Assessment of Key Factors Impacting Variability in AAV Vector Genome Titration by Digital PCR
Abstract
:1. Introduction
2. Results
2.1. Comparison between Genome Extraction Protocol and Nonextraction Protocol
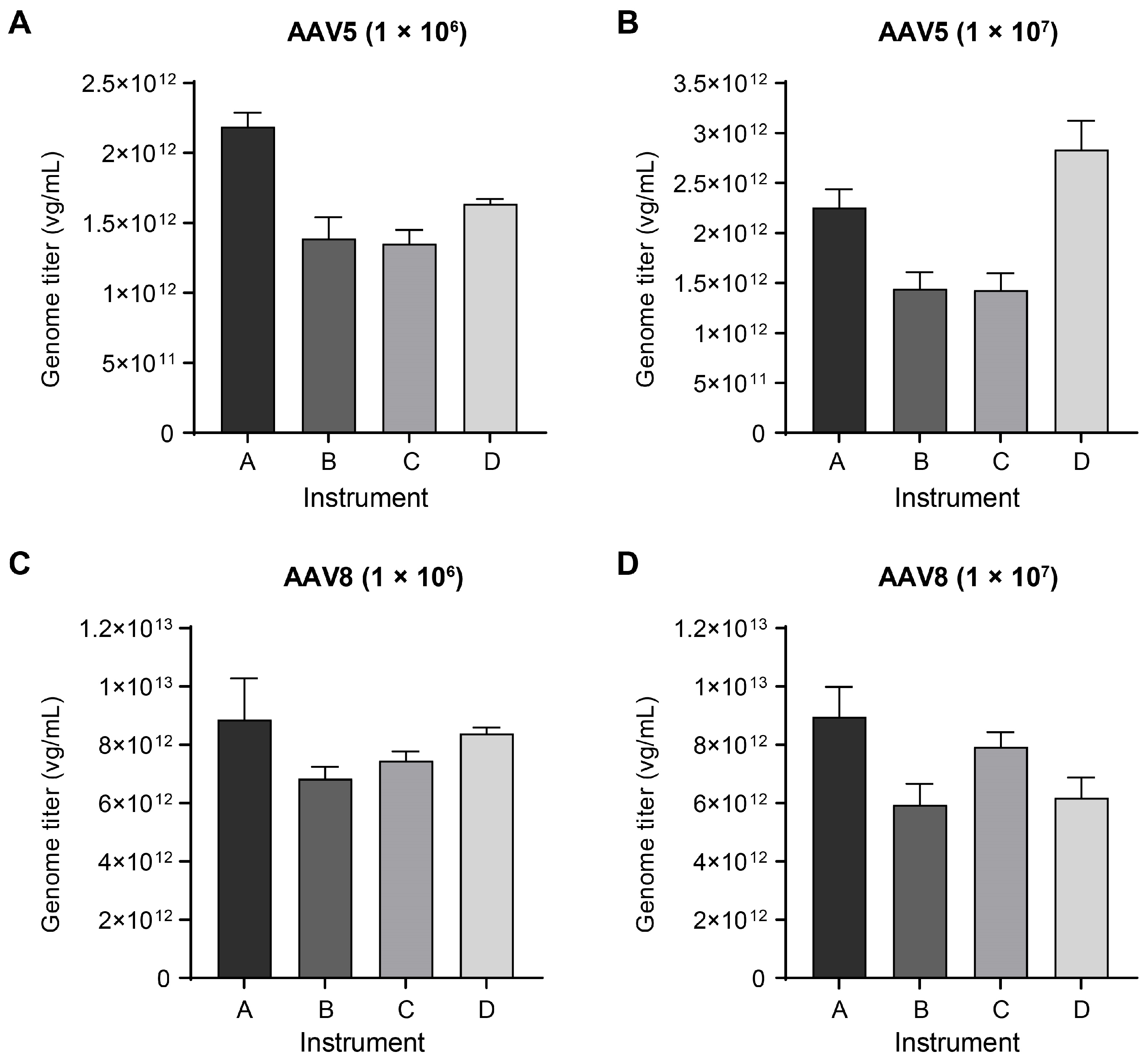
2.2. Checking Titer Variations across Different Instruments
2.3. Testing DNase I Treatment Time and Inactivation
2.4. Dilution Buffer Components Do Not Cause Variations in Genome Titers
2.5. Testing the Order of Serial Dilution and Thermal Capsid Lysis
2.6. Freeze–Thawing Cycles Compromised AAV Genome Titers
2.7. Validation of AAV Genome Titer Assay Using dPCR
3. Discussion
4. Materials and Methods
4.1. RAAV Materials
4.2. Primers and Probes
4.3. AAV Genome Extraction
4.4. AAV Genome Titration by dPCR
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Menon, N.; Shen, S.; Feschenko, M.; Bergelson, S. A qPCR Method for AAV Genome Titer with ddPCR-Level of Accuracy and Precision. Mol. Ther. Methods Clin. Dev. 2020, 19, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Dobnik, D.; Kogovsek, P.; Jakomin, T.; Kosir, N.; Tusek Znidaric, M.; Leskovec, M.; Kaminsky, S.M.; Mostrom, J.; Lee, H.; Ravnikar, M. Accurate Quantification and Characterization of Adeno-Associated Viral Vectors. Front. Microbiol. 2019, 10, 1570. [Google Scholar] [CrossRef]
- Lock, M.; Alvira, M.R.; Chen, S.J.; Wilson, J.M. Absolute determination of single-stranded and self-complementary adeno-associated viral vector genome titers by droplet digital PCR. Hum. Gene Ther. Methods 2014, 25, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Sanmiguel, J.; Gao, G.; Vandenberghe, L.H. Quantitative and Digital Droplet-Based AAV Genome Titration. Methods Mol. Biol. 2019, 1950, 51–83. [Google Scholar] [CrossRef] [PubMed]
- Prantner, A.; Maar, D. Genome concentration, characterization, and integrity analysis of recombinant adeno-associated viral vectors using droplet digital PCR. PLoS ONE 2023, 18, e0280242. [Google Scholar] [CrossRef]
- Suoranta, T.; Laham-Karam, N.; Yla-Herttuala, S. Optimized Protocol for Accurate Titration of Adeno-Associated Virus Vectors. Hum. Gene Ther. 2021, 32, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Zanker, J.; Lazaro-Petri, S.; Huser, D.; Heilbronn, R.; Savy, A. Insight and Development of Advanced Recombinant Adeno-Associated Virus Analysis Tools Exploiting Single-Particle Quantification by Multidimensional Droplet Digital PCR. Hum. Gene Ther. 2022, 33, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Meierrieks, F.; Kour, A.; Patz, M.; Pflanz, K.; Wolff, M.W.; Pickl, A. Unveiling the secrets of adeno-associated virus: Novel high-throughput approaches for the quantification of multiple serotypes. Mol. Ther. Methods Clin. Dev. 2023, 31, 101118. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Patel, S.; Mietzsch, M.; Jose, A.; Lins-Austin, B.; Yu, J.C.; Bothner, B.; McKenna, R.; Agbandje-McKenna, M. Thermal Stability as a Determinant of AAV Serotype Identity. Mol. Ther. Methods Clin. Dev. 2017, 6, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Mayor, H.D.; Torikai, K.; Melnick, J.L.; Mandel, M. Plus and minus single-stranded DNA separately encapsidated in adeno-associated satellite virions. Science 1969, 166, 1280–1282. [Google Scholar] [CrossRef] [PubMed]
- Nakai, H.; Storm, T.A.; Kay, M.A. Recruitment of single-stranded recombinant adeno-associated virus vector genomes and intermolecular recombination are responsible for stable transduction of liver in vivo. J. Virol. 2000, 74, 9451–9463. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.L.; Loganathan, N.; Agarwalla, S.; Yang, C.; Yuan, W.; Zeng, J.; Wu, R.; Wang, W.; Duraiswamy, S. Current commercial dPCR platforms: Technology and market review. Crit. Rev. Biotechnol. 2023, 43, 433–464. [Google Scholar] [CrossRef] [PubMed]






| Serotype | Experiment_1 | Experiment_2 | Experiment_3 | Avg (vg/mL) | RSD (%) | |
|---|---|---|---|---|---|---|
| Extraction | AAV5 | 1.05 × 1012 | 1.02 × 1012 | 9.68 × 1011 | 1.01 × 1012 | 4.06 |
| AAV8 | 9.11 × 1012 | 1.31 × 1013 | 7.58 × 1012 | 9.92 × 1012 | 28.54 | |
| Without extraction | AAV5 | 1.40 × 1012 | 1.38 × 1012 | 1.37 × 1012 | 1.38 × 1012 | 1.24 |
| AAV8 | 1.00 × 1013 | 9.16 × 1012 | 9.28 × 1012 | 9.49 × 1012 | 4.99 |
| Serotype | Test_1 | Test _2 | Test _3 | Avg (vg/mL) | RSD (%) |
|---|---|---|---|---|---|
| AAV2 | 1.36 × 1013 | 1.37 × 1013 | 1.41 × 1013 | 1.38 × 1013 | 1.96 |
| AAV5(a) | 1.15 × 1013 | 1.08 × 1013 | 1.19 × 1013 | 1.14 × 1013 | 4.76 |
| AAV6 | 8.47 × 1012 | 9.01 × 1012 | 8.30 × 1012 | 8.59 ×1012 | 4.32 |
| AAV8 | 1.45 × 1013 | 1.48 × 1013 | 1.53 × 1013 | 1.49 × 1013 | 2.66 |
| AAV9(a) | 8.70 × 1012 | 9.07 × 1012 | 8.33 × 1012 | 8.70 × 1012 | 4.25 |
| AAV5(b) | 3.30 × 1013 | 3.36 × 1013 | 3.36 × 1013 | 3.34 × 1013 | 1.04 |
| AAV9(b) | 3.37 × 1013 | 3.32 × 1013 | 3.21 × 1013 | 3.30 × 1013 | 2.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Ma, Q.; Wei, C.; Yu, L.; Bi, H.; Jin, J.; Qin, X.; Zhou, Y.; Wang, J. Assessment of Key Factors Impacting Variability in AAV Vector Genome Titration by Digital PCR. Int. J. Mol. Sci. 2024, 25, 5149. https://doi.org/10.3390/ijms25105149
Wang G, Ma Q, Wei C, Yu L, Bi H, Jin J, Qin X, Zhou Y, Wang J. Assessment of Key Factors Impacting Variability in AAV Vector Genome Titration by Digital PCR. International Journal of Molecular Sciences. 2024; 25(10):5149. https://doi.org/10.3390/ijms25105149
Chicago/Turabian StyleWang, Guangyu, Qiang Ma, Changlong Wei, Lei Yu, Hua Bi, Jing Jin, Xi Qin, Yong Zhou, and Junzhi Wang. 2024. "Assessment of Key Factors Impacting Variability in AAV Vector Genome Titration by Digital PCR" International Journal of Molecular Sciences 25, no. 10: 5149. https://doi.org/10.3390/ijms25105149






