Surviving a Double-Edged Sword: Response of Horticultural Crops to Multiple Abiotic Stressors
Abstract
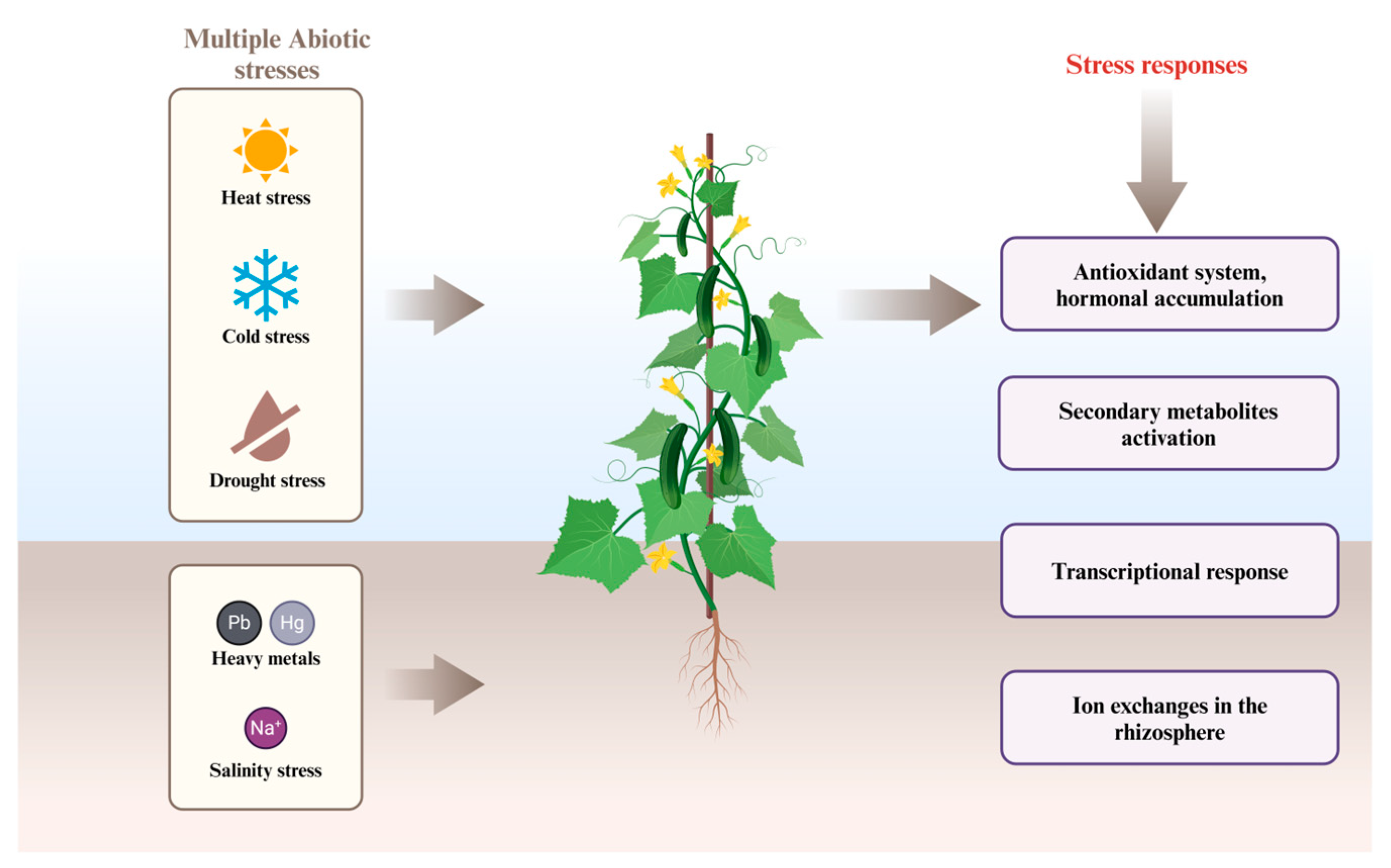
:1. Introduction
2. Common Types of Combined Multiple Abiotic Stresses
2.1. Heat and Drought Stress
2.2. Heat–Drought–Light and Salt Stress
2.3. Heat and Waterlogging Stress
2.4. Heat and Salinity Stress
3. Different Strategies to Study the Stress Response
3.1. Evaluation of Phenotypic/Morphological Changes
3.2. Transcriptomic Analysis
3.3. Proteomic Analysis
3.4. Metabolomics
3.5. Omics Approaches
4. Response Mechanism of Horticultural Crops to Multiple Stresses
4.1. Physiological and Biochemical Response
4.1.1. Antioxidant System
4.1.2. Hormonal Response
4.1.3. Compatible Solutes
4.2. Molecular Response to Multiple Abiotic Stresses
4.2.1. Signal-Sensing Mechanisms
4.2.2. Signal Transduction Mechanism
4.2.3. Mechanisms of Gene Expression Modulation
5. Breeding Strategies Using Molecular Approaches
6. Conclusions and Future Perspectives
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and Salinity Stress Responses and Microbe-Induced Tolerance in Plants. Front Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Subudhi, P.K.; Varshney, R.K.; Jain, M. Editorial: Abiotic Stress: Molecular Genetics and Genomics, Volume II. Front Plant Sci. 2022, 13, 1101139. [Google Scholar] [CrossRef]
- He, X.; Wang, C.; Wang, H.; Li, L.; Wang, C. The Function of MAPK Cascades in Response to Various Stresses in Horticultural Plants. Front Plant Sci. 2020, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Chirivì, D.; Betti, C. Molecular Links between Flowering and Abiotic Stress Response: A Focus on Poaceae. Plants 2023, 12, 331. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, X.; Cui, X.; Wang, K.; Wang, Y.; He, Y. Phytohormones Regulate the Abiotic Stress: An Overview of Physiological, Biochemical, and Molecular Responses in Horticultural Crops. Front Plant Sci. 2022, 13, 1095363. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Mittler, R. Plant Responses to Multifactorial Stress Combination. New Phytol. 2022, 234, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global Warming, Climate Change, and Environmental Pollution: Recipe for a Multifactorial Stress Combination Disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Balfagon, D.; Gomez-Cadenas, A.; Rambla, J.L.; Granell, A.; de Ollas, C.; Bassham, D.C.; Mittler, R.; Zandalinas, S.I. γ-Aminobutyric Acid Plays a Key Role in Plant Acclimation to a Combination of High Light and Heat Stress. Plant Physiol. 2022, 188, 2026–2038. [Google Scholar] [CrossRef] [PubMed]
- Fabian, A.; Safran, E.; Szabo-Eitel, G.; Barnabas, B.; Jager, K. Stigma Functionality and Fertility Are Reduced by Heat and Drought Co-stress in Wheat. Front Plant Sci. 2019, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Sengupta, S.; Fritschi, F.B.; Azad, R.K.; Nechushtai, R.; Mittler, R. The Impact of Multifactorial Stress Combination on Plant Growth and Survival. New Phytol. 2021, 230, 1034–1048. [Google Scholar] [CrossRef]
- Correia, B.; Hancock, R.D.; Amaral, J.; Gomez-Cadenas, A.; Valledor, L.; Pinto, G. Combined Drought and Heat Activates Protective Responses in Eucalyptus globulus That Are Not Activated When Subjected to Drought or Heat Stress Alone. Front Plant Sci. 2018, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Tabassum, J.; Fakhar, A.Z.; Sharif, R.; Chen, H.; Zhang, C.; Ju, L.; Fotopoulos, V.; Siddique, K.H.M.; Singh, R.K.; et al. Smart Reprograming of Plants against Salinity Stress Using Modern Biotechnological Tools. Crit. Rev. Biotechnol. 2022, 43, 1035–1062. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of Combined Abiotic and Biotic Stresses on Plant Growth and Avenues for Crop Improvement by Exploiting Physio-morphological Traits. Front Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef]
- Mickelbart, M.V.; Hasegawa, P.M.; Bailey-Serres, J. Genetic Mechanisms of Abiotic Stress Tolerance that Translate to Crop Yield Stability. Nat. Rev. Genet. 2015, 16, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Veal, A.J. Climate Change 2021: The Physical Science Basis, 6th Report. World Leis. J. 2021, 63, 443–444. [Google Scholar] [CrossRef]
- Patz, J.A.; Frumkin, H.; Holloway, T.; Vimont, D.J.; Haines, A. Climate Change: Challenges and Opportunities for Global Health. JAMA 2014, 312, 1565–1580. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, M.F.; Qureshi, R.; Muqaddasi, Q.H.; Shaheen, H.; Kousar, R.; Roder, M.S. Genome-wide Association Mapping in Bread Wheat Subjected to Independent and Combined High Temperature and Drought stress. PLoS ONE 2018, 13, e0199121. [Google Scholar] [CrossRef]
- Bérard, A.; Ben Sassi, M.; Kaisermann, A.; Renault, P. Soil Microbial Community Responses to Heat Wave Components: Drought and High Temperature. Clim. Res. 2015, 66, 243–264. [Google Scholar] [CrossRef]
- Balfagón, D.; Zandalinas, S.I.; Mittler, R.; Gómez-Cadenas, A. High Temperatures Modify Plant Responses to Abiotic Stress Conditions. Physiol. Plant. 2020, 170, 335–344. [Google Scholar] [CrossRef]
- Sinha, R.; Fritschi, F.B.; Zandalinas, S.I.; Mittler, R. The Impact of Stress Combination on Reproductive Processes in Crops. Plant Sci. 2021, 311, 111007. [Google Scholar] [CrossRef]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Front Chem. 2018, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Kane, J.M.; Anderegg, L.D.L. Consequences of Widespread Tree Mortality Triggered by Drought and Temperature Stress. Nat. Clim. Chang. 2013, 3, 30–36. [Google Scholar] [CrossRef]
- Teskey, R.; Wertin, T.; Bauweraerts, I.; Ameye, M.; McGuire, M.A.; Steppe, K. Responses of Tree Species to Heat Waves and Extreme Heat Events. Plant Cell Environ. 2015, 38, 1699–1712. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.T.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Parasuraman, B.; Rajamanickam, V.; Rathinavelu, S.; Geethanjali, S.; Alagarswamy, S. Interactive Effect of Drought and High Temperature on Physiological Traits of Soybean (Glycine max). Plant Physiol. Rep. 2024, 29, 116–124. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S.; Kataria, S.; Rastogi, A. The Interactive Effect of High Temperature and Water Deficit Stress on Nitrogen Fixation, Photosynthesis, Chlorophyll Fluorescence, Seed Yield and Quality in Soybean (Glycine max). Plant Physiol. Rep. 2023, 29, 125–140. [Google Scholar] [CrossRef]
- Balfagon, D.; Zandalinas, S.I.; Dos Reis de Oliveira, T.; Santa-Catarina, C.; Gomez-Cadenas, A. Reduction of Heat Stress Pressure and Activation of Photosystem II Repairing System Are Crucial for Citrus Tolerance to Multiple Abiotic Stress Combination. Physiol. Plant. 2022, 174, e13809. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.R.; Joshi, G.; Pandey, R.; Kukreja, B.; Goel, S.; Jagannath, A.; Kumar, A.; Katiyar-Agarwal, S.; Agarwal, M. A Genome-Wide Perspective of miRNAome in Response to High Temperature, Salinity and Drought Stresses in Brassica juncea (Czern) L. PLoS ONE 2014, 9, e92456. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Santos, C.; Serodio, J.; Silva, A.M.S.; Dias, M.C. Physiological Performance of Drought-Stressed Olive Plants When Exposed to a Combined Heat-UV-B Shock and after Stress Relief. Funct. Plant Biol. 2018, 45, 1233–1240. [Google Scholar] [CrossRef]
- Sewelam, N.; Oshima, Y.; Mitsuda, N.; Ohme-Takagi, M. A Step towards Understanding Plant Responses to Multiple Environmental Stresses: A Genome-Wide Study. Plant Cell Environ. 2014, 37, 2024–2035. [Google Scholar] [CrossRef]
- Teuling, A.J. A Hot Future for European Droughts. Nat. Clim. Chang. 2018, 8, 364–365. [Google Scholar] [CrossRef]
- Shaar-Moshe, L.; Blumwald, E.; Peleg, Z. Unique Physiological and Transcriptional Shifts under Combinations of Salinity, Drought, and Heat. Plant Physiol. 2017, 174, 421–434. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Coito, J.L.; Goncalves, E.F.; Chaves, M.M.; Amancio, S. Differential Physiological Response of the Grapevine Varieties Touriga Nacional and Trincadeira to Combined Heat, Drought and Light Stresses. Plant Biol. 2016, 18 (Suppl. S1), 101–111. [Google Scholar] [CrossRef] [PubMed]
- Lesk, C.; Anderson, W.; Rigden, A.; Coast, O.; Jaegermeyr, J.; McDermid, S.; Davis, K.F.; Konar, M. Compound Heat and Moisture Extreme Impacts on Global Crop Yields under Climate Change. Nat. Rev. Earth Environ. 2022, 3, 872–889. [Google Scholar] [CrossRef]
- Ning, G.C.; Luo, M.; Zhang, W.; Liu, Z.; Wang, S.G.; Gao, T. Rising Risks of Compound Extreme Heat-Precipitation Events in China. Int. J. Climatol. 2022, 42, 5785–5795. [Google Scholar] [CrossRef]
- Lü, M.Q.; Wu, S.J.; Chen, J.L.; Chen, C.D.; Wen, Z.F.; Huang, Y.Y. Changes in Extreme Precipitation in the Yangtze River Basin and its Association with Global Mean Temperature and ENSO. Int. J. Climatol. 2018, 38, 1989–2005. [Google Scholar] [CrossRef]
- Xu, X.; Ji, J.; Xu, Q.; Qi, X.; Chen, X. Inheritance and Quantitative Trail Loci Mapping of Adventitious Root Numbers in Cucumber Seedlings under Waterlogging Conditions. Mol. Genet. Genom. 2017, 292, 353–364. [Google Scholar] [CrossRef]
- Talanova, V.V.; Topchieva, L.V.; Titov, A.F. Effect of Abscisic Acid on the Resistance of Cucumber Seedlings to Combined Exposure to High Temperature and Chloride. Bio. Bull. 2006, 33, 619–622. [Google Scholar] [CrossRef]
- Liu, S.; Sun, B.; Cao, B.; Lv, Y.; Chen, Z.; Xu, K. Effects of Soil Waterlogging and High-Temperature Stress on Photosynthesis and Photosystem II of Ginger (Zingiber officinale). Protoplasma 2023, 260, 405–418. [Google Scholar] [CrossRef]
- Jorge, T.F.; Ramalho, J.C.; Alseekh, S.; Pais, I.P.; Leitao, A.E.; Rodrigues, A.P.; Scotti-Campos, P.; Ribeiro-Barros, A.I.; Fernie, A.R.; Antonio, C. Will Casuarina glauca Stress Resilience Be Maintained in the Face of Climate Change? Metabolites 2021, 11, 593. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mestre, T.C.; Mittler, R.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The Combined Effect of Salinity and Heat Reveals a Specific Physiological, Biochemical and Molecular Response in Tomato Plants. Plant Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Balfagon, D.; Zandalinas, S.I.; Gomez-Cadenas, A. High Temperatures Change the Perspective: Integrating Hormonal Responses in Citrus Plants under Co-occurring Abiotic Stress Conditions. Physiol. Plant. 2019, 165, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Angon, P.B.; Tahjib-Ul-Arif, M.; Samin, S.I.; Habiba, U.; Hossain, M.A.; Brestic, M. How Do Plants Respond to Combined Drought and Salinity Stress?—A Systematic Review. Plants 2022, 11, 2884. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liu, S.; Zhang, C.; He, J.; Ma, D.; Wang, X.; Dong, T.; Guo, F.; Cai, J.; Long, T.; et al. The Unique Sweet Potato NAC Transcription Factor IbNAC3 Modulates Combined Salt and Drought Stresses. Plant Physiol. 2023, 191, 747–771. [Google Scholar] [CrossRef] [PubMed]
- Min, X.; Wang, Q.; Wei, Z.; Liu, Z.; Liu, W. Full-Length Transcriptional Analysis Reveals the Complex Relationship of Leaves and Roots in Responses to Cold-Drought Combined Stress in Common Vetch. Front. Plant Sci. 2022, 13, 976094. [Google Scholar] [CrossRef]
- Noor, I.; Sohail, H.; Zhang, D.; Zhu, K.; Shen, W.; Pan, J.; Hasanuzzaman, M.; Li, G.; Liu, J. Silencing of Ppnramp5 Improves Manganese Toxicity Tolerance in Peach (Prunus persica) Seedlings. J. Hazard. Mater. 2023, 454, 131442. [Google Scholar] [CrossRef]
- Sohail, H.; Noor, I.; Nawaz, M.A.; Ma, M.; Shireen, F.; Huang, Y.; Yang, L.; Bie, Z. Genome-Wide Identification of Plasma-Membrane Intrinsic Proteins in Pumpkin and Functional Characterization of Cmopip1-4 under Salinity Stress. Environ. Exp. Bot. 2022, 202, 104995. [Google Scholar] [CrossRef]
- Roitsch, T.; Cabrera-Bosquet, L.; Fournier, A.; Ghamkhar, K.; Jimenez-Berni, J.; Pinto, F.; Ober, E.S. Review: New Sensors and Data-Driven Approaches-A Path to Next Generation Phenomics. Plant Sci. 2019, 282, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Francesca, S.; Najai, S.; Zhou, R.; Decros, G.; Cassan, C.; Delmas, F.; Ottosen, C.O.; Barone, A.; Rigano, M.M. Phenotyping to Dissect the Biostimulant Action of a Protein Hydrolysate in Tomato Plants under Combined Abiotic Stress. Plant Physiol. Bioch. 2022, 179, 32–43. [Google Scholar] [CrossRef]
- Visser, M.E. Keeping up with a Warming World; Assessing the Rate of Adaptation to Climate Change. Proc. Biol. Sci. 2008, 275, 649–659. [Google Scholar] [CrossRef]
- Ahmad, S.; Chen, Y.; Shah, A.Z.; Wang, H.; Xi, C.; Zhu, H.; Ge, L. The Homeodomain-Leucine Zipper Genes Family Regulates the Jinggangmycin Mediated Immune Response of Oryza sativa to Nilaparvata lugens, and Laodelphax striatellus. Bioengineering 2022, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Zhu, H.; Chen, Y.; Xi, C.; Shah, A.Z.; Ge, L. Comprehensive Bioinformatics and Expression Analysis of the TLP Gene Family Revealed Its Role in Regulating the Response of Oryza sativa to Nilaparvata lugens, Laodelphax striatellus, and Jinggangmycin. Agronomy 2022, 12, 1297. [Google Scholar] [CrossRef]
- Sharif, R.; Su, L.; Chen, X.; Qi, X. Hormonal Interactions Underlying Parthenocarpic Fruit Formation in Horticultural Crops. Hortic. Res. 2022, 9, uhab024. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Singh, G.; Bhattacharya, S.; Singh, A. Comparative Transcriptome Meta-Analysis of Arabidopsis Thaliana under Drought and Cold Stress. PLoS ONE 2018, 13, e0203266. [Google Scholar] [CrossRef] [PubMed]
- Natali, L.; Vangelisti, A.; Guidi, L.; Remorini, D.; Cotrozzi, L.; Lorenzini, G.; Nali, C.; Pellegrini, E.; Trivellini, A.; Vernieri, P.; et al. How Quercus ilex L. Saplings Face Combined Salt and Ozone Stress: A Transcriptome Analysis. BMC Genom. 2018, 19, 872. [Google Scholar] [CrossRef] [PubMed]
- Biancalani, T.; Scalia, G.; Buffoni, L.; Avasthi, R.; Lu, Z.; Sanger, A.; Tokcan, N.; Vanderburg, C.R.; Segerstolpe, A.; Zhang, M.; et al. Deep Learning and Alignment of Spatially Resolved Single-Cell Transcriptomes with Tangram. Nat. Methods 2021, 18, 1352–1362. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Barkley, D.; Franca, G.S.; Yanai, I. Exploring Tissue Architecture Using Spatial Transcriptomics. Nature 2021, 596, 211–220. [Google Scholar] [CrossRef]
- Sun, X.; Feng, D.; Liu, M.; Qin, R.; Li, Y.; Lu, Y.; Zhang, X.; Wang, Y.; Shen, S.; Ma, W.; et al. Single-Cell Transcriptome Reveals Dominant Subgenome Expression and Transcriptional Response to Heat Stress in Chinese Cabbage. Genome Biol. 2022, 23, 262. [Google Scholar] [CrossRef]
- Tenorio Berrio, R.; Verstaen, K.; Vandamme, N.; Pevernagie, J.; Achon, I.; Van Duyse, J.; Van Isterdael, G.; Saeys, Y.; De Veylder, L.; Inze, D.; et al. Single-Cell Transcriptomics Sheds Light on the Identity and Metabolism of Developing Leaf Cells. Plant Physiol. 2022, 188, 898–918. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, D.; Fan, C.; Zhang, C.; Zhang, C.; Liu, Z. Cell Type-Specific Differentiation Between Indica and Japonica Rice Root Tip Responses to Different Environments Based on Single-Cell RNA Sequencing. Front Genet. 2021, 12, 659500. [Google Scholar] [CrossRef]
- Tenorio Berrio, R.; Dubois, M. Single-Cell Transcriptomics Reveal Heterogeneity in Plant Responses to the Environment: A Focus on Biotic and Abiotic Interactions. J. Exp. Bot. 2024, erae107. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Villena, J.J.; Zhou, H.; Gilman, I.S.; Tausta, S.L.; Cheung, C.Y.M.; Edwards, E.J. Spatial Resolution of an Integrated C4+CAM Photosynthetic Metabolism. Sci. Adv. 2022, 8, eabn2349. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, K.R.; Chandna, R.; Ahmad, P.; Iqbal, M.; Ozturk, M. Relevance of Proteomic Investigations in Plant Abiotic Stress Physiology. OMICS 2012, 16, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Lin, K.H.; Chen, S.C.; Shen, Y.H.; Lo, H.F. Proteomic Analysis of Broccoli (Brassica oleracea) under High Temperature and Waterlogging Stresses. Bot. Stud. 2015, 56, 18. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.W.; Chen, Z.Y.; Yan, X.; Bian, Y.W.; Deng, X.; Yan, Y.M. Integrated Physiological and Proteomic Analysis Reveals Underlying Response and Defense Mechanisms of Brachypodium distachyon Seedling Leaves under Osmotic Stress, Cadmium and Their Combined Stresses. J. Proteom. 2018, 170, 1–13. [Google Scholar] [CrossRef]
- Noor, I.; Sohail, H.; Sun, J.; Nawaz, M.A.; Li, G.; Hasanuzzaman, M.; Liu, J. Heavy Metal and Metalloid Toxicity in Horticultural Plants: Tolerance Mechanism and Remediation Strategies. Chemosphere 2022, 303, 135196. [Google Scholar] [CrossRef] [PubMed]
- Shulaev, V.; Cortes, D.; Miller, G.; Mittler, R. Metabolomics for Plant Stress Response. Physiol. Plant. 2008, 132, 199–208. [Google Scholar] [CrossRef]
- Farag, M.A.; Otify, A.; Porzel, A.; Michel, C.G.; Elsayed, A.; Wessjohann, L.A. Comparative Metabolite Profiling and Fingerprinting of Genus Passiflora Leaves Using a Multiplex Approach of UPLC-MS and NMR Analyzed by Chemometric Tools. Anal. Bioanal. Chem. 2016, 408, 3125–3143. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, J.; Shen, T.; Zhao, Y.L.; Zuo, Z.T.; Wang, Y.Z.; Li, W.Y. Investigation of Chemical Diversity in Different Parts and Origins of Ethnomedicine Gentiana Rigescens Franch Using Targeted Metabolite Profiling and Multivariate Statistical Analysis. Biomed. Chromatogr. 2016, 30, 232–240. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, M.C.; Jung, E.S.; Lee, C.H.; Oh, M.M. Physiological and Metabolomic Responses of Kale to Combined Chilling and UV-A Treatment. Int. J. Mol. Sci. 2019, 20, 4950. [Google Scholar] [CrossRef]
- Demirel, U.; Morris, W.L.; Ducreux, L.J.M.; Yavuz, C.; Asim, A.; Tindas, I.; Campbell, R.; Morris, J.A.; Verrall, S.R.; Hedley, P.E.; et al. Physiological, Biochemical, and Transcriptional Responses to Single and Combined Abiotic Stress in Stress-Tolerant and Stress-Sensitive Potato Genotypes. Front. Plant Sci. 2020, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, J.; Ni, S.; Xing, B.; Cheng, K.; Peng, X. Transcriptome and Metabonomics Combined Analysis Revealed the Defense Mechanism Involved in Hydrogen-Rich Water-Regulated Cold Stress Response of Tetrastigma hemsleyanum. Front. Plant Sci. 2022, 13, 889726. [Google Scholar] [CrossRef]
- Zhou, A.; Ma, H.; Liu, E.; Jiang, T.; Feng, S.; Gong, S.; Wang, J. Transcriptome Sequencing of Dianthus spiculifolius and Analysis of the Genes Involved in Responses to Combined Cold and Drought Stress. Int. J. Mol. Sci. 2017, 18, 849. [Google Scholar] [CrossRef]
- Weng, J.; Rehman, A.; Li, P.; Chang, L.; Zhang, Y.; Niu, Q. Physiological and Transcriptomic Analysis Reveals the Responses and Difference to High Temperature and Humidity Stress in Two Melon Genotypes. Int. J. Mol. Sci. 2022, 23, 734. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.J.; Cheng, G.X.; Khan, A.; Wei, A.M.; Yu, Q.H.; Yang, S.B.; Luo, D.X.; Gong, Z.H. CaHSP16.4, A Small Heat Shock Protein Gene in Pepper, Is Involved in Heat and Drought Tolerance. Protoplasma 2019, 256, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Wang, Y.; Ding, Z.; Zhao, L. Global Transcriptional Analysis Reveals the Complex Relationship between Tea Quality, Leaf Senescence and the Responses to Cold-Drought Combined Stress in Camellia sinensis. Front. Plant Sci. 2016, 7, 1858. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yu, X.; Zhao, T.; Ottosen, C.O.; Rosenqvist, E.; Wu, Z. Physiological Analysis and Transcriptome Sequencing Reveal the Effects of Combined Cold and Drought on Tomato Leaf. BMC Plant Biol. 2019, 19, 377. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ji, D.; Turgeon, R.; Chen, J.; Lin, T.; Huang, J.; Luo, J.; Zhu, Y.; Zhang, C.; Lv, Z. Physiological and Proteomic Responses of Mulberry Trees (Morus alba. L.) to Combined Salt and Drought Stress. Int. J. Mol. Sci. 2019, 20, 2486. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, J.; Liu, Q.; Ben, C.; Todd, C.D.; Shi, J.; Yang, Y.; Hu, X. Comparative Proteomic Analysis of the Thermotolerant Plant Portulaca oleracea Acclimation to Combined High Temperature and Humidity Stress. J. Proteome Res. 2012, 11, 3605–3623. [Google Scholar] [CrossRef]
- Wellpott, K.; Jozefowicz, A.M.; Meise, P.; Schum, A.; Seddig, S.; Mock, H.P.; Winkelmann, T.; Bundig, C. Combined Nitrogen and Drought Stress Leads to Overlapping and Unique Proteomic Responses in Potato. Planta 2023, 257, 58. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Rivero, R.M.; Martinez, V.; Gomez-Cadenas, A.; Arbona, V. Tolerance of Citrus Plants to the Combination of High Temperatures and Drought Is Associated to the Increase in Transpiration Modulated by A Reduction in Abscisic Acid Levels. BMC Plant Biol. 2016, 16, 105. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Caparros, P.; Vogelsang, L.; Persicke, M.; Wirtz, M.; Kumar, V.; Dietz, K.J. Differential Sensitivity of Metabolic Pathways in Sugar Beet Roots to Combined Salt, Heat, and Light Stress. Physiol. Plant. 2022, 174, e13786. [Google Scholar] [CrossRef] [PubMed]
- Safronov, O.; Kreuzwieser, J.; Haberer, G.; Alyousif, M.S.; Schulze, W.; Al-Harbi, N.; Arab, L.; Ache, P.; Stempfl, T.; Kruse, J.; et al. Detecting Early Signs of Heat and Drought Stress in Phoenix dactylifera (Date Palm). PLoS ONE 2017, 12, e0177883. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, O.; Lemaitre-Guillier, C.; Songy, A.; Robert-Siegwald, G.; Lebrun, M.H.; Schmitt-Kopplin, P.; Larignon, P.; Adrian, M.; Fontaine, F. The Combination of Both Heat and Water Stresses May Worsen Botryosphaeria Dieback Symptoms in Grapevine. Plants 2023, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Singiri, J.R.; Swetha, B.; Sikron-Persi, N.; Grafi, G. Differential Response to Single and Combined Salt and Heat Stresses: Impact on Accumulation of Proteins and Metabolites in Dead Pericarps of Brassica juncea. Int. J. Mol. Sci. 2021, 22, 7076. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Ramegowda, V.; Senthil-Kumar, M. Shared and Unique Responses of Plants to Multiple Individual Stresses and Stress Combinations: Physiological and Molecular Mechanisms. Front. Plant Sci. 2015, 6, 723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic Stress Responses in Plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Winter, K.; Garcia, M.; Holtum, J.A. Drought-Stress-Induced Up-Regulation of CAM in Seedlings of a Tropical Cactus, Opuntia Elatior, Operating Predominantly in the C3 Mode. J. Exp. Bot. 2011, 62, 4037–4042. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.M.; Ansary, M.M.U.; Keya, S.S.; Abdelrahman, M.; Miah, M.G.; Phan Tran, L.S. Silicon in Mitigation of Abiotic Stress-Induced Oxidative Damage in Plants. Crit. Rev. Biotechnol. 2021, 41, 918–934. [Google Scholar] [CrossRef]
- Shen, Y.; Cong, W.; Zhang, A.H.; Meng, X. Complexity of Active Medicinal Ingredients in Radix Scutellariae with Sodium Hydrosulfite Exposure. PLoS ONE 2020, 15, e0238927. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Ma, X.; Ouyang, Z.; Deng, L.; Shen, S.; Dong, X.; Du, N.; Dong, H.; Guo, Z.; et al. Melatonin Alleviates Copper Toxicity via Improving ROS Metabolism and Antioxidant Defense Response in Tomato Seedlings. Antioxidants 2022, 11, 758. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Yan, R.; Xing, M.; Liao, S.; Chen, J.; Gan, Z. Fucoidan Treatment Alleviates Chilling Injury in Cucumber by Regulating ROS Homeostasis and Energy Metabolism. Front. Plant Sci. 2022, 13, 1107687. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Sun, C.; Li, K.; Sun, Q.; Gao, M.; Wu, T.; Zhang, X.; Xu, X.; Wang, Y.; Han, Z. MxRop1-MxrbohD1 Interaction Mediates ROS Signaling in Response to Iron Deficiency in the Woody Plant Malus xiaojinensis. Plant Sci. 2021, 313, 111071. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive Oxygen Species Homeostasis and Signalling During Drought and Salinity Stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory Burst Oxidases: The Engines of ROS Signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.H.; Kim, C.K. Abiotic Stress-Induced Anthocyanins in Plants: Their Role in Tolerance to Abiotic Stresses. Physiol. Plant. 2021, 172, 1711–1723. [Google Scholar] [CrossRef]
- Lin, H.H.; Lin, K.H.; Syu, J.Y.; Tang, S.Y.; Lo, H.F. Physiological and Proteomic Analysis in Two Wild Tomato Lines under Waterlogging and High Temperature Stress. J. Plant Biochem. Biot. 2016, 25, 87–96. [Google Scholar] [CrossRef]
- Jia, P.; Sharif, R.; Li, Y.; Sun, T.; Li, S.; Zhang, X.; Dong, Q.; Luan, H.; Guo, S.; Ren, X.; et al. The BELL1-like Homeobox Gene Mdblh14 from Apple Controls Flowering and Plant Height via Repression of MdGA20ox3. Int. J. Biol. Macromol. 2023, 242, 124790. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant Hormone Regulation of Abiotic Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Niu, C.Y.; Yang, C.R.; Jinn, T.L. The Heat Stress Factor HSFA6b Connects ABA Signaling and ABA-Mediated Heat Responses. Plant Physiol. 2016, 172, 1182–1199. [Google Scholar] [CrossRef]
- Hussain, Q.; Asim, M.; Zhang, R.; Khan, R.; Farooq, S.; Wu, J. Transcription Factors Interact with ABA through Gene Expression and Signaling Pathways to Mitigate Drought and Salinity Stress. Biomolecules 2021, 11, 1159. [Google Scholar] [CrossRef]
- Jung, C.; Nguyen, N.H.; Cheong, J.J. Transcriptional Regulation of Protein Phosphatase 2C Genes to Modulate Abscisic Acid Signaling. Int. J. Mol. Sci. 2020, 21, 9517. [Google Scholar] [CrossRef]
- Jia, J.; Zhou, J.; Shi, W.; Cao, X.; Luo, J.; Polle, A.; Luo, Z.B. Comparative Transcriptomic Analysis Reveals the Roles of Overlapping Heat-/Drought-Responsive Genes in Poplars Exposed to High Temperature and Drought. Sci. Rep. 2017, 7, 43215. [Google Scholar] [CrossRef]
- Wurms, K.V.; Reglinski, T.; Buissink, P.; Ah Chee, A.; Fehlmann, C.; McDonald, S.; Cooney, J.; Jensen, D.; Hedderley, D.; McKenzie, C.; et al. Effects of Drought and Flooding on Phytohormones and Abscisic Acid Gene Expression in Kiwifruit. Int. J. Mol. Sci. 2023, 24, 7580. [Google Scholar] [CrossRef]
- Jia, P.; Wang, Y.; Sharif, R.; Ren, X.; Qi, G. MdIPT1, an Adenylate Isopentenyltransferase Coding Gene from Malus domestica, Is Involved in Branching and Flowering Regulation. Plant Sci. 2023, 333, 111730. [Google Scholar] [CrossRef]
- Li, S.M.; Zheng, H.X.; Zhang, X.S.; Sui, N. Cytokinins as Central Regulators During Plant Growth and Stress Response. Plant Cell Rep. 2021, 40, 271–282. [Google Scholar] [CrossRef]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmulling, T. Cytokinin Action in Response to Abiotic and Biotic Stresses in Plants. Plant Cell Environ. 2019, 42, 998–1018. [Google Scholar] [CrossRef]
- Mushtaq, N.; Wang, Y.; Fan, J.; Li, Y.; Ding, J. Down-Regulation of Cytokinin Receptor Gene SlHK2 Improves Plant Tolerance to Drought, Heat, and Combined Stresses in Tomato. Plants 2022, 11, 154. [Google Scholar] [CrossRef]
- Bouzroud, S.; Gasparini, K.; Hu, G.; Barbosa, M.A.M.; Rosa, B.L.; Fahr, M.; Bendaou, N.; Bouzayen, M.; Zsogon, A.; Smouni, A.; et al. Down Regulation and Loss of Auxin Response Factor 4 Function Using CRISPR/Cas9 Alters Plant Growth, Stomatal Function and Improves Tomato Tolerance to Salinity and Osmotic Stress. Genes 2020, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wu, Z.; Xiang, J.; Zhang, D.; Teng, N. Overexpression of a Novel Heat-Inducible Ethylene-Responsive Factor Gene LlERF110 from Lilium longiflorum Decreases Thermotolerance. Plant Sci. 2022, 319, 111246. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; He, J.; Ping, Y.; Guo, J.; Hou, N.; Cao, F.; Li, X.; Geng, D.; Wang, S.; Chen, P.; et al. The Positive Feedback Regulatory Loop of miR160-Auxin Response Factor 17-HYPONASTIC LEAVES 1 Mediates Drought Tolerance in Apple Trees. Plant Physiol. 2022, 188, 1686–1708. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Li, S.; Cao, X.; Li, H.; Shi, W.; Polle, A.; Liu, T.X.; Peng, C.; Luo, Z.B. Physiological and Transcriptional Regulation in Poplar Roots and Leaves During Acclimation to High Temperature and Drought. Physiol. Plant. 2016, 157, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Xie, C.; Zhang, H.; Arnao, M.B.; Ali, M.; Ali, Q.; Muhammad, I.; Shalmani, A.; Nawaz, M.A.; Chen, P.; et al. Melatonin and Its Effects on Plant Systems. Molecules 2018, 23, 2352. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; Nieves-Cordones, M.; Lopez-Delacalle, M.; Rodenas, R.; Mestre, T.C.; Garcia-Sanchez, F.; Rubio, F.; Nortes, P.A.; Mittler, R.; Rivero, R.M. Tolerance to Stress Combination in Tomato Plants: New Insights in the Protective Role of Melatonin. Molecules 2018, 23, 535. [Google Scholar] [CrossRef] [PubMed]
- Parwez, R.; Aftab, T.; Gill, S.S.; Naeem, M. Abscisic Acid Signaling and Crosstalk with Phytohormones in Regulation of Environmental Stress Responses. Environ. Exp. Bot. 2022, 199, 104885. [Google Scholar] [CrossRef]
- Cui, L.; Zou, Z.; Zhang, J.; Zhao, Y.; Yan, F. 24-Epibrassinoslide Enhances Plant Tolerance to Stress from Low Temperatures and Poor Light Intensities in Tomato (Lycopersicon esculentum Mill.). Funct. Integr. Genom. 2016, 16, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Tang, Y.; Yuan, Y.; Sun, J.; Zhong, M.; Guo, S. The Role of 24-Epibrassinolide in the Regulation of Photosynthetic Characteristics and Nitrogen Metabolism of Tomato Seedlings under a Combined Low Temperature and Weak Light Stress. Plant Physiol. Biochem. 2016, 107, 344–353. [Google Scholar] [CrossRef]
- Castagna, A.; Di Baccio, D.; Ranieri, A.M.; Sebastiani, L.; Tognetti, R. Effects of Combined Ozone and Cadmium Stresses on Leaf Traits in Two Poplar Clones. Environ. Sci. Pollut. Res. Int. 2015, 22, 2064–2075. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Enhancement of Tolerance of Abiotic Stress by Metabolic Engineering of Betaines and Other Compatible Solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A.; Witczak, J.; Ahmad, M. Analysis of Genetic Variation and Enhancement of Salt Tolerance in French Pea (Pisum Sativum L.). Int. J. Mol. Sci. 2018, 19, 2433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dong, X.M.; Zhang, Y.W.; Fan, Z.X.; Zhang, S.B. Age-related Differences in Physiological and Metabolic Responses of Pleione aurita (Orchidaceae) Pseudobulbs to Drought Stress and Recovery. Plant Physiol. Biochem. 2023, 197, 107655. [Google Scholar] [CrossRef] [PubMed]
- Giri, J. Glycinebetaine and Abiotic Stress Tolerance in Plants. Plant Signal. Behav. 2011, 6, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Al Hassan, M.; Naranjo, M.A.; Agrawal, V.; Boscaiu, M.; Vicente, O. Effects of Salinity and Drought on Growth, Ionic Relations, Compatible Solutes and Activation of Antioxidant Systems in Oleander (Nerium oleander L.). PLoS ONE 2017, 12, e0185017. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Rehman, H.M.; Ahmed, N.; Nawaz, S.; Saleem, F.; Ahmad, S.; Uzair, M.; Rana, I.A.; Atif, R.M.; Zaman, Q.U.; et al. Using Exogenous Melatonin, Glutathione, Proline, and Glycine Betaine Treatments to Combat Abiotic Stresses in Crops. Int. J. Mol. Sci. 2022, 23, 12913. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell’Aversana, E.; Carillo, P. Spatial and Temporal Profile of Glycine Betaine Accumulation in Plants under Abiotic Stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, F.; Wang, J.; Zhang, W.; Meng, Q.; Chen, T.H.; Murata, N.; Yang, X. Glycinebetaine Enhances the Tolerance of Tomato Plants to High Temperature During Germination of Seeds and Growth of Seedlings. Plant Cell Environ. 2011, 34, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, K.N.; Lal, M.K.; Tiwari, R.K.; Dev, D.; Kardile, H.B.; Patil, V.U.; Kumar, A.; Vanishree, G.; Kumar, D.; Bhardwaj, V.; et al. Salinity Stress in Potato: Understanding Physiological, Biochemical and Molecular Responses. Life 2021, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Godoy, F.; Olivos-Hernandez, K.; Stange, C.; Handford, M. Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef]
- Geng, S.; Sohail, H.; Cao, H.; Sun, J.; Chen, Z.; Zhou, L.; Wang, W.; Ye, R.; Yang, L.; Bie, Z. An Efficient Root Transformation System for CRISPR/Cas9-based Analyses of Shoot-Root Communication in Cucurbit Crops. Hortic. Res. 2022, 9, uhab082. [Google Scholar] [CrossRef]
- Granda, E.; Camarero, J.J. Drought Reduces Growth and Stimulates Sugar Accumulation: New Evidence of Environmentally Driven Non-Structural Carbohydrate Use. Tree Physiol. 2017, 37, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Yobi, A.; Wone, B.W.; Xu, W.; Alexander, D.C.; Guo, L.; Ryals, J.A.; Oliver, M.J.; Cushman, J.C. Metabolomic Profiling in Selaginella lepidophylla at Various Hydration States Provides New Insights Into the Mechanistic Basis of Desiccation Tolerance. Mol. Plant 2013, 6, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhou, K.; Li, Y.; Chen, X.; Liu, B.; Li, C.; Gong, X.; Ma, F. Exogenous Myo-Inositol Alleviates Salinity-Induced Stress in Malus hupehensis Rehd. Plant Physiol. Biochem. 2018, 133, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, N.; Li, Y.; Zhu, S.; Zhang, S.; Sun, Y.; Zhang, H.X.; Wang, L.; Su, H. Overexpression of PeMIPS1 Confers Tolerance to Salt and Copper Stresses by Scavenging Reactive Oxygen Species in Transgenic Poplar. Tree Physiol. 2018, 38, 1566–1577. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, Physiochemical and Antioxidant Responses of Maclura pomifera to Drought Stress. Sci Rep. 2019, 9, 19250. [Google Scholar] [CrossRef] [PubMed]
- Alhaithloul, H.A.; Soliman, M.H.; Ameta, K.L.; El-Esawi, M.A.; Elkelish, A. Changes in Ecophysiology, Osmolytes, and Secondary Metabolites of the Medicinal Plants of Mentha piperita and Catharanthus roseus Subjected to Drought and Heat Stress. Biomolecules 2019, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, A Multifaceted Signalling Molecule in Plant Responses to Abiotic Stress: Understanding the Physiological Mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Sun, X.; Lin, H.; Chen, J.; Ren, J.; Hu, X.; Yang, Y. Comparative Physiological and Proteomic Analyses of Poplar (Populus yunnanensis) Plantlets Exposed to High Temperature and Drought. PLoS ONE 2014, 9, e107605. [Google Scholar] [CrossRef]
- Xu, T.; Niu, J.; Jiang, Z. Sensing Mechanisms: Calcium Signaling Mediated Abiotic Stress in Plants. Front. Plant Sci. 2022, 13, 925863. [Google Scholar] [CrossRef]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F.; et al. Plant Abiotic Stress Response and Nutrient Use Efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Ku, Y.S.; Sintaha, M.; Cheung, M.Y.; Lam, H.M. Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lan, W.; Chen, B.; Fang, W.; Luan, S. A Calcium Sensor-Regulated Protein Kinase, CALCINEURIN B-LIKE PROTEIN-INTERACTING PROTEIN KINASE19, is Required for Pollen Tube Growth and Polarity. Plant Physiol. 2015, 167, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Tafreshi, S.A.; Aghaie, P.; Ebrahimi, M.A.; Haerinasab, M. Regulation of Drought-Related Responses in Tomato Plants by Two Classes of Calcineurin B-like (SlCBL1/2) Proteins. Plant Physiol Biochem. 2021, 162, 431–446. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; He, Q.; Chai, M.; Huang, Y.; Chen, F.; Wang, X.; Liu, Y.; Cai, H.; Qin, Y. Genome-Wide Investigation of Calcium-Dependent Protein Kinase Gene Family in Pineapple: Evolution and Expression Profiles During Development and Stress. BMC Genom. 2020, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 Mediates Osmotic-Stress-Evoked Ca2+ Increases Vital for Osmosensing in Arabidopsis. Nature 2014, 514, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Li, F.; Han, Y.; Yao, Z.; Xu, Z.; Chen, X.; Liu, J.; Zhang, Y.; Wang, A. Identification of OSCA Gene Family in Solanum habrochaites and Its Function Analysis under Stress. BMC Genom. 2022, 23, 547. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yuan, F.; Wang, X.; Zhu, S.; Pei, Z.M. Evolution of Osmosensing OSCA1 Ca2+ Channel Family Coincident with Plant Transition from Water to Land. Plant Genome 2022, 15, e20198. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhu, C.; Chen, J.; Zhao, J.; Hu, Z.; Liu, S.; Zhou, Y. Identification and Expression Profile Analysis of the OSCA Gene Family Related to Abiotic and Biotic Stress Response in Cucumber. Biology 2022, 11, 1134. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, M.; Kong, L.; Lv, Y.; Zou, M.; Lu, G.; Cao, J.; Yu, X. Genome-Wide Identification, Phylogeny, Duplication, and Expression Analyses of Two-Component System Genes in Chinese Cabbage (Brassica rapa ssp. pekinensis). DNA Res. 2014, 21, 379–396. [Google Scholar] [CrossRef]
- He, Y.; Liu, X.; Ye, L.; Pan, C.; Chen, L.; Zou, T.; Lu, G. Genome-Wide Identification and Expression Analysis of Two-Component System Genes in Tomato. Int. J. Mol. Sci. 2016, 17, 1204. [Google Scholar] [CrossRef]
- Huo, R.; Zhao, Y.; Liu, T.; Xu, M.; Wang, X.; Xu, P.; Dai, S.; Cui, X.; Han, Y.; Liu, Z.; et al. Genome-Wide Identification and Expression Analysis of Two-Component System Genes in Sweet Potato (Ipomoea batatas L.). Front. Plant Sci. 2022, 13, 1091620. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, X.; Zou, T.; Pan, C.; Qin, L.; Chen, L.; Lu, G. Genome-Wide Identification of Two-Component System Genes in Cucurbitaceae Crops and Expression Profiling Analyses in Cucumber. Front. Plant Sci. 2016, 7, 899. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ding, Y.; Shi, Y.; Zhang, X.; Zhang, S.; Gong, Z.; Yang, S. MPK3- and MPK6-Mediated ICE1 Phosphorylation Negatively Regulates ICE1 Stability and Freezing Tolerance in Arabidopsis. Dev. Cell 2017, 43, 630–642.e634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, Y.; Yang, T.; Zhang, L.; Xu, S.; Xue, L.; An, L. Diverse Signals Converge at MAPK Cascades in Plant. Plant Physiol. Biochem. 2006, 44, 274–283. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, S. Mitogen-activated Protein Kinase Cascades in Signaling Plant Growth and Development. Trends Plant Sci. 2015, 20, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wu, J.; Jiang, M.; Wang, Y. Plant Mitogen-Activated Protein Kinase Cascades in Environmental Stresses. Int. J. Mol. Sci. 2021, 22, 1543. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, J.; Pan, C.; Guan, X.; Wang, Y.; Liu, S.; He, Y.; Chen, J.; Chen, L.; Lu, G. Genome-Wide Identification of Mapkk and Mapkkk Gene Families in Tomato and Transcriptional Profiling Analysis During Development and Stress Response. PLoS ONE 2014, 9, e103032. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, C.; Wang, Y.; Ye, L.; Wu, J.; Chen, L.; Zou, T.; Lu, G. Genome-Wide Identification of MAPK, MAPKK, and MAPKKK Gene Families and Transcriptional Profiling Analysis During Development and Stress Response in Cucumber. BMC Genom. 2015, 16, 386. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Kilickaya, O. Mitogen-Activated Protein Kinase Cascades in Vitis vinifera. Front. Plant Sci. 2015, 6, 556. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, P.; An, G.; Wang, P.; Song, C.P. The Involvement of a P38-like MAP Kinase in ABA-Induced and H2O2-Mediated Stomatal Closure in Vicia faba L. Plant Cell Rep. 2008, 27, 377–385. [Google Scholar] [CrossRef]
- Wang, G.; Lovato, A.; Polverari, A.; Wang, M.; Liang, Y.H.; Ma, Y.C.; Cheng, Z.M. Genome-Wide Identification and Analysis of Mitogen Activated Protein Kinase Kinase Kinase Gene Family in Grapevine (Vitis vinifera). BMC Plant Biol. 2014, 14, 219. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, J.; Tschaplinski, T.J.; Tuskan, G.A.; Chen, J.G.; Muchero, W. Regulation of Lignin Biosynthesis and Its Role in Growth-Defense Tradeoffs. Front. Plant Sci. 2018, 9, 1427. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wuyun, T.; Chen, J.; Yu, S.; Zhang, X.; Zhang, L. Responses of Trollius Chinensis to Drought Stress and Rehydration: From Photosynthetic Physiology to Gene Expression. Plant Physiol. Biochem. 2023, 201, 107841. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, G.; Zhang, J.; Zhang, Y.; Xie, Q.; Zhao, Z.; Pan, Y.; Hu, Z. The Abiotic Stress-Responsive NAC-Type Transcription Factor SlNAC4 Regulates Salt and Drought Tolerance and Stress-Related Genes in Tomato (Solanum lycopersicum). Plant Cell Rep. 2014, 33, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Broft, P.; Rosenkranz, R.; Schleiff, E.; Hengesbach, M.; Schwalbe, H. Structural Analysis of Temperature-Dependent Alternative Splicing of HsfA2 pre-mRNA from Tomato Plants. RNA Biol. 2022, 19, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Qin, G.; Cao, F.; He, J.; Shen, X.; Chen, P.; Niu, C.; Zhang, D.; Ren, T.; Zhi, F.; et al. MdZAT5 Regulates Drought Tolerance via Mediating Accumulation of Drought-Responsive miRNAs and mRNAs in Apple. New Phytol. 2022, 236, 2131–2150. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Jaiswal, A.; Taj, G.; Jaiswal, J.P.; Qureshi, M.I.; Singh, N.K. DREB1/CBF Transcription Factors: Their Structure, Function and Role in Abiotic Stress Tolerance in Plants. J. Genet. 2012, 91, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, S.; Yu, X.; Du, W.; Li, H.; Sun, Y.; Sun, H.; Ruan, C. Role of Xanthoceras sorbifolium MYB44 in Tolerance to Combined Drought and Heat Stress via Modulation of Stomatal Closure and ROS Homeostasis. Plant Physiol. Biochem. 2021, 162, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Yang, S.; Yan, Y.; Xiao, Z.; Cheng, J.; Wu, J.; Qiu, A.; Lai, Y.; Mou, S.; Guan, D.; et al. CaWRKY6 Transcriptionally Activates CaWRKY40, Regulates Ralstonia solanacearum Resistance, and Confers High-Temperature and High-Humidity Tolerance in Pepper. J. Exp. Bot. 2015, 66, 3163–3174. [Google Scholar] [CrossRef]
- Chaudhari, R.S.; Jangale, B.L.; Azeez, A.; Krishna, B.; Sane, P.V.; Sane, A.P. Differential Regulation of the Banana Stress NAC Family by Individual and Combined Stresses of Drought and Heat in Susceptible and Resistant Genotypes. Plant Physiol. Biochem. 2019, 145, 184–194. [Google Scholar] [CrossRef]
- Samarina, L.S.; Bobrovskikh, A.V.; Doroshkov, A.V.; Malyukova, L.S.; Matskiv, A.O.; Rakhmangulov, R.S.; Koninskaya, N.G.; Malyarovskaya, V.I.; Tong, W.; Xia, E. Comparative Expression Analysis of Stress-Inducible Candidate Genes in Response to Cold and Drought in Tea Plant [Camellia sinensis (L.) Kuntze]. Front. Genet. 2020, 11, 611283. [Google Scholar] [CrossRef]
- Hussain, K.; Kumar, A.; Fayaz, M.; Misra, P.; Ashraf, N. CstMYB14 Links ROS Signaling, Apocarotenoid Metabolism, and Stress Response in Crocus sativus L. Physiol. Plant. 2022, 174, e13712. [Google Scholar] [CrossRef]
- Liu, X.; Bulley, S.M.; Varkonyi-Gasic, E.; Zhong, C.; Li, D. Kiwifruit bZIP Transcription Factor AcePosF21 Elicits Ascorbic Acid Biosynthesis During Cold Stress. Plant Physiol. 2023, 192, 982–999. [Google Scholar] [CrossRef]


| Method | Species | Stress |
|---|---|---|
| Phenotypics | Tomato | Heat + Drought [49] |
| Transcriptomics | Dianthus spiculifolius Melon Pepper Quercus ilex Tea Plant Tomato | Cold + Drought [73] Heat + Humidity [74] Heat + Drought [75] Salt + Ozone [55] Cold + Drought [76] Cold + Drought [77] |
| Proteomics | Broccoli Brachypodium distachyon Mulberry Portulaca oleracea Potato | Heat + Waterlogging [64] Drought + Cd2+ [65] Salt + Drought [78] Heat + Humidity [79] Drought + Nitrogen [80] |
| Metabolomics | Citrus Kale Sugar beet | Heat + Drought [81] Cold + UV-A [70] Salt + Heat + Light [82] |
| Transcriptomics + Proteomics | Citrus | Drought + Heat + High irradiance [27] |
| Transcriptomics + Metabolomics | Date palm Grapevine Potato | Heat + Drought [83] Heat + Drought [84] Heat + Drought [71] |
| Proteomics + Metabolomics | Brassica juncea | Heat + Salt [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, W.; Sharif, R.; Sohail, H.; Zhu, Y.; Chen, X.; Xu, X. Surviving a Double-Edged Sword: Response of Horticultural Crops to Multiple Abiotic Stressors. Int. J. Mol. Sci. 2024, 25, 5199. https://doi.org/10.3390/ijms25105199
Yan W, Sharif R, Sohail H, Zhu Y, Chen X, Xu X. Surviving a Double-Edged Sword: Response of Horticultural Crops to Multiple Abiotic Stressors. International Journal of Molecular Sciences. 2024; 25(10):5199. https://doi.org/10.3390/ijms25105199
Chicago/Turabian StyleYan, Wenjing, Rahat Sharif, Hamza Sohail, Yu Zhu, Xuehao Chen, and Xuewen Xu. 2024. "Surviving a Double-Edged Sword: Response of Horticultural Crops to Multiple Abiotic Stressors" International Journal of Molecular Sciences 25, no. 10: 5199. https://doi.org/10.3390/ijms25105199






