MicroRNA-10 Family Promotes Renal Fibrosis through the VASH-1/Smad3 Pathway
Abstract
:1. Introduction
2. Results
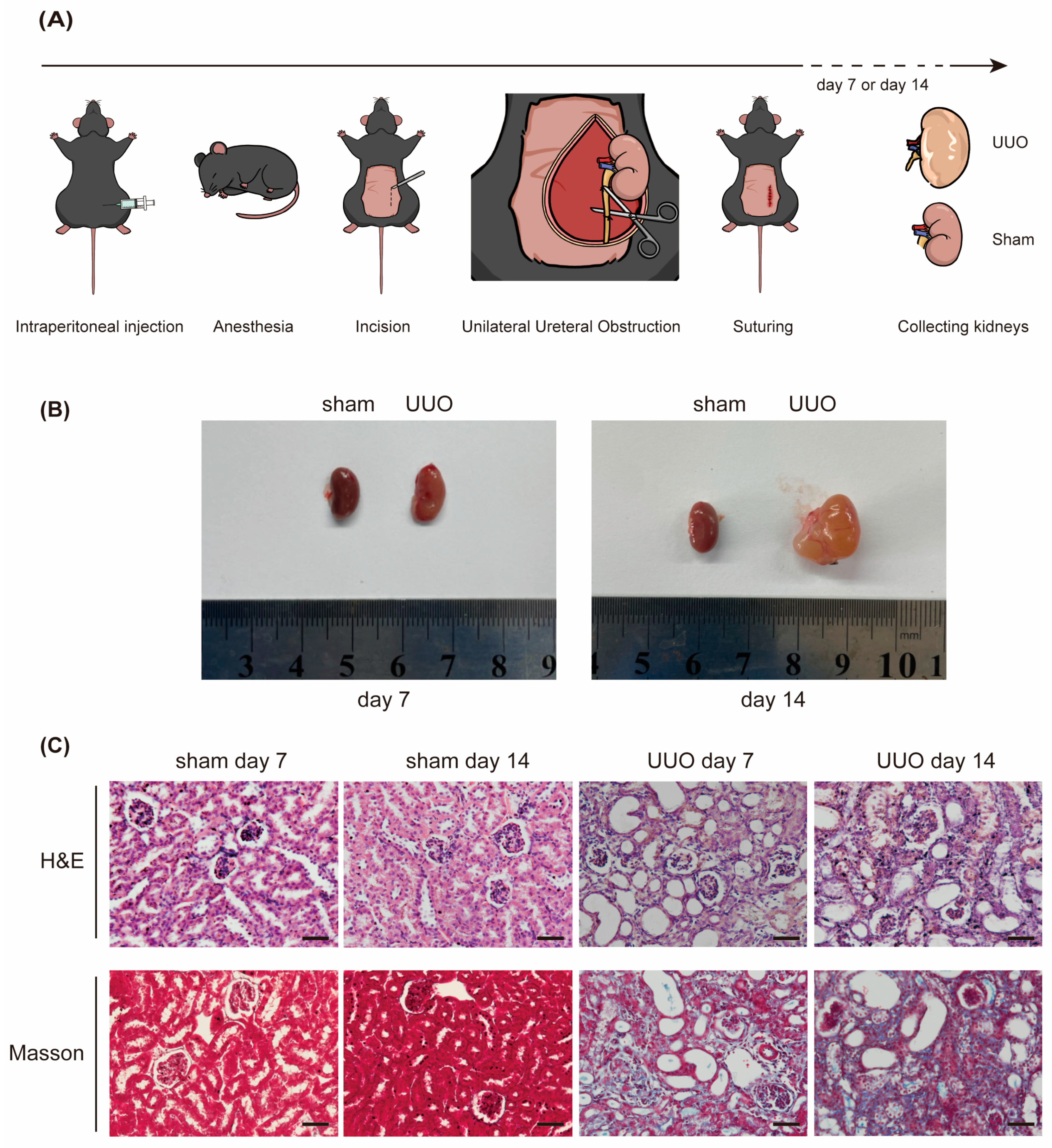
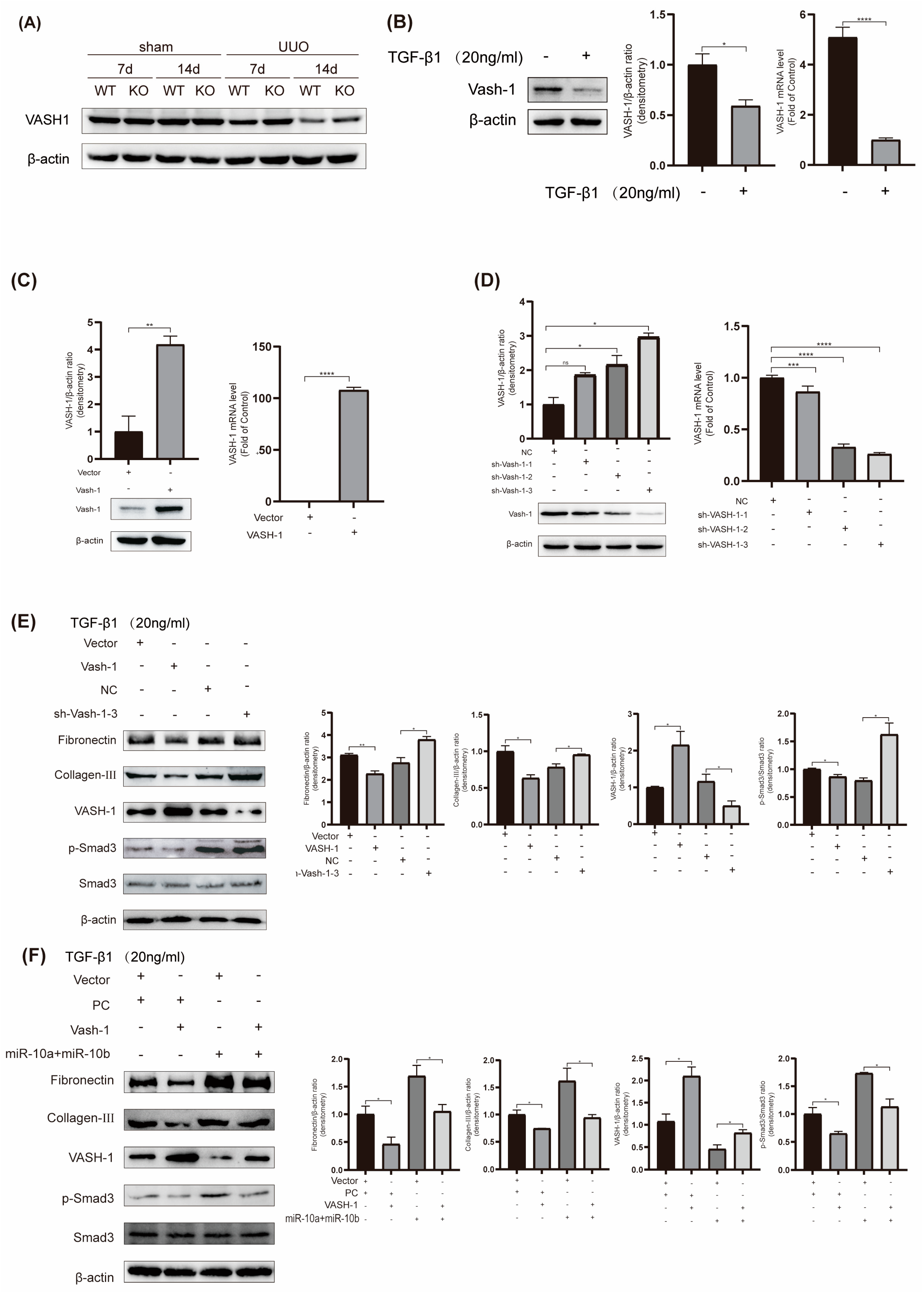
2.1. Elevated Expression of miR-10a and miR-10b in UUO Mice
2.2. Depletion of miR-10a and miR-10b Alleviates Renal Fibrosis and Smad3 Phosphorylation Induced by UUO
2.3. Overexpression of miR-10 Promotes TGF-β1-Induced Fibrosis and Smad3 Phosphorylation in HK-2 Cells
2.4. miR-10a and miR-10b Target and Downregulate VASH-1 Expression
2.5. miR-10 Family Promotes Fibrosis by Modulating the VASH-1/Smad3 Pathway
3. Discussion
4. Materials and Methods
4.1. Gene Knockout Mice
4.2. Experimental Animal Grouping and Model Establishment
4.3. Western Blotting
4.4. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.5. Hematoxylin and Eosin (H&E) Staining and Masson’s Staining
4.6. Cell Culture and Transfection
4.7. Plasmid Preparation and Oligonucleotide Synthesis
4.8. EGFP Fluorescent Reporter Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, M.H. Renal fibrosis. Korean J. Pediatr. 2010, 53, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E. Fibrosis causes progressive kidney failure. Med. Hypotheses 1995, 45, 459–462. [Google Scholar] [CrossRef]
- Naeli, P.; Winter, T.; Hackett, A.P.; Alboushi, L.; Jafarnejad, S.M. The intricate balance between microRNA-induced mRNA decay and translational repression. FEBS J. 2023, 290, 2508–2524. [Google Scholar] [CrossRef] [PubMed]
- Hinamoto, N.; Maeshima, Y.; Saito, D.; Yamasaki, H.; Tanabe, K.; Nasu, T.; Watatani, H.; Ujike, H.; Kinomura, M.; Sugiyama, H.; et al. Urinary and plasma levels of Vasohibin-1 can predict renal functional deterioration in patients with renal disorders. PLoS ONE 2014, 9, e96932. [Google Scholar] [CrossRef]
- Huang, Y.; He, Y.; Li, J. Microrna-21: A central regulator of fibrotic diseases via various targets. Curr. Pharm. Des. 2015, 21, 2236–2242. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Li, J.; Li, Y.; Yang, M.; Nie, S.; Zhou, M.; Zhou, Z.; Yang, X.; Liu, Y.; Hou, F.F. MicroRNA-10 negatively regulates inflammation in diabetic kidney via targeting activation of the NLRP3 inflammasome. Mol. Ther. 2021, 29, 2308–2320. [Google Scholar] [CrossRef]
- Nishimura, R.; Hata, K.; Ikeda, F.; Matsubara, T.; Yamashita, K.; Ichida, F.; Yoneda, T. The role of Smads in BMP signaling. Front. Biosci. 2003, 8, s275–s284. [Google Scholar] [CrossRef] [PubMed]
- Luo, K. Signaling Cross Talk between TGF-β/Smad and Other Signaling Pathways. Cold Spring Harb. Perspect. Biol. 2017, 9, a022137. [Google Scholar] [CrossRef]
- Qu, X.; Li, X.; Zheng, Y.; Ren, Y.; Puelles, V.G.; Caruana, G.; Nikolic-Paterson, D.J.; Li, J. Regulation of renal fibrosis by Smad3 Thr388 phosphorylation. Am. J. Pathol. 2014, 184, 944–952. [Google Scholar] [CrossRef]
- Roberts, A.B.; Tian, F.; Byfield, S.D.; Stuelten, C.; Ooshima, A.; Saika, S.; Flanders, K.C. Smad3 is key to TGF-beta-mediated epi-thelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev. 2006, 17, 19–27. [Google Scholar] [CrossRef]
- Zhong, X.; Chung, A.C.; Chen, H.-Y.; Meng, X.-M.; Lan, H.Y. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J. Am. Soc. Nephrol. 2011, 22, 1668–1681. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Kasajima, A.; Kawagishi, N.; Sekiguchi, S.; Fujishima, F.; Watanabe, M.; Sato, Y.; Ohuchi, N.; Sasano, H. The prognostic significance of vasohibin 1–associated angiogenesis in patients with hepatocellular carcinoma. Hum. Pathol. 2014, 45, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y. Is vasohibin-1 for more than angiogenesis inhibition? J. Biochem. 2011, 149, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, T.; Kimura, H.; Heishi, T.; Suzuki, Y.; Miyashita, H.; Ohta, H.; Sonoda, H.; Moriya, T.; Suzuki, S.; Kondo, T.; et al. Vasohibin-1 Expression in Endothelium of Tumor Blood Vessels Regulates Angiogenesis. Am. J. Pathol. 2009, 175, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Koyanagi, T.; Suzuki, Y.; Saga, Y.; Kanomata, N.; Moriya, T.; Suzuki, M.; Sato, Y. Vasohibin-2 Expressed in human serous ovarian adenocarcinoma accelerates tumor growth by promoting angiogenesis. Mol. Cancer Res. 2012, 10, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Miyashita, H.; Suzuki, Y.; Kobayashi, M.; Satomi, S.; Sato, Y. Enhanced cancer metastasis in mice deficient in vasohibin-1 gene. PLoS ONE 2013, 8, e73931. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shuai, Y.; Zhao, C.; Yang, F.; Su, W.; Ning, Z.; Li, G. MicroRNA-10 Family Promotes the Epithelial-to-Mesenchymal Transition in Renal Fibrosis by the PTEN/Akt Pathway. Curr. Issues Mol. Biol. 2022, 44, 6059–6074. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Shao, Y.; Wu, C.; Lv, C.; Zhou, Y.; Wang, Q. VASH-1 Regulates Oxidative Stress and Fibrosis in Diabetic Kidney Disease via SIRT1/HIF1α and TGFβ1/Smad3 Signaling Pathways. Front. Mol. Biosci. 2020, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Watatani, H.; Maeshima, Y.; Hinamoto, N.; Yamasaki, H.; Ujike, H.; Tanabe, K.; Sugiyama, H.; Otsuka, F.; Sato, Y.; Makino, H. Vasohibin-1 deficiency enhances renal fibrosis and inflammation after unilateral ureteral obstruction. Physiol. Rep. 2014, 2, e12054. [Google Scholar] [CrossRef]
- Nogueira, A.; Pires, M.J.; Oliveira, P.A. Pathophysiological Mechanisms of Renal Fibrosis: A Review of Animal Models and Therapeutic Strategies. In Vivo 2017, 31, 1–22. [Google Scholar] [CrossRef]
- Isaka, Y. Targeting TGF-β Signaling in Kidney Fibrosis. Int. J. Mol. Sci. 2018, 19, 2532. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Kagami, S.; Urushihara, M.; Kitamura, A.; Shimizu, M.; Strutz, F.; Müller, G.A.; Kuroda, Y. Transforming growth fac-tor-beta1 stimulates collagen matrix remodeling through increased adhesive and contractive potential by human renal fibro-blasts. Biochim. Biophys. Acta 2004, 1693, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Xiong, X.; Chen, Y.-G. Feedback regulation of TGF-β signaling. Acta Biochim. Biophys. Sin. 2018, 50, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.E.; Ding, Y.; Kim, S.I. TGF-β Signaling via TAK1 pathway: Role in kidney fibrosis. Semin. Nephrol. 2012, 32, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.; Knaus, P. Integration of the TGF-β pathway into the cellular signalling network. Cell Signal 2002, 14, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Kajdaniuk, D.; Marek, B.; Borgiel-Marek, H.; Kos-Kudła, B. Transforming growth factor β1 (TGFβ1) in physiology and pathology. Endokrynol. Pol. 2013, 64, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Meng, X.-M.; Tang, P.M.-K.; Li, J.; Lan, H.Y. TGF-β/Smad signaling in renal fibrosis. Front. Physiol. 2015, 6, 82. [Google Scholar] [CrossRef]
- Lund, A.H. miR-10 in development and cancer. Cell Death Differ. 2010, 17, 209–214. [Google Scholar] [CrossRef]
- Jiajie, T.; Yanzhou, Y.; Hoi-Hung, A.C.; Zi-Jiang, C.; Wai-Yee, C. Conserved miR-10 family represses proliferation and induces apoptosis in ovarian granulosa cells. Sci. Rep. 2017, 7, 41304. [Google Scholar] [CrossRef]
- Liu, H.; Wang, D.; Tang, J.; Yu, L.; Su, S. Differences and Clinical Significance of Serum 25-Hydroxyvitamin D3 and Vasohibin-1 (VASH-1) Levels in Patients with Diabetic Nephropathy and Different Renal Injuries. Diabetes Metab. Syndr. Obes. Targets Ther. 2023, 16, 1085–1091. [Google Scholar] [CrossRef]
- Fukui, Y.; Nakamura, K.; Hirabayashi, M.; Miyagawa, T.; Toyama, S.; Omatsu, J.; Awaji, K.; Ikawa, T.; Norimatsu, Y.; Yoshizaki, A.; et al. Serum vasohibin-1 levels: A potential marker of dermal and pulmonary fibrosis in systemic sclerosis. Exp. Dermatol. 2021, 30, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Saito, D.; Maeshima, Y.; Nasu, T.; Yamasaki, H.; Tanabe, K.; Sugiyama, H.; Sonoda, H.; Sato, Y.; Makino, H.; Kinomura, M.; et al. Amelioration of renal alterations in obese type 2 diabetic mice by vasohibin-1, a negative feedback regulator of angiogenesis. Am. J. Physiol. Physiol. 2011, 300, F873–F886. [Google Scholar] [CrossRef]
- Liu, Y. Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney Int. 2006, 69, 213–217. [Google Scholar] [CrossRef]
- Chuang, P.Y.; Menon, M.C.; He, J.C. Molecular targets for treatment of kidney fibrosis. J. Mol. Med. 2013, 91, 549–559. [Google Scholar] [CrossRef]
- Klein, J.; Kavvadas, P.; Prakoura, N.; Karagianni, F.; Schanstra, J.P.; Bascands, J.-L.; Charonis, A. Renal fibrosis: Insight from proteomics in animal models and human disease. Proteomics 2011, 11, 805–815. [Google Scholar] [CrossRef]






| Name | Primer |
|---|---|
| miR-10a-PCR-S | CCAAGAACGGACCCACAGT |
| miR-10a-PCR-A | AGTGAACAAGGACCCAAGC |
| miR-10b-PCR-F | CCAGAAAGGTAAATGCTCG |
| miR-10b-PCR-R | ATGAGTGTGGGCAATGTG |
| Name | Primer |
|---|---|
| miR-10 RT | GTCGTATCCAGTGCAGGGTCCGAGGTGCACTGGATACGACAATTTGTG |
| U6 RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATATGGAAC |
| miR-10a-Forward | TGCGGTACCCTGTAGATCCGAATTTGTG |
| miR-10b-Forward | TGCGGTACCCTGTAGAACCGAATTTGTG |
| U6-Forward | TGCGGGTGCTCGCTTCGGCAGC |
| U6-Reverse | CCAGTGCAGGGTCCGAGGT |
| Fibronectin-F-human/mouse | GCTCAGCAAATCGTGCAGC |
| Fibronectin-R-human/mouse | CTAGGTAGGTCCGTTCCCACT |
| COL-III-F-human/mouse | CTGTAACATGGAAACTGGGGAAA |
| COL-III-R-human/mouse | CCATAGCTGAACTGAAAACCACC |
| VASH-1-F-human | GGTGGGCTACCTGTGGATG |
| VASH-1-R-human | CACTCGGTATGGGGATCTTGG |
| VASH-1-F-mouse | TGGGAATTTACCTCACCAACA |
| VASH-1-R-mouse | CCATAGGCCGCCTCATAG |
| β-actin-F-human/mouse | GGCTGTATTCCCCTCCATCG |
| β-actin-R-human/mouse | CCAGTTGGTAACAATGCCATGT |
| Name | Primer |
|---|---|
| VASH-1-S | GTTAAGCTTGAATTCATGCCAGGGGGGAAG |
| VASH-1-A | CTTGTAATCCTCGAGGACCCGGATCTGGTA |
| shVASH-1-F | GATCCCCTGGGAATTTACCTCACCAACTCGAGTTGGTGAGGTAAATTCCCAGGTTTTTGA |
| shVASH-1-R | AGCTTCAAAAACCTGGGAATTTACCTCACCAACTCGAGTTGGTGAGGTAAATTCCCAGGG |
| shVASH-2-F | GATCCCTGCCAATCAAATGCCTGGAACTCGAGTTCCAGGCATTTGATTGGCAGTTTTTGA |
| shVASH-2-R | AGCTTCAAAAACTGCCAATCAAATGCCTGGAACTCGAGTTCCAGGCATTTGATTGGCAGG |
| shVASH-3-F | GATCCCCTACTTCTCAGGGAACTACTCTCGAGAGTAGTTCCCTGAGAAGTAGGTTTTTGA |
| shVASH-3-R | AGCTTCAAAAACCTACTTCTCAGGGAACTACTCTCGAGAGTAGTTCCCTGAGAAGTAGGG |
| pcDNA3 EGFP-VASH-1-3′UTR-WT-F | AGCTGTACAAGTAAAGGATCCCCAGGGAAAGGGAAGCAGGGTAGGAATTCTGCAGATATCCAGCA |
| pcDNA3 EGFP-VASH-1-3′UTR-WT-R | TGCTGGATATCTGCAGAATTCCTACCCTGCTTCCCTTTCCCTGGGGATCCTTTACTTGTACAGCT |
| pcDNA3 EGFP-VASH-1-3′UTR-MUT-F | AGCTGTACAAGTAAAGGATCCCCAGGGAAAGGGAAGGTCCCATGGAATTCTGCAGATATCCAGCA |
| pcDNA3 EGFP-VASH-1-3′UTR-MUT-R | TGCTGGATATCTGCAGAATTCCATGGGACCTTCCCTTTCCCTGGGGATCCTTTACTTGTACAGCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuai, Y.; Xu, N.; Zhao, C.; Yang, F.; Ning, Z.; Li, G. MicroRNA-10 Family Promotes Renal Fibrosis through the VASH-1/Smad3 Pathway. Int. J. Mol. Sci. 2024, 25, 5232. https://doi.org/10.3390/ijms25105232
Shuai Y, Xu N, Zhao C, Yang F, Ning Z, Li G. MicroRNA-10 Family Promotes Renal Fibrosis through the VASH-1/Smad3 Pathway. International Journal of Molecular Sciences. 2024; 25(10):5232. https://doi.org/10.3390/ijms25105232
Chicago/Turabian StyleShuai, Yichen, Na Xu, Chuan Zhao, Fengrui Yang, Zhifen Ning, and Guoxia Li. 2024. "MicroRNA-10 Family Promotes Renal Fibrosis through the VASH-1/Smad3 Pathway" International Journal of Molecular Sciences 25, no. 10: 5232. https://doi.org/10.3390/ijms25105232





