Penifuranone A: A Novel Alkaloid from the Mangrove Endophytic Fungus Penicillium crustosum SCNU-F0006
Abstract
:1. Introduction
2. Results and Discussion
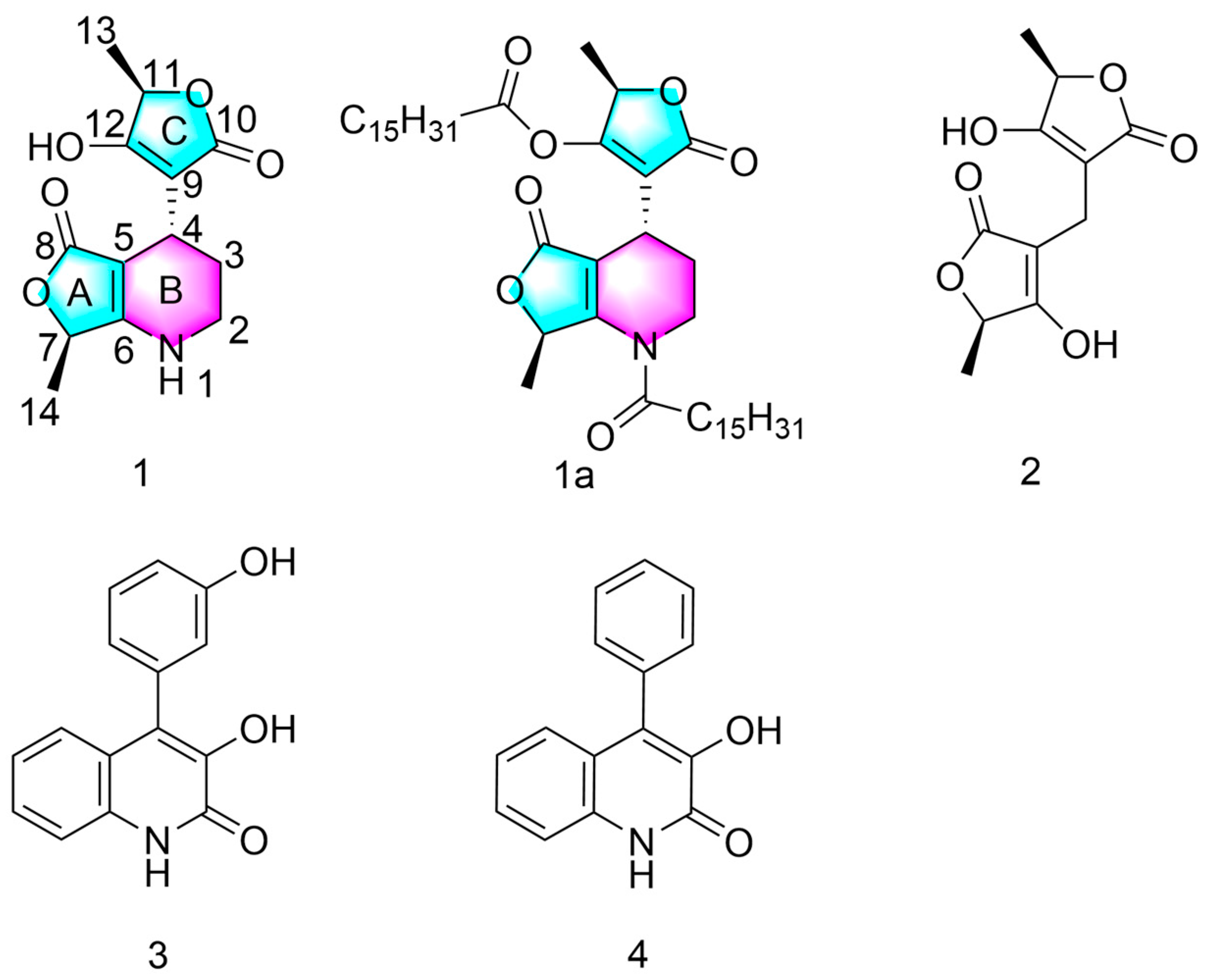
2.1. Structure of Compound 1
2.2. Semi-Synthesis of Compound 1a
2.3. Antimicrobial Assay
2.4. Anti-Inflammatory Assay
2.5. DPPH Scavenging Assay
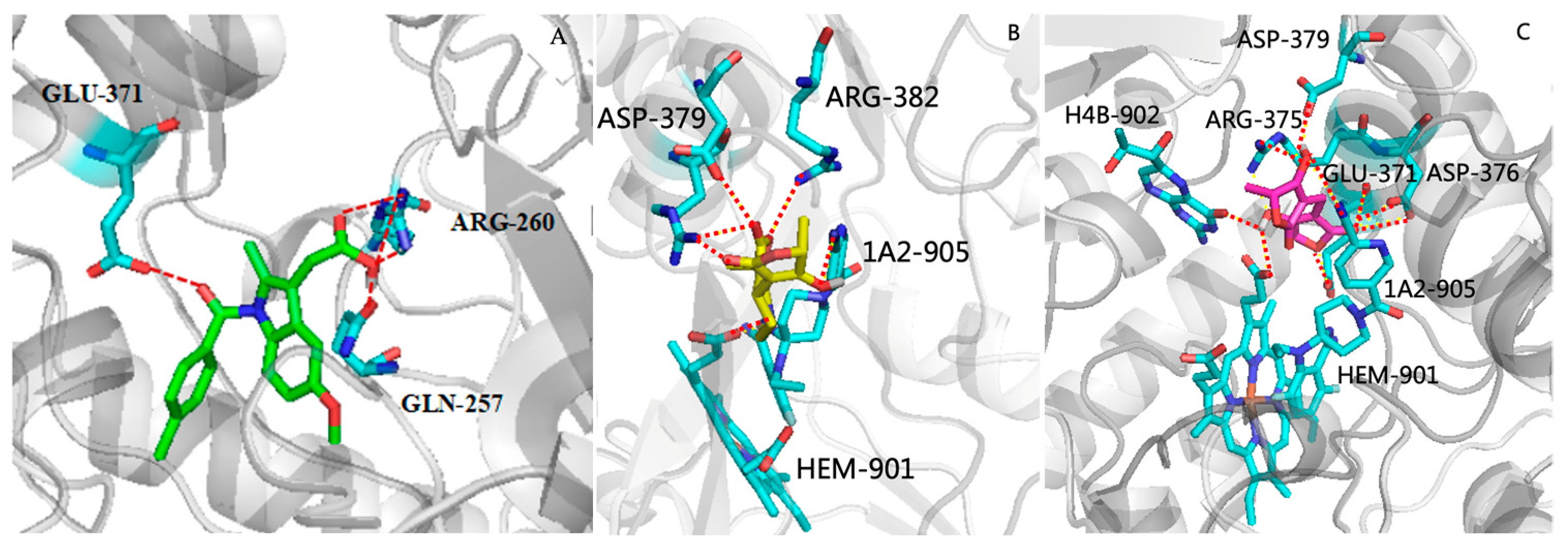
2.6. Molecular Docking Studies
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Fungal Material
3.4. Fermentation and Isolation
3.5. Infrared Spectroscopy
3.6. LC-MS Parameters
3.7. Synthetic Method and Spectral and Physical Data of Compounds 1 and 1a
3.8. X-ray Crystal Data for Compound 1
3.9. In Vitro Antifungal Activity
3.10. In Vitro Antibacterial Activity
3.11. Anti-Inflammatory Assay
3.12. DPPH Scavenging Assay
3.13. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2023, 40, 275–325. [Google Scholar] [CrossRef] [PubMed]
- Ancheeva, E.; Daletos, G.; Proksch, P. Lead compounds from mangrove-associated microorganisms. Mar. Drugs 2018, 16, 319. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiao, Q.; Xu, J.; Li, M.Y.; Pan, J.Y.; Yang, M.H. Natural products from true mangrove flora: Source, chemistry and bioactivities. Nat. Prod. Rep. 2008, 25, 955–981. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cai, R.; Liu, Z.; Cui, H.; She, Z.G. Secondary metabolites from mangrove-associated fungi: Source, chemistry and bioactivities. Nat. Prod. Rep. 2022, 39, 560–595. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, C.; Tao, H.; Lin, X.; Yang, B.; Zhou, X.; Liu, Y. Structurally diverse diketopiperazine alkaloids from the marine-derived fungus Aspergillus versicolor SCSIO 41016. Org. Chem. Front. 2019, 6, 736–740. [Google Scholar] [CrossRef]
- Zhang, P.; Meng, L.H.; Mándi, A.; Kurtán, T.; Li, X.M.; Liu, Y.; Li, X.; Li, C.S.; Wang, B.G. Brocaeloids A–C, 4-oxoquinoline and indole alkaloids with C-2 reversed prenylation from the mangrove-derived endophytic fungus Penicillium brocae. Eur. J. Org. Chem. 2014, 2014, 4029–4036. [Google Scholar] [CrossRef]
- Lin, X.; Ai, W.; Li, M.; Zhou, X.; Liao, S.; Wang, J.; Liu, J.; Yang, B.; Liu, Y. Collacyclumines A-D from the endophytic fungus Colletotrichum salsolae SCSIO 41021 isolated from the mangrove Kandelia candel. Phytochemistry 2020, 171, 112237. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zou, L.; Lei, X.; Su, J.; Yang, R.; Xie, W.; Li, W.; Chen, G. OSMAC strategy integrated with molecular networking discovery peniciacetals A-I, nine new meroterpenoids from the mangrove-derived fungus Penicillium sp. HLLG-122. Bioorg. Chem. 2023, 130, 106271. [Google Scholar] [CrossRef]
- Hang, L.; Liu, N.; Tang, Y. Coordinated and iterative enzyme catalysis in fungal polyketide biosynthesis. ACS. Catal. 2016, 6, 5935–5945. [Google Scholar] [CrossRef]
- Gao, J.M.; Yang, S.X.; Qin, J.C. Azaphilones: Chemistry and biology. Chem. Rev. 2013, 113, 4755–4811. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, S.; Li, N.; Li, F.; Zhu, T.; Gu, Q.; Guo, P.; Li, D. Saroclides A and B, Cyclic depsipeptides from the mangrove-derived fungus Sarocladium kiliense HDN11-112. J. Nat. Prod. 2018, 81, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Gang, D.; Kim, D.W.; Park, H.S. Cyclic peptides: Promising scaffolds for biopharmaceuticals. Genes 2018, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, P.; Lin, X.; Salendra, L.; Kong, F.; Liao, S.; Yang, B.; Zhou, X.; Wang, J.; Liu, Y. Phloroglucinol heterodimers and bis-indolyl alkaloids from the sponge-derived fungus Aspergillus sp. SCSIO 41018. Org. Chem. Front. 2019, 6, 3053–3059. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, S.; Ali, A.; Gupta, A.C.; Shanker, K.; Bawankule, D.U. Microwave-assisted Single Step Cinnamic Acid Derivatization and Evaluation for Cytotoxic Potential. Curr. Pharm. Biotechno. 2020, 21, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Howell, G.B.J.; De, S.R.D.; Farley, J.M.; Liu, H.L.; Yi, G.B.; Rockhold, R.W. Cardiodepressant and neurologic actions of Solenopsis invicta (imported fire ant) venom alkaloids. Ann. Allergy. Asthma. Immunol. 2005, 94, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Faisal, S.; Badshah, S.L.; Kubra, B.; Emwas, A.H.; Jaremko, M. Alkaloids as potential antivirals. A comprehensive review. Nat. Prod. Bioprospect. 2023, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Tuzimski, T.; Petruczynik, A. New trends in the practical use of isoquinoline alkaloids as potential drugs applicated in infectious and non-infectious diseases. Biomed. Pharmacother. 2023, 168, 115704. [Google Scholar] [CrossRef]
- Jia, Y.L.; Wei, M.Y.; Chen, H.Y.; Guan, F.F.; Wang, C.Y.; Shao, C.L. (+)—And (-)—Pestaloxazine A, a pair of antiviral enantiomeric alkaloid dimers with a symmetric spiro [oxazinane—Piperazinedione] skeleton from Pestalotiopsis sp. Org. Lett. 2015, 17, 4216–4219. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.F.; Yu, Y.; Zhang, Q.; Huang, L.; Hao, C.; Shao, C.L.; Wang, W. Inhibition of influenza a virus replication by a marine derived quinolone alkaloid targeting virus nucleoprotein. J. Med. Virol. 2023, 95, e28499. [Google Scholar] [CrossRef]
- Lin, C.; Huang, R.; Liu, J.; Li, H.; Zhu, L.; Huang, X.; Ding, B.; Liu, L.; Huang, H.; Tao, Y. Antibacterial polyketides isolated from the marine-derived fungus Fusarium solani 8388. J. Fungi. 2023, 9, 875. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, S.; Li, S.; Hao, X.; Wang, A.; Si, S.; Xu, Y.; Shu, J.; Gan, M. Antimicrobial and anti-inflammatory cyclic tetrapeptides from the co-cultures of two marine-derived fungi. J. Nat. Prod. 2024, 87, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yuan, Y.; Li, Z.; Zhu, J.; She, Z.; Chen, Y. Cytosporones with anti-Inflammatory activities from the mangrove endophytic fungus Phomopsis sp. QYM-13. Mar. Drugs 2023, 21, 631. [Google Scholar] [CrossRef] [PubMed]
- Cui, E.; Qian, S.; Li, J.; Jiang, X.; Wang, H.; Du, S.; Du, L. Discovery of coixol derivatives as potent anti-inflammatory agents. J. Nat. Prod. 2023, 86, 1950–1959. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Sarai, R.J.; David, O.S.; Julia, P.R.; Leonor, S.P.; Salud, P.G.; Nimsi, C.X. Anti-inflammatory and cytotoxic compounds isolated from plants of euphorbia genus. Molecules 2024, 29, 1083. [Google Scholar] [CrossRef] [PubMed]
- Takuya, H.T.S.; Hiroyuki, O. RK-682, a potent inhibitor of tyrosine phosphatase, arrested the mammalian cell cycle progression at G phase. FEBS. Lett. 1995, 372, 54–58. [Google Scholar]
- Romano, A.; William, A.; Latchezar, S. Two new tetronic acids: 5-Hydroxyvertinolide. Aust. J. Chem. 1997, 50, 255–257. [Google Scholar]
- Schobert, R.; Schlenk, A. Tetramic and tetronic acids: An update on new derivatives and biological aspects. Bioorg. Med. Chem. 2008, 16, 4203–4221. [Google Scholar] [CrossRef]
- Carneiro, V.M.; Trivella, D.B.; Scorsato, V.; Beraldo, V.L.; Dias, M.P.; Sobreira, T.J.; Aparicio, R.; Pilli, R.A. Is RK-682 a promiscuous enzyme inhibitor? Synthesis and in vitro evaluation of protein tyrosine phosphatase inhibition of racemic RK-682 and analogues. Eur. J. Med. Chem. 2015, 97, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, A.; Peczek, F. Furoquinoline alkaloids: Insights into chemistry, occurrence, and biological properties. Int. J. Mol. Sci. 2023, 24, 12811. [Google Scholar] [CrossRef] [PubMed]
- Ramachary, D.B.; Vijayendar, R.Y. A general approach to chiral building blocks via direct amino acid-catalyzed cascade three-component reductive alkylations: Formal total synthesis of HIV-1 protease inhibitors, antibiotic agglomerins, brefeldin A, and (R)-gamma-hexanolide. J. Org. Chem. 2010, 75, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Prabhaker, N.C.S.; Buckelew, L. Baseline susceptibility of bemisia tabaci B biotype (hemiptera: aleyrodidae) populations from California and Arizona to spiromesifen. J. Econ. Entomol. 2008, 101, 174–181. [Google Scholar] [CrossRef]
- Guo, H.X.; Yan, Z.Y.; Chen, T.; Li, W.Y.; Long, Y.H. One new tetronic acid dimer from a mangrove endophytic fungus Penicillium sp. SCNU-F0003. Chin. J. Mar. Drugs 2020, 39, 1–6. [Google Scholar]
- Bracken, A.P.A.; Raistrick, H. Studies in the biochemistry of microorganisms. Cyclopenin, a nitrogen-containing metabolic product of Penicillium cyclopium Westling. Biochem. J. 1954, 57, 587–595. [Google Scholar] [CrossRef]
- Mei, Y.W.; Chang, L.S.; Chang, Y.W.; Dong, S.D.; Zhi, G.S. Isolation, structure elucidation, crystal structure, and biological activity of a marine natural alkaloid, viridicatol. Chem. Nat. Compd. 2011, 47, 322–325. [Google Scholar]
- Mitchell, M.A.; Iannetta, A.A.; Jennings, M.C.; Fletcher, M.H.; Wuest, W.M.; Minbiole, K.P. Scaffold-Hopping of Multicationic Amphiphiles Yields Three New Classes of Antimicrobials. Chembiochem 2015, 16, 2299–2303. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Leiper, J. Blocking NO synthesis: How, where and why? Nat. Rev. Drug. Discov. 2002, 1, 939–950. [Google Scholar] [CrossRef]
- Eiserich, J.P.; Hristova, M.; Cross, C.E.; Jones, A.D.; Freeman, B.A.; Halliwell, B.; Vliet, A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 1998, 391, 393–397. [Google Scholar] [CrossRef]
- Garcin, E.D.; Arvai, A.S.; Rosenfeld, R.J.; Kroeger, M.D.; Crane, B.R.; Andersson, G.; Andrews, G.; Hamley, P.J.; Mallinder, P.R.; Nicholls, D.J. Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase. Nat. Chem. Biol. 2008, 4, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Thippeswamy, T.; McKay, J.S.; Quinn, J.P. Nitric oxide, a biological double-faced janus-is this good or bad? Histol. Histopathol. 2006, 21, 445–458. [Google Scholar] [PubMed]
- Duncan, A.J.; Heales, S.J. Nitric oxide and neurological disorders. Mol. Aspects. Med. 2005, 26, 67–96. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Jiang, M.; Guo, H.; Wu, Q.; Lu, X.; Zou, Y.; Fu, Q.; Chen, S.; Liu, L.; Peng, B.; Chen, S. Anti-inflammatory acetylenic meroterpenoids from the ascidian-derived fungus Amphichorda felina SYSU-MS7908. Bioorg. Chem. 2023, 139, 106715. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, H.; Wu, Z.; Wu, Q.; Jiang, M.; Li, H.; Liu, L. Targeted discovery of sorbicillinoid pigments with anti-inflammatory activity from the sponge-derived fungus Stagonospora sp. SYSU-MS7888 using the PMG strategy. J. Agric. Food. Chem. 2022, 70, 15116–15125. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Qiu, P.; Xu, B.; Zhao, Q.; Gu, Y.C.; Fu, L.; Bi, S.; Lan, L.; Wang, C.Y.; Guo, Y.W. Cytotoxic and antibacterial isomalabaricane terpenoids from the sponge rhabdastrella globostellata. J. Nat. Prod. 2022, 85, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Shi, Z.M.; Lei, Y.H.; Si-Tu, M.X.; Zhou, F.G.; Feng, C.; Wei, X.; Shao, X.H.; Chen, Y.; Zhang, C.X. Rare carbon-bridged citrinin dimers from the starfish-derived symbiotic fungus Penicillium sp. GGF16-1-2. Mar. Drugs 2022, 20, 443. [Google Scholar] [CrossRef]
- Jiao, Z.Z.; Yue, S.; Sun, H.X.; Jin, T.Y.; Wang, H.N.; Zhu, R.X.; Xiang, L. Indoline amide glucosides from portulaca oleracea: Isolation, structure, and DPPH radical scavenging activity. J. Nat. Prod. 2015, 78, 2588–2597. [Google Scholar] [CrossRef]




| Position | 1 | |
|---|---|---|
| δH (J in Hz) | δC, Type | |
| 2 | 3.2, s | 39.8, CH2 |
| 3 | 1.7, m 1.8, m | 26.3, CH2 |
| 4 | 3.3, t (5.8) | 23.3, CH |
| 5 | 88.0, C | |
| 6 | 167.7, C | |
| 7 | 4.6, q (6.6) | 71.8, CH |
| 8 | 171.3, C | |
| 9 | 100.1, C | |
| 10 | 172.6, C | |
| 11 | 4.7, q (6.7) | 72.8, CH |
| 12 | 176.6, CH | |
| 13 | 1.3, d (6.7) | 18.1, CH3 |
| 14 | 1.3, d (6.6) | 18.9, CH3 |
| 1-NH | 7.4, s | |
| 12-OH | 11.5, s | |
| Compound | Fusarium oxysporum | Penicillium italicum | Banana Colletotrichum gloeosporioides | Colletrichum litchi Tra |
|---|---|---|---|---|
| 1 | 0.25 | 0.25 | >1 | 1 |
| 1a | 0.25 | 0.125 | >1 | 1 |
| 2 | 0.25 | 0.25 | >1 | 0.5 |
| 3 | 0.25 | 0.5 | >1 | 1 |
| 4 | 0.25 | 0.25 | >1 | 1 |
| Carbendazim b | <0.03 | <0.03 | <0.03 | <0.03 |
| Compound | Pseudomonas aeruginosa | Salmonella typhimurium | Escherichia coli | Staphylococcus aureus |
|---|---|---|---|---|
| 1 | 0.5 | 1 | 1 | >1 |
| 1a | 0.125 | 1 | >1 | >1 |
| 2 | 0.5 | 1 | 1 | >1 |
| 3 | 0.125 | 1 | 1 | >1 |
| 4 | 0.5 | 1 | >1 | 1 |
| Ciprofloxacin b | <0.03 | <0.03 | <0.03 | <0.03 |
| Compound | Inhibition of NO Production (IC50/μM) b | Cytotoxicity (CC50/μM) b |
|---|---|---|
| 1 | 42.22 ± 2.26 | >50 |
| 1a | - c | <50 |
| 2 | 37.01 ± 0.88 | >50 |
| 3 | - | <50 |
| 4 | - | <50 |
| Dexamethasone a | 58.21 ± 1.74 | >50 |
| Indomethacin a | 35.8 ± 5.7 | >50 |
| Compounds | Radical Scavenging Activity (IC50/μM) b |
|---|---|
| 1 | 180.2 |
| 1a | >200 |
| 2 | 120.5 |
| 3 | >200 |
| 4 | >200 |
| Vitamin C a | 68.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, H.; Wu, L.; Liu, R.; Li, J.; Liu, L.; Chen, C.; Li, J.; Zhang, K.; Liao, J.; Long, Y. Penifuranone A: A Novel Alkaloid from the Mangrove Endophytic Fungus Penicillium crustosum SCNU-F0006. Int. J. Mol. Sci. 2024, 25, 5032. https://doi.org/10.3390/ijms25095032
Jia H, Wu L, Liu R, Li J, Liu L, Chen C, Li J, Zhang K, Liao J, Long Y. Penifuranone A: A Novel Alkaloid from the Mangrove Endophytic Fungus Penicillium crustosum SCNU-F0006. International Journal of Molecular Sciences. 2024; 25(9):5032. https://doi.org/10.3390/ijms25095032
Chicago/Turabian StyleJia, Hao, Li Wu, Rongrong Liu, Jialin Li, Lingling Liu, Chen Chen, Junsen Li, Kai Zhang, Junjiang Liao, and Yuhua Long. 2024. "Penifuranone A: A Novel Alkaloid from the Mangrove Endophytic Fungus Penicillium crustosum SCNU-F0006" International Journal of Molecular Sciences 25, no. 9: 5032. https://doi.org/10.3390/ijms25095032





