv. serotina) grown on different soils and at different elevations. Ital. J. Food Sci. 2003, 15, 197–205. [Google Scholar]
- Fernandes, F.; Valentao, P.; Sousa, C.; Pereira, J.A.; Seabra, R.M.; Andrade, P.B. Chemical and antioxidative assessment of dietary turnip (Brassica rapa var. rapa L.). Food Chem. 2007, 105, 1003–1010. [Google Scholar] [CrossRef]
- Francisco, M.; Moreno, D.A.; Cartea, M.E.; Ferreres, F.; Garcia-Viguera, C.; Velasco, P. Simultaneous identification of glucosinolates and phenolic compounds in a representative collection of vegetable Brassica rapa. J. Chromatogr. 2009, 1216, 6611–6619. [Google Scholar] [CrossRef]
- Velasco, P.; Francisco, M.; Moreno, D.A.; Ferreres, F.; García-Viguera, C.; Cartea, M.E. Phytochemical fingerprinting of vegetable Brassica oleracea and Brassica napus by simultaneous identification of glucosinolates and phenolics. Phytochemical Analysis (in press) 2010. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, J.K.; Olsen, C.E.; Petersen, M.K. Acylated flavonol glycosides from cabbage leaves. Phytochemistry 1993, 34, 539–544. [Google Scholar] [CrossRef]
- Ferreres, F.; Sousa, C.; Vrchovska, V.; Valentao, P.; Pereira, J.A.; Seabra, R.M.; Andrade, P.B. Chemical composition and antioxidant activity of tronchuda cabbage internal leaves. Eur. Food Res. Technol. 2006, 222, 88–98. [Google Scholar] [CrossRef]
- Martinez, S.; Olmos, I.; Carballo, J.; Franco, I. Quality parameters of Brassica spp. grown in northwest Spain. Int. J. Food Sci. Technol. 2010, 45, 776–783. [Google Scholar] [CrossRef]
- Sousa, C.; Valentao, P.; Rangel, J.; Lopes, G.; Pereira, J.A.; Ferreres, F.; Seabra, R.A.; Andrade, P.B. Influence of two fertilization regimens on the amounts of organic acids and phenolic compounds of tronchuda cabbage (Brassica oleracea L. var. costata DC). J. Agric. Food Chem. 2005, 53, 9128–9132. [Google Scholar]
- Sousa, C.; Lopes, G.; Pereira, D.M.; Taveira, M.; Valentao, P.; Seabra, R.M.; Pereira, J.A.; Baptista, P.; Ferreres, F.; Andrade, P.B. Screening of antioxidant compounds during sprouting of Brassica oleracea L. var. costata DC. Comb. Chem. High Throughput Scr. 2007, 10, 377–386. [Google Scholar] [CrossRef]
- Sousa, C.; Taveira, M.; Valentao, P.; Fernandes, F.; Pereira, J.A.; Estevinho, L.; Bento, A.; Ferreres, F.; Seabra, R.M.; Andrade, P.B. Inflorescences of Brassicaceae species as source of bioactive compounds: A comparative study. Food Chem. 2008, 110, 953–961. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomas-Barberan, F.; Garcia-Viguera, C. Health-promoting compounds in broccoli as influenced by refrigerated transport and retail sale period. J. Agric. Food Chem. 2003, 51, 3029–3034. [Google Scholar] [CrossRef]
- Gratacos-Cubarsi, M.; Ribas-Agusti, A.; Garcia-Regueiro, J.A.; Castellari, M. Simultaneous evaluation of intact glucosinolates and phenolic compounds by UPLC-DAD-MS/MS in Brassica oleracea L. var. botrytis. Food Chem. 2010, 121, 257–263. [Google Scholar] [CrossRef]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Vincieri, F.F.; Romani, A. Antiradical activity and polyphenol composition of local Brassicaceae edible varieties. Food Chem. 2006, 99, 464–469. [Google Scholar] [CrossRef]
- Ferreres, F.; Sousa, C.; Pereira, D.M.; Valentao, P.; Taveira, M.; Martins, A.; Pereira, J.A.; Seabra, R.M.; Andrade, P.B. Screening of antioxidant phenolic compounds produced by in vitro shoots of Brassica oleracea L. var. costata DC. Comb. Chem. High Throughput Scr. 2009, 12, 230–240. [Google Scholar] [CrossRef]
- Taveira, M.; Pereira, D.M.; Sousa, C.; Ferreres, F.; Andrade, P.B.; Martins, A.; Pereira, J.A.; Valentao, P. In Vitro Cultures of Brassica oleracea L. var. costata DC: Potential Plant Bioreactor for Antioxidant Phenolic Compounds. J. Agric. Food Chem. 2009, 57, 1247–1252. [Google Scholar] [CrossRef]
- Wu, X.L.; Prior, R.L. Identification and characterization of anthocyanins by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry in common foods in the United States: Vegetables, nuts, and grains. J. Agric. Food Chem. 2005, 53, 3101–3113. [Google Scholar] [CrossRef]
- Vallejo, F.; Garcia-Viguera, C.; Tomas-Barberan, F.A. Changes in broccoli (Brassica oleracea L. var. italica) health-promoting compounds with inflorescence development. J. Agric. Food Chem. 2003, 51, 3776–3782. [Google Scholar] [CrossRef]
- Harbaum, B.; Hubbermann, E.M.; Zhu, Z.J.; Schwarz, K. Free and bound phenolic compounds in leaves of pak choi (Brassica campestris L. ssp chinensis var. communis) and Chinese leaf mustard (Brassica juncea Coss). Food Chem. 2008, 110, 838–846. [Google Scholar] [CrossRef]
- Romani, A.; Vignolini, P.; Isolani, L.; Ieri, F.; Heimler, D. HPLC-DAD/MS characterization of flavonoids and hydroxycinnamic derivatives in turnip tops (Brassica rapa L. subsp sylvestris L.). J. Agric. Food Chem. 2006, 54, 1342–1346. [Google Scholar]
- Ferreres, F.; Valentao, P.; Pereira, J.A.; Bento, A.; Noites, A.; Seabra, R.M.; Andrade, P.B. HPLC-DAD-MS/MS-ESI screening of phenolic compounds in Pieris brassicae L. reared on Brassica rapa var. rapa L. J. Agric. Food Chem. 2008, 56, 844–853. [Google Scholar] [CrossRef]
- Szydlowska-Czerniak, A.; Trokowski, K.; Karlovits, G.; Szlyk, E. Determination of Antioxidant Capacity, Phenolic Acids, and Fatty Acid Composition of Rapeseed Varieties. J. Agric. Food Chem. 2010, 58, 7502–7509. [Google Scholar] [CrossRef]
- Khattab, R.; Eskin, M.; Aliani, M.; Thiyam, U. Determination of Sinapic Acid Derivatives in Canola Extracts Using High-Performance Liquid Chromatography. J. Am. Oil Chem. Soc. 2010, 87, 147–155. [Google Scholar] [CrossRef]
- Li, X.; Gao, M.J.; Pan, H.Y.; Cui, D.J.; Gruber, M.Y. Purple Canola: Arabidopsis PAP1 Increases Antioxidants and Phenolics in Brassica napus Leaves. J. Agric. Food Chem. 2010, 58, 1639–1645. [Google Scholar] [CrossRef]
- Harbaum, B.; Hubbermann, E.M.; Zhu, Z.J.; Schwarz, K. Impact of fermentation on phenolic compounds in leaves of pak choi (Brassica campestris L. ssp chinensis var. communis) and Chinese leaf mustard (Brassica juncea coss). J. Agric. Food Chem. 2008, 56, 148–157. [Google Scholar] [CrossRef]
- Fang, Z.X.; Hu, Y.X.; Liu, D.H.; Chen, J.C.; Ye, X.Q. Changes of phenolic acids and antioxidant activities during potherb mustard (Brassica juncea, Coss.) pickling. Food Chem. 2008, 108, 811–817. [Google Scholar] [CrossRef]
- U, N. Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Jpn. J. Bot. 1935, 7, 389–452. [Google Scholar]
- Aguinagalde, I.; Gomezcampo, C.; Sanchezyelamo, M.D. A chemosystematic survey on wild relatives of Brassica oleracea L. Bot. J. Linn. Soc. 1992, 109, 57–67. [Google Scholar] [CrossRef]
- Braham, H.; Mighri, Z.; Ben Jannet, H.; Matthew, S.; Abreu, P.M. Antioxidant phenolic glycosides from Moricandia arvensis. J. Nat. Prod. 2005, 68, 517–522. [Google Scholar] [CrossRef]
- Weckerle, B.; Michel, K.; Balazs, B.; Schreier, P.; Toth, G. Quercetin 3,3 ',4 '-tri-O-beta-D-glucopyranosides from leaves of Eruca sativa (Mill.). Phytochemistry 2001, 57, 547–551. [Google Scholar] [CrossRef]
- Bennett, R.N.; Rosa, E.A.S.; Mellon, F.A.; Kroon, P.A. Ontogenic profiling of glucosinolates, flavonoids, and other secondary metabolites in Eruca sativa (salad rocket), Diplotaxis erucoides (wall rocket), Diplotaxis tenuifolia (wild rocket), and Bunias orientalis (Turkish rocket). J. Agric. Food Chem. 2006, 54, 4005–4015. [Google Scholar] [CrossRef]
- Martinez-Sanchez, A.; Llorach, R.; Gil, M.I.; Ferreres, F. Identification of new flavonoid glycosides and flavonoid profiles to characterize rocket leafy salads (Eruca vesicaria and Diplotaxis tenuifolia). J. Agric. Food Chem. 2007, 55, 1356–1363. [Google Scholar] [CrossRef]
- Onyilagha, J.; Bala, A.; Hallett, R.; Gruber, M.; Soroka, J.; Westcott, N. Leaf flavonoids of the cruciferous species, Camelina sativa, Crambe sp. Thlaspi arvense and several other genera of the family Brassicaceae. Biochem. Syst. Ecol. 2003, 31, 1309–1322. [Google Scholar] [CrossRef]
- Francisco, M.; Velasco, P.; Moreno, D.A.; Garcia-Viguera, C.; Cartea, M.E. Cooking methods of Brassica rapa affect the preservation of glucosinolates, phenolics and vitamin C. Food Res. Int. 2010, 43, 1455–1463. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U. Effect of hydrothermal treatment on the antioxidant properties of broccoli (Brassica oleracea var. botrytis italica) florets. Food Chem. 2008, 109, 393–401. [Google Scholar] [CrossRef]
- De Pascale, S.; Maggio, A.; Pernice, R.; Fogliano, V.; Barbieri, G. Sulphur fertilization may improve the nutritional value of Brassica rapa L. subsp sylvestris. Eur. J. Agron. 2007, 26, 418–424. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Z.; Gerendas, J. Effects of nitrogen and sulfur on total phenolics and antioxidant activity in two genotypes of leaf mustard. J. Plant Nutr. 2008, 31, 1642–1655. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomas-Barberan, F.A.; Garcia-Viguera, C. Effect of climatic and sulphur fertilisation conditions, on phenolic compounds and vitamin C, in the inflorescences of eight broccoli cultivars. Eur. Food Res. Technol. 2003, 216, 395–401. [Google Scholar]
- Zhao, X.; Nechols, J.R.; Williams, K.A.; Wang, W.Q.; Carey, E.E. Comparison of phenolic acids in organically and conventionally grown pak choi (Brassica rapa L. chinensis). J. Sci. Food Agric. 2009, 89, 940–946. [Google Scholar] [CrossRef]
- Young, J.E.; Zhao, X.; Carey, E.E.; Welti, R.; Yang, S.S.; Wang, W.Q. Phytochemical phenolics in organically grown vegetables. Mol. Nutr. Food Res. 2005, 49, 1136–1142. [Google Scholar] [CrossRef]
- Podsedek, A.; Sosnowska, D.; Redzynia, M.; Koziolkiewicz, M. Effect of domestic cooking on the red cabbage hydrophilic antioxidants. Int. J. Food Sci. Technol. 2008, 43, 1770–1777. [Google Scholar] [CrossRef]
- Zhang, D.L.; Hamauzu, Y. Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking. Food Chem. 2004, 88, 503–509. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomas-Barberan, F.A.; Garcia-Viguera, C. Phenolic compound contents in edible parts of broccoli inflorescences after domestic cooking. J. Sci. Food Agric. 2003, 83, 1511–1516. [Google Scholar] [CrossRef]
- Lin, C.H.; Chang, C.Y. Textural change and antioxidant properties of broccoli under different cooking treatments. Food Chem. 2005, 90, 9–15. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Iqbal, S. Effect of different cooking methods on the antioxidant activity of some vegetables from Pakistan. Int. J. Food Sci. Technol. 2008, 43, 560–567. [Google Scholar] [CrossRef]
- Gliszczynska-Swiglo, A.; Ciska, E.; Pawlak-Lemanska, K.; Chmielewski, J.; Borkowski, T.; Tyrakowska, B. Changes in the content of health-promoting compounds and antioxidant activity of broccoli after domestic processing. Food Addit. Contam. 2006, 23, 1088–1098. [Google Scholar] [CrossRef]
- Natella, F.; Belelli, F.; Ramberti, A.; Scaccini, C. Microwave and traditional cooking methods: Effect of cooking on antioxidant capacity and phenolic compounds content of seven vegetables. J. Food Biochem. 2010, 34, 796–810. [Google Scholar]
- Starzynska, A.; Leja, M.; Mareczek, A. Physiological changes in the antioxidant system of broccoli flower buds senescing during short-term storage, related to temperature and packaging. Plant Sci. 2003, 165, 1387–1395. [Google Scholar] [CrossRef]
- Meyer, P.; Heidmann, I.; Forkmann, G.; Saedler, H. A new petunia flower color generated by transformation of a mutant with a maize gene. Nature 1987, 330, 677–678. [Google Scholar] [CrossRef]
- Jung, W.; Yu, O.; Lau, S.M.C.; O'Keefe, D.P.; Odell, J.; Fader, G.; McGonigle, B. Identification and expression of isoflavone synthase, the key enzyme for biosynthesis of isoflavones in legumes (vol 18, pg 211, 2000). Nat. Biotechnol. 2000, 18, 559–559. [Google Scholar] [CrossRef]
- Schijlen, E.G.W.; de Vos, C.H.R.; van Tunen, A.J.; Bovy, A.G. Modification of flavonoid biosynthesis in crop plants. Phytochemistry 2004, 65, 2631–2648. [Google Scholar] [CrossRef]
- Husken, A.; Baumert, A.; Strack, D.; Becker, H.C.; Mollers, C.; Milkowski, C. Reduction of sinapate ester content in transgenic oilseed rape (Brassica napus) by dsRNAi-based suppression of BnSGT1 gene expression. Mol. Breed. 2005, 16, 127–138. [Google Scholar] [CrossRef]
- Milkowski, C.; Strack, D. Sinapate esters in brassicaceous plants: Biochemistry, molecular biology, evolution and metabolic engineering. Planta 2010, 232, 19–35. [Google Scholar] [CrossRef]
- Velasco, L.; Mollers, C. Nondestructive assessment of sinapic acid esters in Brassica species: II. Evaluation of germplasm and identification of phenotypes with reduced levels. Crop Sci. 1998, 38, 1650–1654. [Google Scholar] [CrossRef]
- Nair, R.B.; Joy, R.W.; Kurylo, E.; Shi, X.H.; Schnaider, J.; Datla, R.S.S.; Keller, W.A.; Selvaraj, G. Identification of a CYP84 family of cytochrome P450-dependent mono-oxygenase genes in Brassica napus and perturbation of their expression for engineering sinapine reduction in the seeds. Plant Physiol. 2000, 123, 1623–1634. [Google Scholar] [CrossRef]
- Bhinu, V.S.; Schafer, U.A.; Li, R.; Huang, J.; Hannoufa, A. Targeted modulation of sinapine biosynthesis pathway for seed quality improvement in Brassica napus. Transgenic Res. 2009, 18, 31–44. [Google Scholar] [CrossRef]
- Sample availability: Not available.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2011, 16, 251-280. https://doi.org/10.3390/molecules16010251
Cartea ME, Francisco M, Soengas P, Velasco P. Phenolic Compounds in Brassica Vegetables. Molecules. 2011; 16(1):251-280. https://doi.org/10.3390/molecules16010251
Chicago/Turabian StyleCartea, María Elena, Marta Francisco, Pilar Soengas, and Pablo Velasco. 2011. "Phenolic Compounds in Brassica Vegetables" Molecules 16, no. 1: 251-280. https://doi.org/10.3390/molecules16010251
APA StyleCartea, M. E., Francisco, M., Soengas, P., & Velasco, P. (2011). Phenolic Compounds in Brassica Vegetables. Molecules, 16(1), 251-280. https://doi.org/10.3390/molecules16010251