A Practical Method for Synthesizing Iptacopan
Abstract
:1. Introduction
2. Results and Discussion
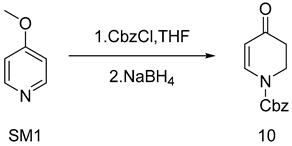
2.1. Optimization of Reaction Conditions for Benzyl 4-Oxo-3,4-dihydropyridine-1(2H)-carboxylate
2.2. Effect of Reaction Conditions on the Synthesis of Compound 11
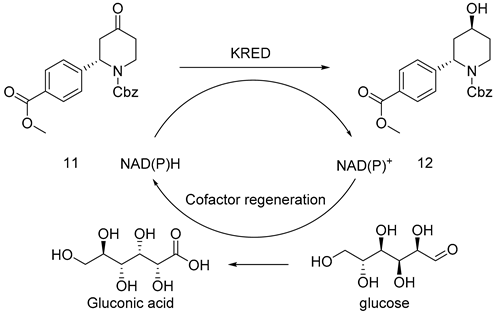
2.3. Screening of Ketoreductases
2.4. Determination of the Absolute Configuration
2.5. Screening of a WTEA Variant Library
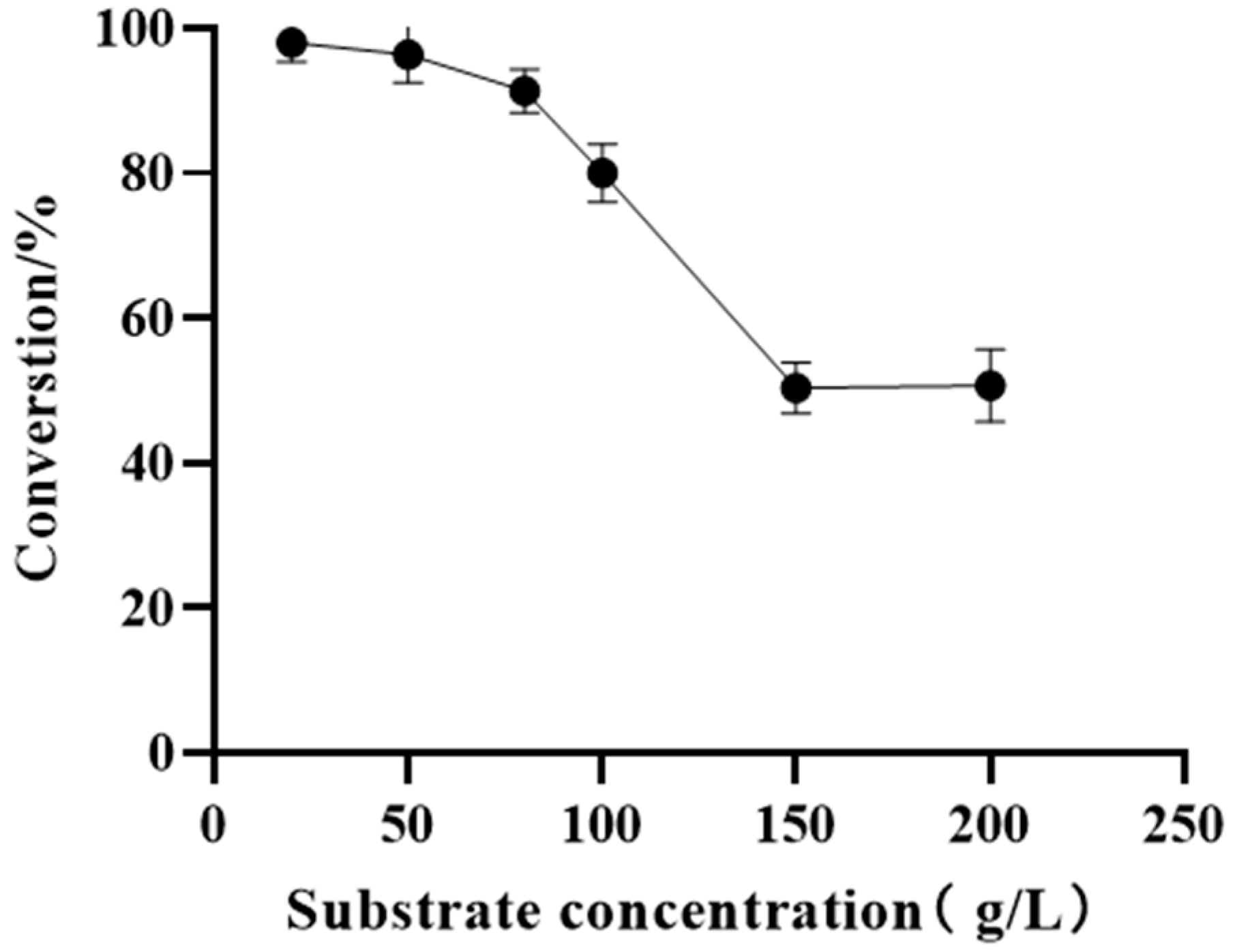
2.6. Optimization of the Reaction Conditions of Enzyme Catalysis: Optimization of the Substrate Concentration
2.7. Selection and Optimization of the Reaction Co-Solvent
2.8. Optimization of the pH and Temperature
2.9. Optimization of the Biocatalyst Loading
2.10. Scaling up the Enzymatic Process
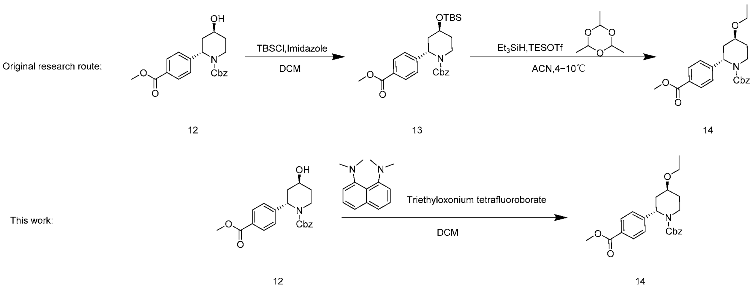
2.11. Optimization of the Synthesis of Compound 14
3. Conclusions
4. Experimental Section
4.1. Materials and Methods
4.2. Screening of the Variant(s) Library
4.3. Culture of E. coli/pET-28a (+)-M8 Cells in a 10 L Bioreactor
4.4. Synthesis of Benzyl 4-Oxo-3,4-dihydropyridine-1(2H)-carboxylate
4.5. Synthesis of Benzyl (S)-2-(4-(Methoxycarbonyl)-phenyl)-4-oxopiperidine-1-carboxylate
4.6. Synthesis of Benzyl (2S,4S)-4-Hydroxy-2-(4-(methoxycarbonyl)-phenyl)-piperidine-1-carboxylate
4.7. Synthesis of Benzyl (2S,4S)-4-Ethoxy-2-(4-(methoxycarbonyl)-phenyl)-piperidine-1-carboxylate
4.8. Synthesis of Methyl 4-((2S,4S)-4-Ethoxypiperidin-2-yl)-benzoate Hydrochloride
4.9. Synthesis of Tert-Butyl 4-(((2S,4S)-4-ethoxy-2-(4-(methoxycarbonyl) phenyl) piperidin-1-yl)-methyl)-5-methoxy-7-methyl-1H-indole-1-carboxylate
4.10. Synthesis of Iptacopan Hydrochloride (LNP023)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mamidi, S.; Hone, S.; Kirschfink, M. The complement system in cancer: Ambivalence between tumour destruction and promotion. Immunobiology 2017, 222, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Schubart, A.; Anderson, K.; Mainolfi, N.; Sellner, H.; Ehara, T.; Adams, C.M.; Mac Sweeney, A.; Liao, S.M.; Crowley, M.; Littlewood-Evans, A.; et al. Small-molecule factor B inhibitor for the treatment of complement-mediated diseases. Proc. Natl. Acad. Sci. USA 2019, 116, 7926–7931. [Google Scholar] [CrossRef] [PubMed]
- Mainolfi, N.; Ehara, T.; Karki, R.G.; Anderson, K.; Mac Sweeney, A.; Liao, S.M.; Argikar, U.A.; Jendza, K.; Zhang, C.; Powers, J.; et al. Discovery of 4-((2S,4S)-4-Ethoxy-1-((5-methoxy-7-methyl-1H-indol-4-yl)methyl)piperidin-2-yl)benzoic Acid (LNP023), a Factor B Inhibitor Specifically Designed To Be Applicable to Treating a Diverse Array of Complement Mediated Diseases. J. Med. Chem. 2020, 63, 5697–5722. [Google Scholar] [CrossRef]
- Santos, C.C.F.; Paradela, L.S.; Novaes, L.F.T.; Dias, S.M.G.; Pastre, J.C. Design and synthesis of cenocladamide analogues and their evaluation against breast cancer cell lines. MedChemComm 2017, 8, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-S.; Huang, L.-Z.; Jeon, H.J.; Xuan, Z.; Lee, S.-g. Cooperative Pd(0)/Rh(II) Dual Catalysis: Interceptive Capturing of π-Allyl Pd(II) Complexes with α-Imino Rh(II) Carbenoids. ACS Catal. 2016, 6, 4914–4919. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, Y.; Qiu, S.; Zhai, Q.-Y.; Liu, H.-T.; Li, S.-F.; Weng, C.-Y.; Wang, Y.-J.; Zheng, Y.-G. Controlling Stereopreferences of Carbonyl Reductases for Enantioselective Synthesis of Atorvastatin Precursor. ACS Catal. 2021, 11, 2572–2582. [Google Scholar] [CrossRef]
- Huang, W.Y.; Lu, C.H.; Ghorai, S.; Li, B.; Li, C. Regio- and Enantioselective Allylic Alkylation of Terminal Alkynes by Synergistic Rh/Cu Catalysis. J. Am. Chem. Soc. 2020, 142, 15276–15281. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.H.; Fuentes, J.A.; Cordes, D.B.; Slawin, A.M.Z.; Clarke, M.L. Phospholane-Phosphite Ligands for Rh Catalyzed Enantioselective Conjugate Addition: Unusually Reactive Catalysts for Challenging Couplings. Eur. J. Org. Chem. 2020, 2020, 3071–3076. [Google Scholar] [CrossRef]
- Jagt, R.B.C.; de Vries, J.G.; Feringa, B.L.; Minnaard, A.J. Enantioselective Synthesis of 2-Aryl-4-piperidones via Rhodium/Phosphoramidite-Catalyzed Conjugate Addition of Arylboroxines. Org. Lett. 2005, 7, 2433–2435. [Google Scholar] [CrossRef] [PubMed]
- Shintani, R.; Tokunaga, N.; Doi, H.; Hayashi, T. A new entry of nucleophiles in rhodium-catalyzed asymmetric 1,4-addition reactions: Addition of organozinc reagents for the synthesis of 2-aryl-4-piperidones. J. Am. Chem. Soc. 2004, 126, 6240–6241. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, R.; Zhang, T.; Shi, M. Asymmetric 1,4-addition of arylboronic acids to 2,3-dihydro-4-pyridones catalyzed by axially chiral NHC-Pd(II) complexes. J. Org. Chem. 2010, 75, 3935–3937. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.E.; Boehnke, J.; Glen, P.E.; Levey, S.; Patrick, L.; Jordan-Hore, J.A.; Lee, A.-L. Ligand- and Base-Free Pd(II)-Catalyzed Controlled Switching between Oxidative Heck and Conjugate Addition Reactions. Org. Lett. 2013, 15, 1886–1889. [Google Scholar] [CrossRef] [PubMed]
- Gini, F.; Hessen, B.; Minnaard, A.J. Palladium-Catalyzed Enantioselective Conjugate Addition of Arylboronic Acids. Org. Lett. 2005, 7, 5309–5312. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chen, L.; Zhang, L.; Ni, G.; Yu, J.; Wang, H.; Zhang, F.; Yuan, S.; Feng, M.; Chen, S. Structure-guided evolution of a ketoreductase for efficient and stereoselective bioreduction of bulky α-amino β-keto esters. Catal. Sci. Technol. 2021, 11, 6755–6769. [Google Scholar] [CrossRef]
- Peng, X.; Lu, C.; Pang, J.; Liu, Z.; Lu, D. A distal regulatory strategy of enzymes: From local to global conformational dynamics. Phys. Chem. Chem. Phys. 2021, 23, 22451–22465. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Chu-Moyer, M.Y.; Danishefsky, S.J.; Schulte, G.K. The total synthesis of indolizomycin. J. Am. Chem. Soc. 1993, 115, 30–39. [Google Scholar] [CrossRef]
- Pichlmair, S. R3O+BF4-: Meerwein’s Salt. Synlett 2004, 2004, 195–196. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, J.; Feng, M.; Chen, S. Engineering Methyltransferase and Sulfoxide Synthase for High-Yield Production of Ergothioneine. J. Agric. Food Chem. 2023, 71, 671–679. [Google Scholar] [CrossRef]








 | ||||
|---|---|---|---|---|
| Entry | Solvent | Temperature b (°C) | Reaction Time (h) | Yield c (%) |
| 1 | MeOH/THF (5/1) | −78 | 3 | 41 |
| 2 | THF | −78 | 3.5 | 50 |
| 3 | THF | −78 | 4.5 | 60 |
| 4 | THF | −78 | 7.5 | 62 |
| 5 | THF | −78 | 4.5 | 63 |
| 6 | THF | −20 | 2 | 85 |
| 7 | THF | 0 | 2 | 51 |
| 8 | THF | 25 | 2 | 23 |
 | |||||
|---|---|---|---|---|---|
| Entry | 16/17(Equiv.) | Catalyst(Equiv.) | Base(Equiv.) | Solvent(Proportion) | Yield |
| 1 | 16(3) | Rh(Acac)(C2H5)2(0.03) | - | 2-Methyl-2-butanol:H2O = 10:1 | - |
| 2 | 16(3) | Rh(Acac)(C2H5)2(0.03) | Cs2CO3(3) | 1,4-Dioxane:H2O = 5:1 | 15% |
| 3 | 17(4) | Pd(OAc)2(0.03) | Cs2CO3(3) | 1,4-Dioxane:H2O = 5:1 | - |
| 4 | 17(4) | Pd[P(C6H5)3]4(0.03) | Cs2CO3(3) | 1,4-Dioxane:H2O = 5:1 | - |
| 5 | 17(4) | Pd(C5H7O2)2(0.03) | Cs2CO3(3) | 1,4-Dioxane:H2O = 5:1 | - |
| 6 | 17(2) | Rh(Acac)(C2H5)2(0.03) | Cs2CO3(3) | 1,4-Dioxane:H2O = 5:1 | 33% |
| 7 | 17(2.5) | Rh(Acac)(C2H5)2(0.03) | Cs2CO3(3) | 1,4-Dioxane:H2O = 5:1 | 79% |
| 8 | 17(3) | Rh(Acac)(C2H5)2(0.03) | Cs2CO3(3) | 1,4-Dioxane:H2O = 5:1 | 80% |
| 9 | 17(4) | Rh(Acac)(C2H5)2(0.03) | Cs2CO3(3) | 1,4-Dioxane:H2O = 5:1 | 81% |
| 10 | 17(4) | Rh(Acac)(C2H5)2(0.03) | Cs2CO3(3) | 1,4-Dioxane | - |
| 11 | 17(4) | Rh(Acac)(C2H5)2(0.03) | Cs2CO3(3) | 1,4-Dioxane:H2O = 10:1 | 36% |
| 12 | 17(4) | Rh(Acac)(C2H5)2(0.03) | Cs2CO3(3) | 1,4-Dioxane:H2O = 20:1 | 34% |
| 13 | 17(4) | Rh(Acac)(C2H5)2(0.03) | Cs2CO3(3) | Tol:H2O = 5:1 | 85% |
| 14 | 17(4) | Rh(Acac)(C2H5)2(0.03) | Cs2CO3(3) | ACN:H2O = 5:1 | 25% |
| 15 | 17(4) | Rh(Acac)(C2H5)2(0.03) | Cs2CO3(3) | THF:H2O = 5:1 | 50% |
| 16 | 17(4) | Rh(Acac)(C2H5)2(0.03) | TEA(3) | 1,4-Dioxane:H2O = 5:1 | - |
| 17 | 17(4) | Rh(Acac)(C2H5)2(0.03) | Pyridine(3) | 1,4-Dioxane:H2O = 5:1 | - |
| 18 | 17(4) | Rh(Acac)(C2H5)2(0.03) | K2CO3 | 1,4-Dioxane:H2O = 5:1 | 35% |
 | |||
|---|---|---|---|
| Entry | Conservation (%) | ee c (%) | Configuration |
| K2 a | 95 | 72.94 | R |
| K17 a | 95 | 63.74 | S |
| K30 a | 80 | 79.22 | S |
| K31 b | 80 | 99.99 | R |
| K73 a | 95 | 99.99 | S |
| Entry | KRED-M8 | (S) CBS Catalyst |
|---|---|---|
| Solvent | water/DMSO | THF |
| Catalyst loading | 25 g/L | 1.2 eq |
| Catalyst cost | ¥125 | ¥7840 |
| Co-factor b b cost | ¥66 | 0 |
| Yield | 92% | 65% |
| Purity | 99.50% | >95% |
| ee | 99.99% | 32% |
 | |||
|---|---|---|---|
| Entry | Alkylating Reagent | Conversion (%) | Yield (%) |
| 1 | N-ethylpyridinium tetrafluoroborate | 0 | - |
| 2 | Trifluoromethanesulfonic anhydride/EtOH | 0 | - |
| 3 | Sodium hydrogen/Iodoethane | 0 | - |
| 4 | Triethyloxonium tetrafluoroborate | 99.9 | 75 |
| 5 | Paraldehyde | 83.0 | 44 |
| 6 | Triethyloxonium tetrafluoroborate (No added proton sponge) | 48.5 | - |
| 7 | Triethyloxonium tetrafluoroborate (Added base) | 50.3 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Chu, S.; Wu, X.; Chen, S.; Chen, L.; Tang, J.; Wang, H. A Practical Method for Synthesizing Iptacopan. Molecules 2024, 29, 2289. https://doi.org/10.3390/molecules29102289
Tang Z, Chu S, Wu X, Chen S, Chen L, Tang J, Wang H. A Practical Method for Synthesizing Iptacopan. Molecules. 2024; 29(10):2289. https://doi.org/10.3390/molecules29102289
Chicago/Turabian StyleTang, Zhiwei, Shaojie Chu, Xuesong Wu, Shaoxin Chen, Likuo Chen, Jiawei Tang, and Hongbo Wang. 2024. "A Practical Method for Synthesizing Iptacopan" Molecules 29, no. 10: 2289. https://doi.org/10.3390/molecules29102289





