Seeds Priming with Melatonin Improves Root Hydraulic Conductivity of Wheat Varieties under Drought, Salinity, and Combined Stress
Abstract
:1. Introduction
2. Results
2.1. The Effects of Seed-Priming on Leaf Area and Root Length
2.2. The Effects of Seed-Priming on Root Vitality
2.3. The Effects of Seed-Priming on MDA Content
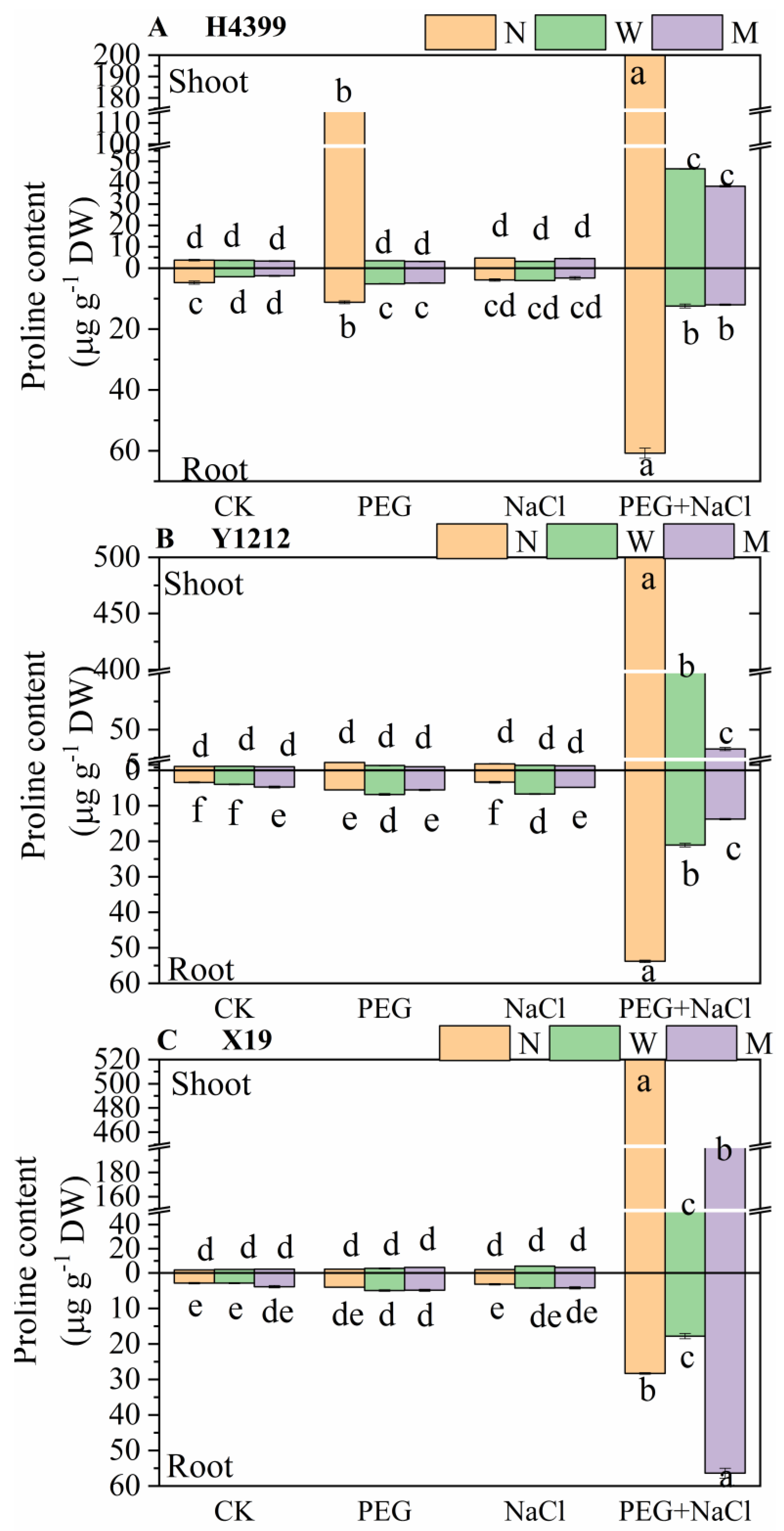
2.4. The Effects of Seed-Priming on Proline Content
2.5. The Effects of Seed-Priming on Soluble Protein and Soluble Sugar
2.6. The Effects of Seed-Priming on Antioxidant Enzyme Activities
2.7. The Effects of Seed-Priming on K+ and Na+ Content
2.8. The Effects of Seed-Priming on Root Hydraulic Conductivity and Theoretical Hydraulic Conductivity
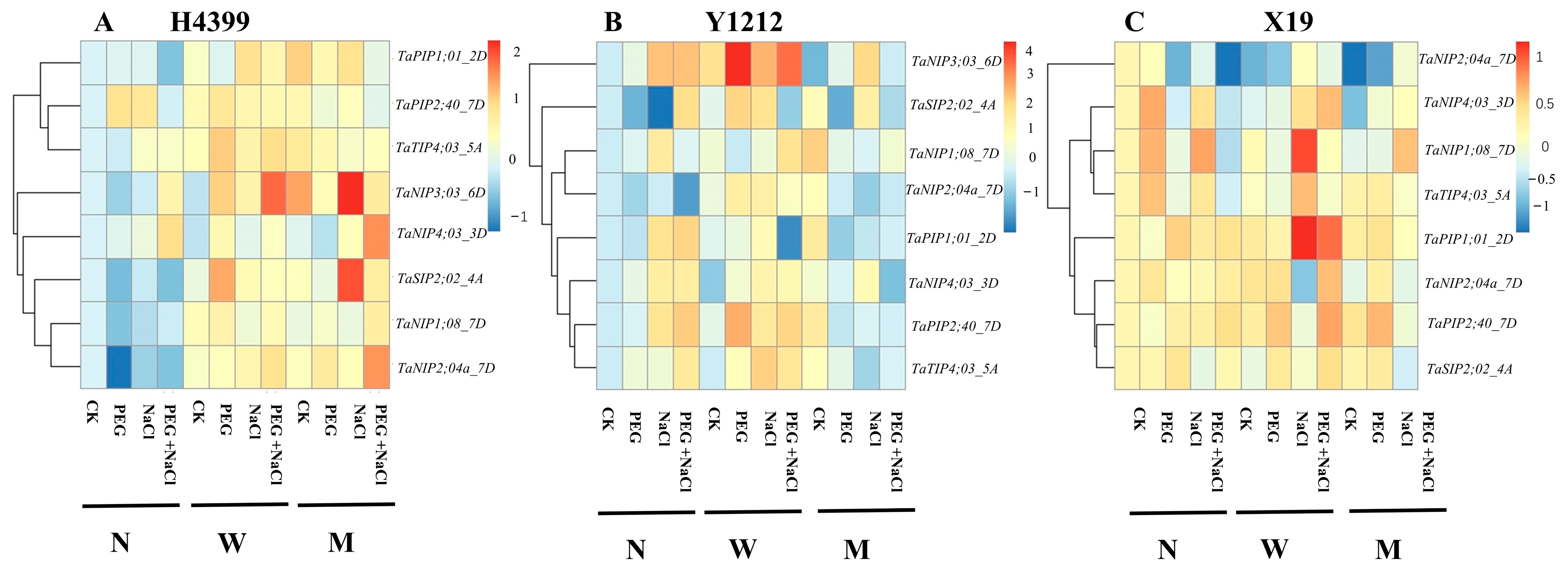
2.9. The Effects of Seed-Priming on Expression of Some Aquaporin Genes in Roots
2.10. The Effects of Seed-Priming on the Relation between Root Hydraulic Conductivity and the Physiological Characteristics
3. Discussion
3.1. Seed-Priming Using Melatonin Increasing Root Hydraulic Conductivity of Wheat Seedlings
3.2. Seed-Priming Using Melatonin Promoting Wheat Growth
3.3. Seed-Priming Inducing Stress Responses
4. Materials and Methods
4.1. Plant Materials
4.2. Experimental Design
4.3. Determination of Root Growth and Leaf Area
4.4. Measurem Ent of Root Viability
4.5. Measurement of Relative Conductivity in Shoot
4.6. Measurement of Root Hydraulic Conductivity and Theoretical Hydraulic Conductivity
4.7. Detection of Antioxidant Enzymes Activity, Levels of Malondialdehyde, Contents of Osmoregulatory Substances, and Amounts of Potassium and Sodium
4.8. qPCR Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alam, J.; Nawaz, H.; Rehman, H.U.; Yousaf, M.M.; Hussain, N. Potential effect of bed-furrow planting improved the wheat grains productivity under drought stress. Turk. J. Field Crops 2022, 27, 242–250. [Google Scholar] [CrossRef]
- Isayenkov, S.V. Genetic sources for the development of salt tolerance in crops. Plant Growth Regul. 2019, 89, 1–17. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Chen, J.; Wang, X.; Cai, J.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Parental Drought-Priming Enhances Tolerance to Post-anthesis Drought in Offspring of Wheat. Front. Plant Sci. 2018, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Qin, C.; Begum, N.; Maodong, Q.; Dong, X.X.; El-Esawi, M.; El-Sheikh, M.A.; Alatar, A.A.; Zhang, L. Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation. BMC Plant Biol. 2019, 19, 479. [Google Scholar] [CrossRef] [PubMed]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Colin, L.; Ruhnow, F.; Zhu, J.-K.; Zhao, C.; Zhao, Y.; Persson, S. The cell biology of primary cell walls during salt stress. Plant Cell 2023, 35, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Alam, M.A.; Syed, M.A.; Hossain, J.; Sarkar, S.; Saha, S.; Bhadra, P. Consequences and Mitigation Strategies of Abiotic Stresses in Wheat (Triticum aestivum L.) under the Changing Climate. Agronomy 2021, 11, 241. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Iqbal, S.; Hafeez, M.B.; Ibrahim, A.M.H.; Raza, A.; Fatima, E.M.; Baloch, H.; Jahanzaib; Woodrow, P.; Ciarmiello, L.F. Effect of Salinity Stress on Physiological Changes in Winter and Spring Wheat. Agronomy 2021, 11, 1193. [Google Scholar] [CrossRef]
- Wang, B.X.; Li, L.Q.; Liu, M.L.; Peng, D.; Wei, A.S.; Hou, B.Y.; Lei, Y.H.; Li, X.J. TaFDL2-1A confers drought stress tolerance by promoting ABA biosynthesis, ABA responses, and ROS scavenging in transgenic wheat. Plant J. 2022, 112, 722–737. [Google Scholar] [CrossRef]
- Zafar, S.; Hasnain, Z.; Anwar, S.; Perveen, S.; Ali, M. Influence of melatonin on antioxidant defense system and yield of wheat (Triticum aestivum L.) genotypes under saline condition. Pak. J. Bot. 2019, 51, 1987–1994. [Google Scholar] [CrossRef]
- Imran, M.; Mahmood, A.; Römheld, V.; Neumann, G. Nutrient seed priming improves seedling development of maize exposed to low root zone temperatures during early growth. Eur. J. Agron. 2013, 49, 141–148. [Google Scholar] [CrossRef]
- Sánchez-Bermúdez, M.; del Pozo, J.C.; Pernas, M. Effects of combined abiotic stresses related to climate change on root growth in crops. Front. Plant Sci. 2022, 13, 918537. [Google Scholar] [CrossRef]
- Castilla, F.J.C.; Rispail, N.; Tejera, O.G.; Arbona, V.; Prats, E. Drought Resistance in Oat Involves ABA-mediated Modulation of Transpiration and Root Hydraulic Conductivity. Environ. Exp. Bot. 2021, 182, 104333. [Google Scholar] [CrossRef]
- Rodriguez-Dominguez, C.M.; Brodribb, T.J. Declining root water transport drives stomatal closure in olive under moderate water stress. New Phytol. 2020, 225, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.; Ahmed, M.A.; Cai, G.; Zarebanadkauki, M.; Carminati, A. Coupled effects of soil drying and salinity on soil-plant hydraulics. Plant Physiol. 2022, 190, 1228–1241. [Google Scholar] [CrossRef]
- Li, Q.-M.; Liu, B.-B. Comparison of Three Methods for Determination of Root Hydraulic Conductivity of Maize (Zea mays L.) Root System. Agric. Sci. China 2010, 9, 1438–1447. [Google Scholar] [CrossRef]
- Meng, D.; Fricke, W. Changes in root hydraulic conductivity facilitate the overall hydraulic response of rice (Oryza sativa L.) cultivars to salt and osmotic stress. Plant Physiol. Biochem. 2017, 113, 64–77. [Google Scholar] [CrossRef]
- Nedjimi, B. Effects of salinity on growth, membrane permeability and root hydraulic conductivity in three saltbush species. Biochem. Syst. Ecol. 2014, 52, 4–13. [Google Scholar] [CrossRef]
- Vitali, V.; Sutka, M.; Ojeda, L.; Aroca, R.; Amodeo, G. Root hydraulics adjustment is governed by a dominant cell-to-cell pathway in Beta vulgaris seedlings exposed to salt stress. Plant Sci. 2021, 306, 110873. [Google Scholar] [CrossRef]
- Yu, S.; Yi, Z.; Weihua, H.; Ru, F.; Yanhong, H.; Jia, G.; Haijun, G. Silicon Enhances Water Stress Tolerance by Improving Root Hydraulic Conductance in Solanum lycopersicum L. Front. Plant Sci. 2016, 7, 196. [Google Scholar] [CrossRef]
- Fu, Y.; Li, P.; Hamani, A.K.M.; Wan, S.; Gao, Y.; Wang, X. Effects of Single and Combined Drought and Salinity Stress on the Root Morphological Characteristics and Root Hydraulic Conductivity of Different Winter Wheat Varieties. Plants 2023, 12, 2694. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Polanco, M.; Ribeyre, Z.; Dauzat, M.; Reyt, G.; Hidalgo-Shrestha, C.; Diehl, P.; Frenger, M.; Simonneau, T.; Muller, B.; Salt, D.E.; et al. Physiological roles of Casparian strips and suberin in the transport of water and solutes. New Phytol. 2021, 232, 2295–2307. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, F.; Tyerman, S.D. Aquaporins: Highly Regulated Channels Controlling Plant Water Relations. Plant Physiol. 2014, 164, 1600–1618. [Google Scholar] [CrossRef]
- Maurel, C.; Nacry, P. Root architecture and hydraulics converge for acclimation to changing water availability. Nat. Plants 2020, 6, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, A.; Odugbemi, A.; Ajewole, T. Structure and Function of Aquaporins: The Membrane Water Channel Proteins. Biointerface Res. Appl. Chem. 2022, 12, 690–705. [Google Scholar] [CrossRef]
- Geng, X.Q.; Shao, G.Y.; Jiang, T.; Yang, B.X. Transport Characteristics of Aquaporins. In Aquaporins, 2nd ed.; Yang, B., Ed.; Springer: Dordrecht, The Netherlands, 2023; pp. 53–64. [Google Scholar]
- Knipfer, T.; Besse, M.; Verdeil, J.L.; Fricke, W. Aquaporin-facilitated water uptake in barley (Hordeum vulgare L.) roots. J. Exp. Bot. 2011, 62, 4115–4126. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Zaplana, A.; Martinez-Garcia, N.; Carvajal, M.; Barzana, G. Relationships between aquaporins gene expression and nutrient concentrations in melon plants (Cucumis melo L.) during typical abiotic stresses. Environ. Exp. Bot. 2022, 195, 104759. [Google Scholar] [CrossRef]
- Mahdieh, M.; Mostajeran, A.; Horie, T.; Katsuhara, M. Drought stress alters water relations and expression of PIP-Type aquaporin genes in nicotiana tabacum plants. Plant Cell Physiol. 2008, 49, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Murai-Hatano, M.; Kuwagata, T.; Sakurai, J.; Nonami, H.; Ahamed, A.; Nagasuga, K.; Matsunami, T.; Fukushi, K.; Maeshima, M.; Okada, M. Effect of low root temperature on hydraulic conductivity of rice plants and the possible role of aquaporins. Plant Cell Physiol. 2008, 49, 1294–1305. [Google Scholar] [CrossRef]
- Paluch-Lubawa, E.; Prosicka, B.; Polcyn, W. Expression patterns of maize PIP aquaporins in middle or upper leaves correlate with their different physiological responses to drought and mycorrhiza. Front. Plant Sci. 2022, 13, 1056992. [Google Scholar] [CrossRef]
- Raturi, G.; Kumawat, S.; Mandlik, R.; Duhan, D.; Thakral, V.; Sudhakaran, S.; Ram, C.; Sonah, H.; Deshmukh, R. Deciphering the Role of Aquaporins Under Different Abiotic Stress Conditions in Watermelon (Citrullus lanatus). J. Plant Growth Regul. 2023, 42, 3137–3149. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Huang, Y.; Li, Y.; Su, C.; Zeng, X. EuPIP1;2, a Plasma Membrane Aquaporin Gene from Eucommia ulmoides, Enhances Drought and Salt Tolerance in Transgenic Tobacco. Agronomy 2022, 12, 615. [Google Scholar] [CrossRef]
- Luo, Y.J.; Ma, L.; Du, W.X.; Yan, S.; Wang, Z.Y.; Pang, Y.Z. Identification and Characterization of Salt- and Drought-Responsive AQP Family Genes in Medicago sativa L. Int. J. Mol. Sci. 2022, 23, 3342. [Google Scholar] [CrossRef] [PubMed]
- Luu, D.; Maurel, C. Aquaporin Trafficking in Plant Cells: An Emerging Membrane-Protein Model. Traffic 2013, 14, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Yang, L.L.; Chai, S.S.; Ren, Y.F.; Guan, M.; Ma, F.W.; Liu, J.Y. An aquaporin gene MdPIP1;2 from Malus domestica confers salt tolerance in transgenic Arabidopsis. J. Plant Physiol. 2022, 273, 153711. [Google Scholar] [CrossRef] [PubMed]
- Bhave, F.M. The PIP and TIP aquaporins in wheat form a large and diverse family with unique gene structures and functionally important features. Funct. Integr. Genom. 2008, 8, 115–124. [Google Scholar]
- Hu, W.; Yuan, Q.; Wang, Y.; Cai, R.; Deng, X.; Wang, J.; Zhou, S.; Chen, M.; Chen, L.; Huang, C.; et al. Overexpression of a Wheat Aquaporin Gene, TaAQP8, Enhances Salt Stress Tolerance in Transgenic Tobacco. Plant Cell Physiol. 2012, 53, 2127–2141. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hu, W.; Deng, X.; Ma, Z.; Chen, L.; Huang, C.; Wang, C.; Wang, J.; He, Y.; Yang, G. Overexpression of the Wheat Aquaporin Gene, TaAQP7, Enhances Drought Tolerance in Transgenic Tobacco. PLoS ONE 2012, 7, e52439. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, M.; Zhou, L.; Quan, T.; Xia, G. Heterologous Expression of the Wheat Aquaporin Gene TaTIP2;2 Compromises the Abiotic Stress Tolerance of Arabidopsis thaliana. PLoS ONE 2013, 8, e79618. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.Q.; An, Y.C.; Wu, J.Y.; He, S.B.; Sun, L.R.; Hao, F.S. Wheat TaTIP4;1 Confers Enhanced Tolerance to Drought, Salt and Osmotic Stress in Arabidopsis and Rice. Int. J. Mol. Sci. 2022, 23, 2085. [Google Scholar] [CrossRef]
- Lu, Y.; Fricke, W. Changes in root hydraulic conductivity in wheat (Triticum aestivum L.) in response to salt stress and day/night can best be explained through altered activity of aquaporins. Plant Cell Environ. 2023, 46, 747–763. [Google Scholar] [CrossRef]
- Mehta, A.; Chowdhury, P. Exogenous Application of Chenopodium album Aqueous Extracts on Salt-Stressed Wheat Seedling. Biosci. Biotechnol. Res. Commun. 2021, 14, 1197–1204. [Google Scholar] [CrossRef]
- Rai-Kalal, P.; Tomar, R.S.; Jajoo, A. Seed nanopriming by silicon oxide improves drought stress alleviation potential in wheat plants. Funct. Plant Biol. 2021, 48, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Ni, J.; Tang, C.G.; Chen, X.; Kan, W.J.; Wu, L.F. Karrikinolide alleviates salt stress in wheat by regulating the redox and K+/Na+ homeostasis. Plant Physiol. Biochem. 2021, 167, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Tian, Z.W.; Xu, L.B.; Abid, M.; Lei, K.Q.; Khanzada, A.; Zeeshan, M.; Sun, C.J.; Yu, J.H.; Dai, T.B. Improving the effects of drought priming against post-anthesis drought stress in wheat (Triticum aestivum L.) using nitrogen. Front. Plant Sci. 2022, 13, 965996. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Lv, P.H.; Yan, D.; Zhang, Z.D.; Xu, X.M.; Wang, T.; Wang, Y.; Peng, Z.; Yu, C.X.; Gao, Y.R.; et al. Exogenous Melatonin Improves Seed Germination of Wheat (Triticum aestivum L.) under Salt Stress. Int. J. Mol. Sci. 2022, 23, 8436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, L.; Sun, H.; Wu, J.; Liu, L.; Wang, J.; Wang, B.; Wang, Q.; Sun, Z.; Li, D. Melatonin Increases Drought Resistance through Regulating the Fine Root and Root Hair Morphology of Wheat Revealed with RhizoPot. Agronomy 2023, 13, 1881. [Google Scholar] [CrossRef]
- Abid, M.; Hakeem, A.; Shao, Y.; Liu, Y.; Zahoor, R.; Fan, Y.; Suyu, J.; Ata-Ul-Karim, S.T.; Tian, Z.; Jiang, D.; et al. Seed osmopriming invokes stress memory against post-germinative drought stress in wheat (Triticum aestivum L.). Environ. Exp. Bot. 2018, 145, 12–20. [Google Scholar] [CrossRef]
- Ellouzi, H.; Oueslati, S.; Hessini, K.; Rabhi, M.; Abdelly, C. Seed-priming with H2O2 alleviates subsequent salt stress by preventing ROS production and amplifying antioxidant defense in cauliflower seeds and seedlings. Sci. Hortic. 2021, 288, 110360. [Google Scholar] [CrossRef]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant. 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Molecular processes induced in primed seeds—Increasing the potential to stabilize crop yields under drought conditions. J. Plant Physiol. 2016, 203, 116–126. [Google Scholar] [CrossRef]
- Zhipeng, D.; Xu, L.; Yujiao, T.; Xue, T.; Hang, Z.; Haihua, L.; Chongjun, Y. Research advances on seed priming in drought stress of crops in China. Seed 2016, 35, 53–58. [Google Scholar] [CrossRef]
- Ahmad, S.; Wang, G.-Y.; Muhammad, I.; Chi, Y.-X.; Zeeshan, M.; Nasar, J.; Zhou, X.-B. Interactive Effects of Melatonin and Nitrogen Improve Drought Tolerance of Maize Seedlings by Regulating Growth and Physiochemical Attributes. Antioxidants 2022, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Wang, G.Y.; Muhammad, I.; Farooq, S.; Kamran, M.; Ahmad, I.; Zeeshan, M.; Javed, T.; Ullah, S.; Huang, J.H.; et al. Application of melatonin-mediated modulation of drought tolerance by regulating photosynthetic efficiency, chloroplast ultrastructure, and endogenous hormones in maize. Chem. Biol. Technol. Agric. 2022, 9, 5. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.B.; Li, L.; Ai, S. Melatonin and Its Protective Role against Biotic Stress Impacts on Plants. Biomolecules 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Sezer, I.; Kiremit, M.S.; Öztürk, E.; Subrata, B.A.G.; Osman, H.M.; Akay, H.; Arslan, H. Role of melatonin in improving leaf mineral content and growth of sweet corn seedlings under different soil salinity levels. Sci. Hortic. 2021, 288, 110376. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, C.; Zheng, C.; Zhang, Y.; Fan, H.; Xia, F. Effects of melatonin priming on germination of lespedezad avurica seeds under drought stress. Chin. J. Grassl. 2022, 44, 114–120. [Google Scholar] [CrossRef]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.; Hu, L. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crops Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Muhammad, I.; Yang, L.; Ahmad, S.; Farooq, S.; Khan, A.; Muhammad, N.; Ullah, S.; Adnan, M.; Ali, S.; Liang, Q.P.; et al. Melatonin-priming enhances maize seedling drought tolerance by regulating the antioxidant defense system. Plant Physiol. 2023, 191, 2301–2315. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.J.; Dong, Y.T.; Zhang, F.; He, Q.L.; Chen, J.H.; Zhu, S.J.; Zhao, T.L. Seed priming with melatonin improves salt tolerance in cotton through regulating photosynthesis, scavenging reactive oxygen species and coordinating with phytohormone signal pathways. Ind. Crops Prod. 2021, 169, 113671. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.J.; Weeda, S.; Yang, C. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2012, 54, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Akbari, G.A.; Heshmati, S.; Soltani, E.; Dehaghi, M.A. Influence of Seed Priming on Seed Yield, Oil Content and Fatty Acid Composition of Safflower (Carthamus tinctorius L.) Grown Under Water Deficit. Int. J. Plant Prod. 2020, 14, 245–258. [Google Scholar] [CrossRef]
- Heshmati, S.; Dehaghi, M.A.; Farooq, M.; Wojtyla, L.; Maleki, K.; Heshmati, S. Role of melatonin seed priming on antioxidant enzymes and biochemical responses of Carthamus tinctorius L. under drought stress conditions. Plant Stress 2021, 2, 100023. [Google Scholar] [CrossRef]
- Wei, X.; Qin, Z.; Liang, L.; Lin, X.; Li, Y. Study on the Mechanism of Melatonin Seed Priming in Improving Salt Tolerance of Capsicum annuum. Biotechnol. Bull. 2023, 39, 69–71. [Google Scholar]
- Yin, S.; Zhou, G.; GU, B.; Wu, C.; Yan, L.; Xie, Y. Effects of Melatonin Priming on Physiological Characteristics of Cucumber Seedlings Under Drought Stress. Chin. Agric. Sci. Bull. 2022, 38, 30–36. [Google Scholar]
- Mao, H.; Jiang, C.; Tang, C.; Nie, X.; Du, L.; Liu, Y.; Cheng, P.; Wu, Y.; Liu, H.; Kang, Z.; et al. Wheat adaptation to environmental stresses under climate change: Molecular basis and genetic improvement. Mol. Plant 2023, 16, 1564–1589. [Google Scholar] [CrossRef] [PubMed]
- Singha, A.; Soothar, R.K.; Wang, C.; Marín, E.E.T.; Tankari, M.; Hao, W.; Wang, Y. Drought priming alleviated salinity stress and improved water use efficiency of wheat plants. Plant Growth Regul. 2022, 96, 357–368. [Google Scholar] [CrossRef]
- Coêlho, M.R.V.; Rivas, R.; Ferreira-Neto, J.R.C.; Bezerra-Neto, J.P.; Pandolfi, V.; Benko-Iseppon, A.M.; Santos, M.G. Salt tolerance of Calotropis procera begins with immediate regulation of aquaporin activity in the root system. Physiol. Mol. Biol. Plants 2021, 27, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.K.; Nguyen, H.T.; Belanger, R.R. Editorial: Aquaporins: Dynamic Role and Regulation. Front. Plant Sci. 2017, 8, 1420. [Google Scholar] [CrossRef]
- Qiao, Y.; Ren, J.; Yin, L.; Liu, Y.; Deng, X.; Liu, P.; Wang, S. Exogenous melatonin alleviates PEG-induced short-term water deficiency in maize by increasing hydraulic conductance. BMC Plant Biol. 2020, 20, 218. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Chourasia, K.N.; Naga, K.C.; Kumar, D.; Das, S.K.; Zinta, G. Mechanistic insights on melatonin-mediated drought stress mitigation in plants. Physiol. Plant. 2021, 172, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.N.; Jin, X.J.; Zhang, Y.X.; Ren, C.Y.; Zhang, M.C.; Wang, M.X. Effects of melatonin on photosynthesis and soybean seed growth during grain filling under drought stress. Photosynthetica 2019, 57, 512–520. [Google Scholar] [CrossRef]
- Çakir, R. Effect of water stress at different development stages on vegetative and reproductive growth of corn. Field Crop. Res. 2004, 89, 1–16. [Google Scholar] [CrossRef]
- Long, J.; Dong, M.; Wang, C.; Miao, Y. Effects of drought and salt stress on seed germination and seedling growth of Elymus nutans. PeerJ 2023, 11, e15968. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Shahid, R.; Ren, M.-X.; Naz, S.; Altaf, M.M.; Khan, L.U.; Tiwari, R.K.; Lal, M.K.; Shahid, M.A.; Kumar, R.; et al. Melatonin improves drought stress tolerance of tomato by modulating plant growth, root architecture, photosynthesis, and antioxidant defense system. Antioxidants 2022, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.H.; Li, D.X.; Liu, L.T.; Sun, H.C.; Zhu, L.X.; Zhang, K.; Zhao, H.M.; Zhang, Y.J.; Li, A.C.; Bai, Z.Y.; et al. Seed Priming With Melatonin Promotes Seed Germination and Seedling Growth of Triticale hexaploide L. Under PEG-6000 Induced Drought Stress. Front. Plant Sci. 2022, 13, 932912. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.N.; Avila, R.G.; de Souza, K.R.D.; Azevedo, L.M.; Alves, J.D. Melatonin reduces oxidative stress and promotes drought tolerance in young Coffea arabica L. plants. Agric. Water Manag. 2019, 211, 37–47. [Google Scholar] [CrossRef]
- Jiang, X.W.; Li, H.Q.; Song, X.Y. Seed priming with melatonin effects on seed germination and seedling growth in maize under salinity stress. Pak. J. Bot. 2016, 48, 1345–1352. [Google Scholar]
- Kubala, S.; Wojtyla, Ł.; Quinet, M.; Lechowska, K.; Lutts, S.; Garnczarska, M. Enhanced expression of the proline synthesis gene P5CSA in relation to seed osmopriming improvement of Brassica napus germination under salinity stress. J. Plant Physiol. 2015, 183, 1–12. [Google Scholar] [CrossRef]
- Chen, K.; Arora, R. Priming memory invokes seed stress-tolerance. Environ. Exp. Bot. 2013, 94, 33–45. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.-K. Epigenetic regulation of stress responses in plants. Curr. Opin. Plant Biol. 2009, 12, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.A.; Matthes, M.C.; Napier, J.A.; Pickett, J.A. Stressful memories of plants: Evidence and possible mechanisms. Plant Sci. 2007, 173, 603–608. [Google Scholar] [CrossRef]
- Corbineau, F.; Taskiran-Özbingöl, N.; El-Maarouf-Bouteau, H. Improvement of seed quality by priming: Concept and biological basis. Seeds 2023, 2, 101–115. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Okamoto, M.; Koshiba, T.; Kamiya, Y.; Nambara, E. Genome-wide profiling of stored mRNA in Ara-bidopsis thaliana seed germination: Epigenetic and genetic regulation of transcription in seed. Plant J. 2005, 41, 697–709. [Google Scholar] [CrossRef]
- Sridha, S.; Wu, K. Identification of AtHD2C as a novel regulator of abscisic acid responses in Arabidopsis. Plant J. 2006, 46, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Liu, X.; Luo, M.; Yang, S.; Wu, K. Involvement of histone modifications in plant abiotic stress responses. J. Integr. Plant Biol. 2013, 55, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Hou, X.; Duan, W.; Yin, B.; Ren, J.; Wang, Y.; Liu, X.; Gu, L.; Zhen, W. Screening and evaluation of drought resistance traits of winter wheat in the North China Plain. Front. Plant Sci. 2023, 14, 1194759. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Dang, H.K.; Zheng, C.L.; Li, K.J.; Ma, J.Y. Fluorescence characteristics and drought resistance of wheat under different irrigation regimes. J. Triticeae Crops 2017, 37, 1434–1444. [Google Scholar]
- Fu, Y.; Lang, X.; Hamani, A.K.M.; Weihao, S.; Wang, H.; Amin, A.S.; Wang, X.; Qin, A.; Gao, Y. Foliar Application of Melatonin Positively Affects the Physio-Biochemical Characteristics of Cotton (Gossypium hirsutum L.) under the Combined Effects of Low Temperature and Salinity Stress. Plants 2023, 12, 3730. [Google Scholar] [CrossRef]
- Gal, A.; Hendel, E.; Peleg, Z.; Schwartz, N.; Sade, N. Measuring the hydraulic conductivity of grass root systems. Curr. Protoc. Plant Biol. 2020, 5, e20110. [Google Scholar] [CrossRef]
- Dey, G.; Basu, R.N. Studies on the maintenance of seed viability of sunflower (Helianthus annuus L.) by physico-chemical treatments. Indian J. Plant Physiol. 1982, 25, 87–97. [Google Scholar]
- Muccifora, S.; Castillo-Michel, H.; Barbieri, F.; Bellani, L.; Castiglione, M.R.; Spanò, C.; del Real, A.E.P.; Giorgetti, L.; Tassi, E.L. Synchrotron Radiation Spectroscopy and Transmission Electron Microscopy Techniques to Evaluate TiO2 NPs Incorporation, Speciation, and Impact on Root Cells Ultrastructure of Pisum sativum L. Plants. Nanomaterials 2021, 11, 921. [Google Scholar] [CrossRef] [PubMed]
- Zotz, G.; Patino, S.; Tyree, M.T. Water relations and hydraulic architecture of woody hemiepiphytes. J. Exp. Bot. 1997, 48, 1825–1833. [Google Scholar] [CrossRef]
- Li, P.H.; Li, H.; Liu, Z.J.; Zhuang, Y.; Wei, M.; Gu, Y.Y.; Liu, Y.X.; Sun, X.Q.; Tang, Y.Y.; Yue, L.; et al. Characterization of the ‘Oat-Like Rice’ Caused by a Novel Allele OsMADS1Olr Reveals Vital Importance of OsMADS1 in Regulating Grain Shape in Oryza sativa L. Rice 2020, 13, 73. [Google Scholar] [CrossRef] [PubMed]









| SOD | POD | CAT | ||||||
|---|---|---|---|---|---|---|---|---|
| (U mg−1 prot−1 DW) | (U g−1 prot−1 DW) | (μmol−1min−1 mg−1 prot−1 DW) | ||||||
| Shoot | Root | Shoot | Root | Shoot | Root | |||
| H 4399 | CK | N | 50.85 ± 2.89 d | 47.58 ± 1.83 ef | 0.93 ± 0.10 e | 16.15 ± 0.24 fg | 2.45 ± 0.10 de | 2.65 ± 0.05 e |
| W | 31.45 ± 0.56 ef | 56.36 ± 1.20 ef | 1.46 ± 0.14 cd | 23.38 ± 0.75 de | 2.27 ± 0.13 e | 2.64 ± 0.32 e | ||
| M | 31.07 ± 0.17 ef | 82.96 ± 3.41 e | 1.02 ± 0.11 de | 26.24 ± 3.41 cd | 2.35 ± 0.11 e | 4.39 ± 0.23 d | ||
| PEG | N | 53.85 ± 1.59 d | 149.60 ± 9.03 d | 1.32 ± 0.05 cde | 15.18 ± 0.39 g | 3.28 ± 0.10 c | 8.29 ± 0.22 b | |
| W | 27.43 ± 4.73 f | 342.25 ± 17.64 b | 1.08 ± 0.07 de | 13.39 ± 0.60 g | 2.32 ± 0.05 e | 4.47 ± 0.26 d | ||
| M | 30.68 ± 0.69 ef | 120.85 ± 6.21 d | 1.20 ± 0.02 cde | 15.01 ± 0.50 g | 2.73 ± 0.26 cde | 4.97 ± 0.25 d | ||
| NaCl | N | 75.12 ± 1.17 b | 187.93 ± 12.61 c | 1.42 ± 0.06 cde | 39.31 ± 0.95 b | 2.32 ± 0.15 e | 7.74 ± 0.81 bc | |
| W | 37.27 ± 1.68 e | 76.90 ± 1.27 e | 1.63 ± 0.11 c | 19.83 ± 0.88 ef | 2.92 ± 0.07 cd | 0.83 ± 0.13 f | ||
| M | 49.16 ± 0.89 d | 149.05 ± 5.36 d | 1.18 ± 0.02 cde | 26.11 ± 1.36 cd | 3.22 ± 0.01 c | 2.58 ± 0.21 e | ||
| PEG + NaCl | N | 94.89 ± 2.09 a | 437.52 ± 35.16 a | 4.92 ± 0.16 b | 44.72 ± 0.84 a | 3.03 ± 0.17 c | 11.91 ± 0.34 a | |
| W | 69.65 ± 3.72 bc | 213.28 ± 7.70 c | 5.80 ± 0.39 a | 29.14 ± 2.53 c | 5.65 ± 0.38 a | 8.81 ± 0.49 b | ||
| M | 65.88 ± 3.85 c | 18.85 ± 0.48 f | 6.13 ± 0.22 a | 17.02 ± 0.60 fg | 4.87 ± 0.19 b | 6.78 ± 0.15 c | ||
| Y 1212 | CK | N | 54.88 ± 1.49 f | 104.14 ± 1.12 g | 1.23 ± 0.10 de | 30.35 ± 0.58 de | 3.12 ± 0.06 f | 5.99 ± 0.62 de |
| W | 124.94 ± 1.84 b | 362.57 ± 5.85 f | 0.79 ± 0.05 g | 29.14 ± 0.41 e | 4.87 ± 0.08 e | 5.24 ± 0.93 ef | ||
| M | 102.24 ± 0.40 c | 458.96 ± 9.00 e | 1.83 ± 0.11 b | 46.52 ± 3.26 a | 4.94 ± 0.06 de | 10.76 ± 1.26 ab | ||
| PEG | N | 74.60 ± 1.39 e | 160.98 ± 11.43 g | 0.92 ± 0.04 fg | 27.25 ± 1.36 e | 3.15 ± 0.04 f | 8.91 ± 1.06 bc | |
| W | 124.17 ± 1.46 b | 2656.57 ± 42.03 a | 1.61 ± 0.03 c | 29.70 ± 1.00 de | 5.30 ± 0.24 cd | 10.92 ± 1.08 ab | ||
| M | 103.34 ± 1.40 c | 1717.18 ± 47.65 b | 1.88 ± 0.02 b | 28.73 ± 0.35 e | 4.60 ± 0.01 e | 7.44 ± 0.56 cde | ||
| NaCl | N | 49.21 ± 7.96 f | 137.01 ± 1.56 g | 1.57 ± 0.03 c | 34.24 ± 0.54 c | 2.63 ± 0.19 g | 3.35 ± 0.64 f | |
| W | 147.93 ± 3.02 a | 902.13 ± 33.12 cd | 2.01 ± 0.08 b | 32.82 ± 0.45 cd | 7.05 ± 0.17 a | 6.63 ± 0.33 cde | ||
| M | 145.31 ± 1.69 a | 520.22 ± 37.91 e | 1.34 ± 0.04 d | 29.56 ± 0.30 de | 5.38 ± 0.23 c | 7.95 ± 0.46 cd | ||
| PEG + NaCl | N | 76.97 ± 3.79 e | 532.12 ± 12.55 e | 4.34 ± 0.16 a | 42.97 ± 0.56 b | 1.86 ± 0.03 h | 12.35 ± 0.45 a | |
| W | 142.15 ± 0.49 a | 852.59 ± 17.53 d | 1.04 ± 0.05 ef | 40.00 ± 1.12 b | 5.98 ± 0.10 b | 10.57 ± 0.48 ab | ||
| M | 87.55 ± 0.87 d | 936.84 ± 22.3 c | 0.51 ± 0.01 h | 40.83 ± 0.09 b | 3.43 ± 0.04 f | 9.05 ± 0.53 bc | ||
| X 19 | CK | N | 57.35 ± 1.72 d | 63.92 ± 0.94 g | 1.09 ± 0.08 e | 21.64 ± 0.15 f | 3.44 ± 0.05 b | 2.65 ± 0.61 fg |
| W | 39.33 ± 1.59 f | 102.09 ± 3.24 efg | 0.91 ± 0.04 ef | 31.12 ± 3.19 cde | 2.60 ± 0.09 d | 3.89 ± 0.12 f | ||
| M | 49.58 ± 0.62 e | 155.56 ± 4.62 d | 0.60 ± 0.02 fg | 39.70 ± 4.72 b | 2.65 ± 0.09 cd | 5.80 ± 0.48 e | ||
| PEG | N | 60.26 ± 1.01 d | 212.85 ± 17.77 c | 1.03 ± 0.01 e | 25.39 ± 0.67 ef | 3.46 ± 0.10 b | 9.63 ± 0.31 b | |
| W | 48.86 ± 1.06 e | 483.80 ± 12.20 a | 0.32 ± 0.04 g | 37.87 ± 1.06 bc | 2.49 ± 0.01 d | 8.68 ± 0.17 bc | ||
| M | 38.67 ± 1.65 f | 160.99 ± 20.26 d | 0.66 ± 0.05 fg | 33.46 ± 2.40 bcd | 2.58 ± 0.10 d | 5.81 ± 0.46 e | ||
| NaCl | N | 66.73 ± 1.12 c | 150.79 ± 3.98 de | 1.02 ± 0.06 e | 37.50 ± 0.44 bcd | 3.33 ± 0.18 b | 1.96 ± 0.52 g | |
| W | 48.95 ± 0.89 e | 104.14 ± 2.95 efg | 0.87 ± 0.04 ef | 35.43 ± 0.63 bcd | 2.91 ± 0.02 c | 3.89 ± 0.51 f | ||
| M | 71.74 ± 0.95 c | 119.81 ± 4.76 def | 1.70 ± 0.17 d | 61.48 ± 1.67 a | 3.58 ± 0.11 b | 7.63 ± 1.12 cd | ||
| PEG + NaCl | N | 145.96 ± 3.51 a | 69.60 ± 0.46 fg | 7.02 ± 0.19 a | 31.17 ± 0.40 cde | 2.94 ± 0.13 c | 6.65 ± 0.12 de | |
| W | 89.85 ± 1.51 b | 346.12 ± 36.88 b | 3.14 ± 0.14 b | 30.57 ± 0.88 de | 4.29 ± 0.03 a | 13.78 ± 0.01 a | ||
| M | 66.03 ± 4.49 c | 496.57 ± 33.80a | 2.17 ± 0.24 c | 25.46 ± 2.77 ef | 4.01 ± 0.07 a | 9.26 ± 0.04 b | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Li, P.; Si, Z.; Ma, S.; Gao, Y. Seeds Priming with Melatonin Improves Root Hydraulic Conductivity of Wheat Varieties under Drought, Salinity, and Combined Stress. Int. J. Mol. Sci. 2024, 25, 5055. https://doi.org/10.3390/ijms25095055
Fu Y, Li P, Si Z, Ma S, Gao Y. Seeds Priming with Melatonin Improves Root Hydraulic Conductivity of Wheat Varieties under Drought, Salinity, and Combined Stress. International Journal of Molecular Sciences. 2024; 25(9):5055. https://doi.org/10.3390/ijms25095055
Chicago/Turabian StyleFu, Yuanyuan, Penghui Li, Zhuanyun Si, Shoutian Ma, and Yang Gao. 2024. "Seeds Priming with Melatonin Improves Root Hydraulic Conductivity of Wheat Varieties under Drought, Salinity, and Combined Stress" International Journal of Molecular Sciences 25, no. 9: 5055. https://doi.org/10.3390/ijms25095055





