Targeting Neutrophil Extracellular Trap Formation: Exploring Promising Pharmacological Strategies for the Treatment of Preeclampsia
Abstract
:1. Introduction
2. Activation of Neutrophils in PE
3. Neutrophils in the Uteroplacental Microvasculature in PE
4. NETs
5. NETs in PE
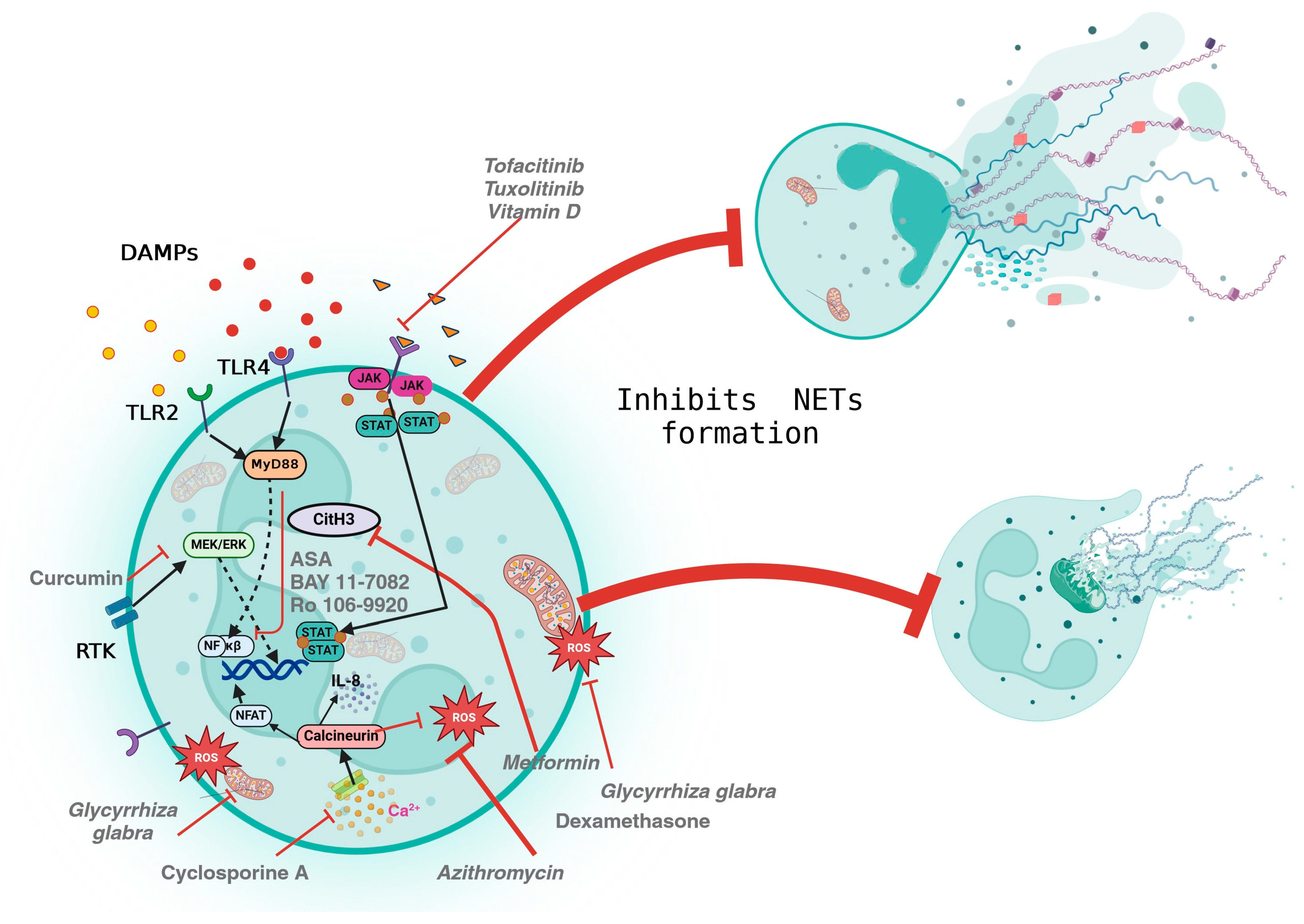
6. Pharmacological Modulation of NETs
| Drugs Substance | Mechanism of Action | Evaluated for NOX-Dependent (NOX-D) and ROS-Independent (ROX-I) NET Formation | Clinical Trials/Models/Examples | Mutagenicity and Genotoxicity In Vitro |
|---|---|---|---|---|
| Drugs | ||||
| Aspirin (ASA) | Human neutrophils were stimulated with Phorbol 12-myristate 13-acetate (PMA) or TNF-α. In addition, ASA, BAY 11-7082, and Ro 106-9920 prevented the formation of NETs by reducing the phosphorylation of the p65 subunit of NF-κβ [84]. | NOX-D | In isolated neutrophils stimulated with sodium hydroxide, ASA can enhance the migration of corneal epithelial cells (HCEs) and reduce the formation of NETs [96]. | ASA can protect against genotoxicity [97]. |
| Dexamethasone | Inhibits neutrophil functions such as intracellular ROS, degranulation, and NETosis. | NOX-D | Neutrophils cultured with dexamethasone showed reduced NET formation, after stimulation with PMA [98]. | It can induce significant DNA damage in human cells; however, it passes the Ames/Salmonella assay [99,100]. |
| Resveratrol | Decreases the release of free DNA from neutrophils and NETosis | NOX-D | During in vitro tests in the presence of PMA, it was shown that resveratrol decreases the formation of NETs and cytokine production in healthy controls and with COVID-19 [101]. | Despite its genotoxic effects, it does not cause mutagenesis and is used for its genotoxic activity against gastric cell adenocarcinoma [102,103]. |
| Cyclosporine A | Inhibits IL-8-induced NETosis by inhibiting the calcineurin pathway. | NOX-D | In isolated neutrophils, stimulated with PMA or ionomycin, treated with Cyclosporine A or ascomycin, the formation of NETs decreases [104]. | It is not genotoxic in humans. It inhibits the protein phosphatase calcineurin and can induce lymphoma in Xpa/p53 mice [105]. |
| Azithromycin | Decreases the production of ROS | NOX-D | Pre-treatment with Azithromycin decreases NETosis in neutrophils isolated from PMA-stimulated healthy subjects. This effect is observed at low doses [106]. | It does not induce mutations or chromosomal aberrations in microbial or mammalian cells [107]. |
| Chloramphenicol | Reduces the formation of NETs, possibly by inhibiting myeloperoxidase (MPO) | NOX-D | Pre-treatment with chloramphenicol reduces PMA-induced NET release [94]. | In rodents and human cells, it is a pro-mutagenic compound [108]. |
| Metformin | Affects nuclear dynamics (delobulation and decondensation) as well as PKC-βII membrane translocation and NADPH oxidase activation in neutrophils [109]. | NOX-D | Metformin decreases NETosis and its components such as elastase, proteinase-3, histones, and double-strand DNA in PMA-stimulated neutrophils in in vitro and clinical trial samples [109]. | There is conflicting evidence about the effects of metformin. Micronucleus assay suggests it may be genotoxic; however, analyses using chromosomal aberration (CA) and cytokinesis-block micronucleus (CBMN) assay report that it has a radioprotective effect on DNA damage and apoptosis in human lymphocytes [110,111]. |
| Hydroxychloroquine | Inhibits the expression of PAC4, Rac2, and the formation of NETs | NOX-D | Hydroxychloroquine alleviates hepatic ischemia/reperfusion (IR) injury in severe combined immunodeficiency (SCID) mice and C57BL/6 mice by inhibiting NETosis [91]. | It induces both oxidative DNA damage detected by 8-oxodG and the induction of mutants in mouse embryonic fibroblasts [112]. |
| Heparin | NET molecules, such as neutrophil elastase, interact with heparin and heparin oligomers to form molecular complexes that can regulate NETosis [113]. Low molecular weight heparin (LMWH) can repair nucleosome/histone 3-mediated damage in trophoblasts [69]. | -- | In vitro studies have shown that heparinized adsorbents such as heparin sepharose deplete PF4, histones/nucleosomes, and HMGB1 [114]. Heparin pre-treatment decreased serum and lung NETs in a C57BL/6J mice model [115]. Circulating histones bound to H3 and H4 nucleosomes are increased in patients with preeclampsia and intrauterine growth restriction. H3 affects extravillous trophoblast migration, invasion, and survival. This effect can be reversed in vitro by LMWH, but not with ASA [69]. | LMWH does not show any mutagenic activity [116,117]. |
| Vitamin D | Vitamin D supplementation has been shown to reduce the risk of preeclampsia [118], as well as decrease the generation of NETs, particularly when combined with omega-3 PUFAs [119]. | NOX-D | PMA-stimulated neutrophils from patients with systemic lupus erythematosus (SLE) and hypovitaminosis D were treated with calcitriol/1,25(OH)2D3. The authors reported a dose-independent decrease in externalised neutrophil elastase (NE) during NETosis [120]. | In cancer rodents treated with cyclophosphamide, it reduced the frequency of chromosomal aberrations in Chinese hamster lung cells and reduced micronuclei and lymphocyte damage in mice [121]. |
| Disulfiram | Inhibits NETs [122]. | NOX-D | It reduced NETs and perivascular fibrosis and downregulated innate immune and complement/coagulation pathways [111]. | -- |
| Curcumin | Inhibits the generation of NETs by suppressing the MEK/ERK pathway [123]. | NOX-D | In a mouse model, curcumin was shown to reduce hepatic ischemia-reperfusion injury by inhibiting NET formation [91]. | High concentrations are cytotoxic and increase the frequency of micronuclei in PC12 cells; at low doses, it reduces the number of micronuclei induced by cisplatin [124]. |
| Phosphodiesterase Type-4 (PDE4) inhibitors such as Roflumilast (Daliresp), Apremilast (Otezla), and Rolipram | Roflumilast blocks PDE4 and reduces in vitro and in vivo NETosis in animal models [125]. Inhibition of PDE4 by rolipram prevents the adhesion of platelets and neutrophils, which involves members of the Src family kinase (SFK) [126,127]. | ROX-I | Clinical trials have been conducted with Roflumilast for severe chronic obstructive pulmonary disease (COPD) and with Otezla for psoriasis [128]. | -- |
| Glycyrrhiza glabra | Inhibits ROS, mitochondrial ROS (mtROS), NET generation, and cytokine release. | -- | In an animal model, Glycyrrhiza glabra was proven to decrease COVID-19 pathology by reducing NETosis [129]. | -- |
| Protein molecules | ||||
| Activated protein C (APC) | Cleaves and detoxifies extracellular histones and its effect on reducing NETs dependent on endothelial protein C receptor (EPCR), protease-activated receptor 3 (PAR3), and macrophage antigen-1 (Mac-1) [130]. | -- | A clinical trial was conducted evaluating the safety and efficacy of recombinant human-activated protein C (rhAPC; drotrecogin alfa [activated]) in preeclampsia, but further studies are needed [131]. APC variants have been designed to have a greater ability to destroy histone H3 with fewer anticoagulant properties [132]. | Drotrecogin alfa (activated) has not been studied for carcinogenicity [133]. |
| Recombinant human DNase I (rhDNase I, rhDNase, Pulmozyme®, dornase alfa) | Hydrolyses extracellular DNA released by neutrophils [134]. | -- | Intravenous administration of rhDNase to mice degraded NETs and attenuated coagulopathy in the acute respiratory distress syndrome (ARDS) model [135]. | It does not show cytotoxicity in human peripheral blood mononuclear cells [136]. |
7. Therapeutic Approaches to Inhibit NET Formation in PE
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robillard, P.Y.; Dekker, G.; Iacobelli, S.; Chaouat, G. An essay of reflection: Why does preeclampsia exist in humans, and why are there such huge geographical differences in epidemiology? J. Reprod. Immunol. 2016, 114, 44–47. [Google Scholar] [CrossRef]
- Elgari, M.M.; Khabour, O.F.; Alhag, S.M. Correlations between changes in hematological indices of mothers with preeclampsia and umbilical cord blood of newborns. Clin. Exp. Hypertens. 2019, 41, 58–61. [Google Scholar] [CrossRef]
- Schindler, A.E. New data about preeclampsia: Some possibilities of prevention. Gynecol. Endocrinol. 2018, 34, 636–637. [Google Scholar] [CrossRef]
- Roberts, J.M.; Rich-Edwards, J.W.; McElrath, T.F.; Garmire, L.; Myatt, L.; Global Pregnancy Collaboration. Subtypes of Preeclampsia: Recognition and Determining Clinical Usefulness. Hypertension 2021, 77, 1430–1441. [Google Scholar] [CrossRef]
- Gyselaers, W. Preeclampsia Is a Syndrome with a Cascade of Pathophysiologic Events. J. Clin. Med. 2020, 9, 2245. [Google Scholar] [CrossRef]
- Shokry, M.; Bedaiwy, M.A.; Fathalla, M.M.; Alsemary, A.; Elwakil, S.; Murphy, A. Maternal serum placental growth factor and soluble fms-like tyrosine kinase 1 as early predictors of preeclampsia. Acta Obstet. Gynecol. Scand. 2010, 89, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Tamás, P.; Kovács, K.; Várnagy, Á.; Farkas, B.; Alemu Wami, G.; Bódis, J. Preeclampsia subtypes: Clinical aspects regarding pathogenesis, signs, and management with special attention to diuretic administration. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 274, 175–181. [Google Scholar] [CrossRef]
- Celik, C.; Gezginç, K.; Altintepe, L.; Tonbul, H.Z.; Yaman, S.T.; Akyürek, C.; Türk, S. Results of the pregnancies with HELLP syndrome. Ren. Fail. 2003, 25, 613–618. [Google Scholar] [CrossRef]
- Dennis, A.T. Cardiac Function in Women with Preeclampsia; University of Melbourne, Department of Pharmacology: Parkville, Australia, 2010. [Google Scholar]
- Wang, Y.; Gu, Y.; Lucas, M.J. Expression of thrombin receptors in endothelial cells and neutrophils from normal and preeclamptic pregnancies. J. Clin. Endocrinol. Metab. 2002, 87, 3728–3734. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, Y.; Philibert, L.; Lucas, M.J. Neutrophil activation induced by placental factors in normal and pre-eclamptic pregnancies in vitro. Placenta 2001, 22, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Crocker, I.P.; Wellings, R.P.; Fletcher, J.; Baker, P.N. Neutrophil function in women with pre-eclampsia. Br. J. Obstet. Gynaecol. 1999, 106, 822–828. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Grieshaber-Bouyer, R.; Nigrovic, P.A. Neutrophil Heterogeneity as Therapeutic Opportunity in Immune-Mediated Disease. Front. Immunol. 2019, 10, 346. [Google Scholar] [CrossRef]
- McKenna, E.; Mhaonaigh, A.U.; Wubben, R.; Dwivedi, A.; Hurley, T.; Kelly, L.A.; Stevenson, N.J.; Little, M.A.; Molloy, E.J. Neutrophils: Need for Standardized Nomenclature. Front. Immunol. 2021, 12, 602963. [Google Scholar] [CrossRef]
- Mihaila, A.C.; Ciortan, L.; Macarie, R.D.; Vadana, M.; Cecoltan, S.; Preda, M.B.; Hudita, A.; Gan, A.M.; Jakobsson, G.; Tucureanu, M.M.; et al. Transcriptional Profiling and Functional Analysis of N1/N2 Neutrophils Reveal an Immunomodulatory Effect of S100A9-Blockade on the Pro-Inflammatory N1 Subpopulation. Front. Immunol. 2021, 10, 708770. [Google Scholar] [CrossRef]
- Vono, M.; Lin, A.; Norrby-Teglund, A.; Koup, R.A.; Liang, F.; Loré, K. Neutrophils acquire the capacity for antigen presentation to memory CD4+ T cells in vitro and ex vivo. Blood 2017, 129, 1991–2001. [Google Scholar] [CrossRef]
- Dolislager, C.G.; Callahan, S.M.; Donohoe, D.R.; Johnson, J.G. Campylobacter jejuni induces differentiation of human neutrophils to the CD16hi /CD62Llo subtype. J. Leukoc. Biol. 2022, 112, 1457–1470. [Google Scholar] [CrossRef]
- Granai, M.; Warm, V.; Vogelsberg, A.; Milla, J.; Greif, K.; Vogel, U.; Bakchoul, T.; Rosenberger, P.; Quintanilla-Martinez, L.; Schürch, C.M.; et al. Impact of P-selectin-PSGL-1 Axis on Platelet-Endothelium-Leukocyte Interactions in Fatal COVID-19. Lab. Investig. 2023, 103, 100179. [Google Scholar] [CrossRef]
- Dehghani, T.; Panitch, A. Endothelial cells, neutrophils and platelets: Getting to the bottom of an inflammatory tri-angle. Open Biol. 2020, 10, 200161. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gebhardt, S.; Hillermann, R.; Holzgreve, W.; Hahn, S. Analysis of plasma elastase levels in early and late onset preeclampsia. Arch. Gynecol. Obstet. 2006, 273, 239–242. [Google Scholar] [CrossRef]
- Zhong, X.Y.; Gebhardt, S.; Hillermann, R.; Tofa, K.C.; Holzgreve, W.; Hahn, S. Circulatory nucleosome levels are significantly increased in early and late-onset preeclampsia. Prenat. Diagn. 2005, 25, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.A.; Jebbink, J.; Nieuwland, R.; Faas, M.M.; Boer, K.; Sturk, A.; Van Der Post, J.A. Leukocyte activation and circulating leukocyte-derived microparticles in preeclampsia. Am. J. Reprod. Immunol. 2009, 61, 346–359. [Google Scholar] [CrossRef]
- von Dadelszen, P.; Watson, R.W.; Noorwali, F.; Marshall, J.C.; Parodo, J.; Farine, D.; Lye, S.J.; Ritchie, J.W.; Rotstein, O.D. Maternal neutrophil apoptosis in normal pregnancy, preeclampsia, and normotensive intrauterine growth restriction. Am. J. Obstet. Gynecol. 1999, 181, 408–414. [Google Scholar] [CrossRef]
- Xin, H.; Wang, H.L. Expression changes and clinical significance of annexin V in maternal blood and placenta in patients with preeclampsia. Zhonghua Fu Chan Ke Za Zhi 2011, 46, 88–93. (In Chinese) [Google Scholar] [PubMed]
- Barden, A.; Ritchie, J.; Walters, B.; Michael, C.; Rivera, J.; Mori, T.; Croft, K.; Beilin, L. Study of plasma factors associated with neutrophil activation and lipid peroxidation in preeclampsia. Hypertension 2001, 38, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, F.; Bretelle, F.; D’ercole, C.; Boubli, L.; Sampol, J.; Dignat-George, F. Neutrophil activation in preeclampsia and isolated intrauterine growth restriction. Am. J. Obstet. Gynecol. 2000, 183, 1558–1563. [Google Scholar] [CrossRef]
- Hu, Y.; Li, H.; Yan, R.; Wang, C.; Wang, Y.; Zhang, C.; Liu, M.; Zhou, T.; Zhu, W.; Zhang, H.; et al. Increased Neutrophil Activation and Plasma DNA Levels in Patients with Pre-Eclampsia. Thromb. Haemost. 2018, 118, 2064–2073. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef]
- Barden, A.; Graham, D.; Beilin, L.J.; Ritchie, J.; Baker, R.; Walters, B.N.; Michael, C.A. Neutrophil CD11B expression and neutrophil activation in pre-eclampsia. Clin. Sci. 1997, 92, 37–44. [Google Scholar] [CrossRef]
- Luppi, P.; Tse, H.; Lain, K.Y.; Markovic, N.; Piganelli, J.D.; DeLoia, J.A. Preeclampsia activates circulating immune cells with engagement of the NF-kappaβ pathway. Am. J. Reprod. Immunol. 2006, 56, 135–144. [Google Scholar] [CrossRef]
- Han, C.; Chen, Y.Y.; Dong, J.F. Prothrombotic state associated with preeclampsia. Curr. Opin. Hematol. 2021, 28, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Mellembakken, J.R.; Aukrust, P.; Olafsen, M.K.; Ueland, T.; Hestdal, K.; Videm, V. Activation of leukocytes during the uteroplacental passage in preeclampsia. Hypertension 2002, 39, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Leik, C.E.; Walsh, S.W. Neutrophils infiltrate resistance-sized vessels of subcutaneous fat in women with preeclampsia. Hypertension 2004, 44, 72–77. [Google Scholar] [CrossRef]
- Shah, T.J.; Walsh, S.W. Activation of NF-kappaβ and expression of COX-2 in association with neutrophil infiltration in systemic vascular tissue of women with preeclampsia. Am. J. Obstet. Gynecol. 2007, 196, 48.e1–48.e18. [Google Scholar] [CrossRef]
- Shukla, J.; Walsh, S.W. Neutrophil release of myeloperoxidase in systemic vasculature of obese women may put them at risk for preeclampsia. Reprod. Sci. 2015, 22, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Cadden, K.A.; Walsh, S.W. Neutrophils, but not lymphocytes or monocytes, infiltrate maternal systemic vasculature in women with preeclampsia. Hypertens. Pregnancy 2008, 27, 396–405. [Google Scholar] [CrossRef]
- Mincheva-Nilsson, L.; Baranov, V. Placenta-derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: Immune modulation for pregnancy success. Am. J. Reprod. Immunol. 2014, 72, 440–457. [Google Scholar] [CrossRef]
- Aly, A.S.; Khandelwal, M.; Zhao, J.; Mehmet, A.H.; Sammel, M.D.; Parry, S. Neutrophils are stimulated by syncytiotrophoblast microvillous membranes to generate superoxide radicals in women with preeclampsia. Am. J. Obstet. Gynecol. 2004, 190, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, Q.; Ding, H.; Zhao, G.; Wang, Z.; Cao, C.; Dai, Y.; Zheng, M.; Zhu, X.; Wu, Q.; et al. Placenta-derived IL-32β activates neutrophils to promote preeclampsia development. Cell. Mol. Immunol. 2021, 18, 979–991. [Google Scholar] [CrossRef]
- Kenny, E.F.; Herzig, A.; Krüger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; Bernuth, H.V.; Zychlinsky, A. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife 2017, 6, e24437. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Van der Zwan, L.P.; Scheffer, P.G.; Dekker, J.M.; Stehouwer, C.D.; Heine, R.J.; Teerlink, T. Hyperglycemia and oxidative stress strengthen the association between myeloperoxidase and blood pressure. Hypertension 2010, 55, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Mahat, R.K.; Singh, N.; Rathore, V. Association of myeloperoxidase with cardiovascular disease risk factors in prediabetic subjects. Diabetes Metab. Syndr. 2019, 13, 396–400. [Google Scholar] [CrossRef]
- Parker, H.; Winterbourn, C.C. Reactive oxidants and myeloperoxidase and their involvement in neutrophil extracellular traps. Front. Immunol. 2013, 3, 424. [Google Scholar] [CrossRef] [PubMed]
- Demkow, U. Molecular Mechanisms of Neutrophil Extracellular Trap (NETs) Degradation. Int. J. Mol. Sci. 2023, 24, 4896. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, J.; Zhang, Y.; Li, T.; Peng, J.; Zhou, H.; Zong, Z. Emerging Role of Neutrophil Extracellular Traps in Gastrointestinal Tumors: A Narrative Review. Int. J. Mol. Sci. 2022, 24, 334. [Google Scholar] [CrossRef]
- Chen, W.A.; Boskovic, D.S. Neutrophil Extracellular DNA Traps in Response to Infection or Inflammation, and the Roles of Platelet Interactions. Int. J. Mol. Sci. 2024, 25, 3025. [Google Scholar] [CrossRef]
- Tan, C.; Aziz, M.; Wang, P. The vitals of NETs. J. Leukoc. Biol. 2021, 110, 797–808. [Google Scholar] [CrossRef]
- Besteman, S.B.; Callaghan, A.; Hennus, M.P.; Westerlaken, G.H.A.; Meyaard, L.; Bont, L.L. Signal inhibitory receptor on leukocytes (SIRL)-1 and leukocyte- associated immunoglobulin-like receptor (LAIR)-1 regulate neutrophil function in infants. Clin. Immunol. 2020, 211, 108324. [Google Scholar] [CrossRef]
- Ode, Y.; Aziz, M.; Jin, H.; Arif, A.; Nicastro, J.G.; Wang, P. Cold-inducible RNA-binding Protein Induces Neutrophil Extracellular Traps in the Lungs during Sepsis. Sci. Rep. 2019, 9, 6252. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Z.; Li, L.; Zhang, Z.; Jin, X.; Wu, P.; Sun, S.; Pan, J.; Su, K.; Jia, F.; et al. Aged neutrophils form mitochondria-dependent vital NETs to promote breast cancer lung metasta-sis. J. Immunother. Cancer 2021, 9, e002875. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, S.; Takagaki, M.; Nakamura, H.; Nishida, T.; Terada, E.; Kadono, Y.; Izutsu, N.; Takenaka, T.; Matsui, Y.; Yamada, S.; et al. Association of Gut Microbiome with Early Brain Injury After Subarachnoid Hemorrhage: An Experimental Study. Transl. Stroke Res. 2024, 15, 87–100. [Google Scholar] [CrossRef]
- de Buhr, N.; von Köckritz-Blickwede, M. Detection, Visualization, and Quantification of Neutrophil Extracellular Traps (NETs) and NET Markers. Methods Mol. Biol. 2020, 2087, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Donkel, S.J.; Wolters, F.J.; Ikram, M.A.; de Maat, M.P.M. Circulating Myeloperoxidase (MPO)-DNA complexes as marker for Neutrophil Extracellular Traps (NETs) levels and the association with cardiovascular risk factors in the general population. PLoS ONE 2021, 16, e0253698. [Google Scholar] [CrossRef]
- Lee, K.H.; Cavanaugh, L.; Leung, H.; Yan, F.; Ahmadi, Z.; Chong, B.H.; Passam, F. Quantification of NETs-associated markers by flow cytometry and serum assays in patients with thrombosis and sepsis. Int. J. Lab. Hematol. 2018, 40, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Grilz, E.; Mauracher, L.M.; Posch, F.; Königsbrügge, O.; Zöchbauer-Müller, S.; Marosi, C.; Lang, I.; Pabinger, I.; Ay, C. Citrullinated histone H3, a biomarker for neutrophil extracellular trap formation, predicts the risk of mortality in patients with cancer. Br. J. Haematol. 2019, 186, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Kanayama, N.; El Maradny, E.; Maehara, K.; Bhuiyan, A.B.; Terao, T. Correlated plasma elastase and sera cytotoxicity in eclampsia. A possible role of endothelin-1 induced neutrophil activation in preeclampsia-eclampsia. Am. J. Hypertens. 1996, 9, 33–38. [Google Scholar] [CrossRef] [PubMed]
- von Leitner, E.C.; Klinke, A.; Atzler, D.; Slocum, J.L.; Lund, N.; Kielstein, J.T.; Maas, R.; Schmidt-Haupt, R.; Pekarova, M.; Hellwinkel, O.; et al. Pathogenic cycle between the endogenous nitric oxide synthase inhibitor asymmetrical dimethylarginine and the leukocyte-derived hemoprotein myeloperoxidase. Circulation 2011, 124, 2735–2745. [Google Scholar] [CrossRef]
- Rocha-Penha, L.; Caldeira-Dias, M.; Tanus-Santos, J.E.; de Carvalho Cavalli, R.; Sandrim, V.C. Myeloperoxidase in Hypertensive Disorders of Pregnancy and Its Relation With Nitric Oxide. Hypertension 2017, 69, 1173–1180. [Google Scholar] [CrossRef]
- Marder, W.; Knight, J.S.; Kaplan, M.J.; Somers, E.C.; Zhang, X.; O’Dell, A.A.; Padmanabhan, V.; Lieberman, R.W. Placental histology and neutrophil extracellular traps in lupus and pre-eclampsia pregnancies. Lupus Sci. Med. 2016, 3, e000134. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Hasler, P.; Gebhardt, S.; Holzgreve, W.; Hahn, S. Occurrence of neutrophil extracellular DNA traps (NETs) in pre-eclampsia: A link with elevated levels of cell-free DNA? Ann. N. Y. Acad. Sci. 2006, 1075, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Moodley, M.; Moodley, J.; Naicker, T. Neutrophil extracellular traps: The synergy source in the placentae of HIV infected women with pre-eclampsia. Pregnancy Hypertens. 2020, 20, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Hasler, P.; Holzgreve, W.; Gebhardt, S.; Hahn, S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum. Immunol. 2005, 66, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Romero, R.; Yeo, L.; Gomez-Lopez, N.; Chaemsaithong, P.; Jaovisidha, A.; Gotsch, F.; Erez, O. The etiology of preeclampsia. Am. J. Obstet. Gynecol. 2022, 226 (Suppl. S2), S844–S866. [Google Scholar] [CrossRef] [PubMed]
- Buttrup Larsen, S.; Wallukat, G.; Schimke, I.; Sandager, A.; Tvilum Christensen, T.; Uldbjerg, N.; Tørring, N. Functional autoantibodies against Endothelin-1 receptor type A and Angiotensin II receptor type 1 in patients with preeclampsia. Pregnancy Hypertens. 2018, 14, 189–194. [Google Scholar] [CrossRef]
- Elliott, S.E.; Parchim, N.F.; Liu, C.; Xia, Y.; Kellems, R.E.; Soffici, A.R.; Daugherty, P.S. Characterization of antibody specificities associated with preeclampsia. Hypertension 2014, 63, 1086–1093. [Google Scholar] [CrossRef]
- Shaarawy, M.; el-Mallah, S.Y.; el-Yamani, A.M. The prevalence of serum antineutrophil cytoplasmic autoantibodies in preeclampsia and eclampsia. J. Soc. Gynecol. Investig. 1997, 4, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Mutua, V.; Gershwin, L.J. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin. Rev. Allergy Immunol. 2021, 61, 194–211. [Google Scholar] [CrossRef]
- Kirchner, T.; Hermann, E.; Möller, S.; Klinger, M.; Solbach, W.; Laskay, T.; Behnen, M. Flavonoids and 5-aminosalicylic acid inhibit the formation of neutrophil extracellular traps. Mediat. Inflamm. 2013, 2013, 710239. [Google Scholar] [CrossRef]
- Palmroth, M.; Kuuliala, K.; Peltomaa, R.; Virtanen, A.; Kuuliala, A.; Kurttila, A.; Kinnunen, A.; Leirisalo-Repo, M.; Silvennoinen, O.; Isomäki, P. Tofacitinib Suppresses Several JAK-STAT Pathways in Rheumatoid Arthritis In Vivo and Baseline Signaling Profile Associates With Treatment Response. Front. Immunol. 2021, 12, 738481. [Google Scholar] [CrossRef] [PubMed]
- Malcova, H.; Milota, T.; Strizova, Z.; Cebecauerova, D.; Striz, I.; Sediva, A.; Horvath, R. Interleukin-1 Blockade in Polygenic Autoinflammatory Disorders: Where Are We now? Front. Pharmacol. 2021, 11, 619273. [Google Scholar] [CrossRef]
- Basyreva, L.Y.; Brodsky, I.B.; Gusev, A.A.; Zhapparova, O.N.; Mikhalchik, E.V.; Gusev, S.A.; Shor, D.B.; Dahan, S.; Blank, M.; Shoenfeld, Y. The effect of Intravenous Immunoglobulin (IVIG) on \textit{ex vivo} activation of human leukocytes. Hum. Antibodies 2016, 24, 39–44. [Google Scholar] [CrossRef]
- Lood, C.; Hughes, G.C. Neutrophil extracellular traps as a potential source of autoantigen in cocaine-associated autoimmunity. Rheumatology 2017, 56, 638–643. [Google Scholar] [CrossRef]
- Hair, P.S.; Enos, A.I.; Krishna, N.K.; Cunnion, K.M. Inhibition of Immune Complex Complement Activation and Neutrophil Extracellular Trap Formation by Peptide Inhibitor of Complement C1. Front. Immunol. 2018, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Hair, P.S.; Enos, A.I.; Krishna, N.K.; Cunnion, K.M. Inhibition of complement activation, myeloperoxidase, NET formation and oxidant activity by PIC1 peptide variants. PLoS ONE 2019, 14, e0226875. [Google Scholar] [CrossRef] [PubMed]
- Kajioka, H.; Kagawa, S.; Ito, A.; Yoshimoto, M.; Sakamoto, S.; Kikuchi, S.; Kuroda, S.; Yoshida, R.; Umeda, Y.; Noma, K.; et al. Targeting neutrophil extracellular traps with thrombomodulin prevents pancreatic cancer metastasis. Cancer Lett. 2021, 497, 1–13. [Google Scholar] [CrossRef]
- Tadie, J.M.; Bae, H.B.; Jiang, S.; Park, D.W.; Bell, C.P.; Yang, H.; Pittet, J.F.; Tracey, K.; Thannickal, V.J.; Abraham, E.; et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 304, L342–L349. [Google Scholar] [CrossRef]
- Wygrecka, M.; Kosanovic, D.; Wujak, L.; Reppe, K.; Henneke, I.; Frey, H.; Didiasova, M.; Kwapiszewska, G.; Marsh, L.M.; Baal, N.; et al. Antihistone Properties of C1 Esterase Inhibitor Protect against Lung Injury. Am. J. Respir. Crit. Care Med. 2017, 196, 186–199. [Google Scholar] [CrossRef]
- Kakigano, A.; Tomimatsu, T.; Mimura, K.; Kanayama, T.; Fujita, S.; Minato, K.; Kumasawa, K.; Taniguchi, Y.; Kanagawa, T.; Endo, M.; et al. Drug Repositioning for Preeclampsia Therapeutics by In Vitro Screening: Phosphodiesterase-5 Inhibitor Vardenafil Restores Endothelial Dysfunction via Induction of Placental Growth Factor. Reprod. Sci. 2015, 22, 1272–1280. [Google Scholar] [CrossRef]
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011, 7, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Koehn, C.D.; Yue, Y.; Li, S.; Thiele, G.M.; Hearth-Holmes, M.P.; Mikuls, T.R.; O’Dell, J.R.; Klassen, L.W.; Zhang, Z.; et al. Celastrol inhibits inflammatory stimuli-induced neutrophil extracellular trap formation. Curr. Mol. Med. 2015, 15, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Lapponi, M.J.; Carestia, A.; Landoni, V.I.; Rivadeneyra, L.; Etulain, J.; Negrotto, S.; Pozner, R.G.; Schattner, M. Regulation of neutrophil extracellular trap formation by anti-inflammatory drugs. J. Pharmacol. Exp. Ther. 2013, 345, 430–437. [Google Scholar] [CrossRef]
- Zafarani, A.; Razizadeh, M.H.; Haghi, A. Neutrophil extracellular traps in influenza infection. Heliyon 2023, 9, e23306. [Google Scholar] [CrossRef] [PubMed]
- Vorobjeva, N.V.; Pinegin, B.V. Effects of the antioxidants Trolox, Tiron and Tempol on neutrophil extracellular trap formation. Immunobiology 2016, 221, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qiao, C.; Zhao, L.; Jing, Q.; Xue, D.; Zhang, Y. Epigallocatechin-3-gallate reduces neutrophil extracellular trap formation and tissue injury in severe acute pancreatitis. J. Leukoc. Biol. 2022, 112, 1427–1443. [Google Scholar] [CrossRef]
- Ostafin, M.; Pruchniak, M.P.; Ciepiela, O.; Reznick, A.Z.; Demkow, U. Different procedures of diphenyleneiodonium chloride addition affect neutrophil extracellular trap formation. Anal. Biochem. 2016, 509, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yang, S.; Meng, D.; Wang, Q.; Ji, J. Targeting NADPH Oxidase and Integrin α5β1 to Inhibit Neutrophil Extracellular Traps-Mediated Metastasis in Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 16001. [Google Scholar] [CrossRef]
- Meier, A.; Chien, J.; Hobohm, L.; Patras, K.A.; Nizet, V.; Corriden, R. Inhibition of Human Neutrophil Extracellular Trap (NET) Production by Propofol and Lipid Emulsion. Front. Pharmacol. 2019, 10, 323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Q.; Wang, F.; Guo, X.; Liu, T.; Zhao, Y.; Gu, B.; Chen, H.; Li, Y. Hydroxychloroquine inhibiting neutrophil extracellular trap formation alleviates hepatic ischemia/reperfusion injury by blocking TLR9 in mice. Clin. Immunol. 2020, 216, 108461, Erratum in Clin. Immunol. 2021, 225, 108681. [Google Scholar] [CrossRef]
- Gavillet, M.; Martinod, K.; Renella, R.; Wagner, D.D.; Williams, D.A. A key role for Rac and Pak signaling in neutrophil extracellular traps (NETs) formation defines a new potential therapeutic target. Am. J. Hematol. 2018, 93, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Ivey, A.D.; Matthew Fagan, B.; Murthy, P.; Lotze, M.T.; Zeh, H.J.; Hazlehurst, L.A.; Geldenhuys, W.J.; Boone, B.A. Chloroquine reduces neutrophil extracellular trap (NET) formation through inhibition of peptidyl arginine deiminase 4 (PAD4). Clin. Exp. Immunol. 2023, 211, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Juha, M.; Molnár, A.; Jakus, Z.; Ledó, N. NETosis: An emerging therapeutic target in renal diseases. Front. Immunol. 2023, 14, 1253667. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, L.; Floresta, G.; Cilibrizzi, A.; Giovannoni, M.P. An Overview of PDE4 Inhibitors in Clinical Trials: 2010 to Early 2022. Molecules 2022, 27, 4964. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Zhang, Y.; Yuan, K.; Min, J.; Mou, Y.; Jin, X. Acetylsalicylic Acid Promotes Corneal Epithelium Migration by Regulating Neutrophil Extracellular Traps in Alkali Burn. Front. Immunol. 2020, 11, 551057. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Swacha, P.; Aung, K.M.; Gekara, N.O. Aspirin protects against genotoxicity by promoting genome repair. Cell Res. 2023, 33, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Hafkamp, F.M.J.; Mol, S.; Waqué, I.; De Jong, E.C. Dexamethasone, but Not Vitamin D or A, Dampens the Inflammatory Neutrophil Response to Protect At-risk COVID-19 Patients. Immune Netw. 2022, 22, e36. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Singh, J.R.; Dhillon, V.S.; Bali, D.; Paul, H. In vitro and in vivo genotoxicity evaluation of hormonal drugs. II. Dexamethasone. Mutat. Res. 1994, 308, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Biswas, D.; Mukherjee, A. High-altitude medicines: A short-term genotoxicity study. Toxicol. Ind. Health 2010, 26, 417–424. [Google Scholar] [CrossRef]
- de Souza Andrade, M.M.; Leal, V.N.C.; Fernandes, I.G.; Gozzi-Silva, S.C.; Beserra, D.R.; Oliveira, E.A.; Teixeira, F.M.E.; Yendo, T.M.; Sousa, M.D.G.T.; Teodoro, W.R.; et al. Resveratrol Downmodulates Neutrophil Extracellular Trap (NET) Generation by Neutrophils in Patients with Severe COVID-19. Antioxidants 2022, 11, 1690. [Google Scholar] [CrossRef]
- Fox, J.T.; Sakamuru, S.; Huang, R.; Teneva, N.; Simmons, S.O.; Xia, M.; Tice, R.R.; Austin, C.P.; Myung, K. High-throughput genotoxicity assay identifies antioxidants as inducers of DNA damage response and cell death. Proc. Natl. Acad. Sci. USA 2012, 109, 5423–5428. [Google Scholar] [CrossRef] [PubMed]
- Mitruţ, P.; Burada, F.; Enescu, A.; Scorei, R.; Badea, D.; Genunche-Dumitrescu, A.; Rogoz, I.; Manea, M. The genotoxicity study of resveratrol in primary gastric adenocarcinoma cell cultures. Rom. J. Morphol. Embryol. 2009, 50, 429–433. [Google Scholar] [PubMed]
- Gupta, A.K.; Giaglis, S.; Hasler, P.; Hahn, S. Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. PLoS ONE 2014, 9, e97088. [Google Scholar] [CrossRef] [PubMed]
- van Kesteren, P.C.; Beems, R.B.; Luijten, M.; Robinson, J.; de Vries, A.; van Steeg, H. DNA repair-deficient Xpa/p53 knockout mice are sensitive to the non-genotoxic carcinogen cyclosporine A: Escape of initiated cells from immunosurveillance? Carcinogenesis 2009, 30, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Bystrzycka, W.; Manda-Handzlik, A.; Sieczkowska, S.; Moskalik, A.; Demkow, U.; Ciepiela, O. Azithromycin and Chloramphenicol Diminish Neutrophil Extracellular Traps (NETs) Release. Int. J. Mol. Sci. 2017, 18, 2666. [Google Scholar] [CrossRef] [PubMed]
- Amacher, D.E.; Ellis, J.H., Jr.; Joyce, A.J.; Muehlbauer, P.A.; Turner, G.N.; Wahrenburg, M.G.; Holden, H.E.; Ray, V.A. Preclinical toxicology studies with azithromycin: Genetic toxicology evaluation. Mutat. Res. 1993, 300, 79–90. [Google Scholar] [CrossRef]
- Martelli, A.; Mattioli, F.; Pastorino, G.; Robbiano, L.; Allavena, A.; Brambilla, G. Genotoxicity testing of chloramphenicol in rodent and human cells. Mutat. Res. 1991, 260, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Menegazzo, L.; Scattolini, V.; Cappellari, R.; Bonora, B.M.; Albiero, M.; Bortolozzi, M.; Romanato, F.; Ceolotto, G.; Vigili de Kreutzeberg, S.; Avogaro, A.; et al. The antidiabetic drug metformin blunts NETosis in vitro and reduces circulating NETosis biomarkers in vivo. Acta Diabetol. 2018, 55, 593–601. [Google Scholar] [CrossRef]
- Harishankar, M.K.; Logeshwaran, S.; Sujeevan, S.; Aruljothi, K.N.; Dannie, M.A.; Devi, A. Genotoxicity evaluation of metformin and glimepiride by micronucleus assay in exfoliated urothelial cells of type 2 diabetes mellitus patients. Food Chem. Toxicol. 2015, 83, 146–150. [Google Scholar] [CrossRef]
- Cheki, M.; Shirazi, A.; Mahmoudzadeh, A.; Bazzaz, J.T.; Hosseinimehr, S.J. The radioprotective effect of metformin against cytotoxicity and genotoxicity induced by ionizing radiation in cultured human blood lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 809, 24–32. [Google Scholar] [CrossRef]
- Besaratinia, A.; Caliri, A.W.; Tommasi, S. Hydroxychloroquine induces oxidative DNA damage and mutation in mammalian cells. DNA Repair 2021, 106, 103180. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Du, Y.; Kaltashov, I.A. Towards better understanding of the heparin role in NETosis: Feasibility of using native mass spectrometry to monitor interactions of neutrophil elastase with heparin oligomers. Int. J. Mass. Spectrom. 2021, 463, 116550. [Google Scholar] [CrossRef]
- Ebeyer-Masotta, M.; Eichhorn, T.; Weiss, R.; Semak, V.; Lauková, L.; Fischer, M.B.; Weber, V. Heparin-Functionalized Adsorbents Eliminate Central Effectors of Immunothrombosis, including Platelet Factor 4, High-Mobility Group Box 1 Protein and Histones. Int. J. Mol. Sci. 2022, 23, 1823. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Mu, S.; Zhang, F.; Qiao, Y.; Wu, Y.; Zhang, Z.; Ma, X. Effect of heparin pretreatment on the level of neutrophil extracellular traps of serum and lung tissue in septic mice. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2017, 29, 337–341. (In Chinese) [Google Scholar] [CrossRef]
- Borelli, G.; Bertoli, D. Toxic and mutagenic effects of a low molecular weight heparin in rats. Arzneimittelforschung 1986, 36, 1256–1260. [Google Scholar]
- Bertoli, D.; Borelli, G. Peri- and postnatal, teratology and reproductive studies of a low molecular weight heparin in rats. Arzneimittelforschung 1986, 36, 1260–1263. [Google Scholar]
- Irwinda, R.; Hiksas, R.; Lokeswara, A.W.; Wibowo, N. Vitamin D supplementation higher than 2000 IU/day compared to lower dose on maternal-fetal outcome: Systematic review and meta-analysis. Womens Health 2022, 18, 17455057221111066. [Google Scholar] [CrossRef]
- Basyreva, L.Y.; Vakhrusheva, T.V.; Letkeman, Z.V.; Maximov, D.I.; Fedorova, E.A.; Panasenko, O.M.; Ostrovsky, E.M.; Gusev, S.A. Effect of Vitamin D3 in combination with Omega-3 Polyunsaturated Fatty Acids on NETosis in Type 2 Diabetes Mellitus Patients. Oxid. Med. Cell Longev. 2021, 2021, 8089696. [Google Scholar] [CrossRef]
- Handono, K.; Sidarta, Y.O.; Pradana, B.A.; Nugroho, R.A.; Hartono, I.A.; Kalim, H.; Endharti, A.T. Vitamin D prevents endothelial damage induced by increased neutrophil extracellular traps formation in patients with systemic lupus erythematosus. Acta Med. Indones. 2014, 46, 189–198. [Google Scholar]
- Liu, H.; Feng, X.; Wu, S.; Zong, T.; Li, B.; Zhang, Z. Vitamin D Resists Cyclophosphamide-Induced Genomic and DNA Damage in CHL Cells In Vitro and in Mice In Vivo. Nutr. Cancer 2019, 71, 1030–1039. [Google Scholar] [CrossRef]
- Adrover, J.M.; Carrau, L.; Daßler-Plenker, J.; Bram, Y.; Chandar, V.; Houghton, S.; Redmond, D.; Merrill, J.R.; Shevik, M.; tenOever, B.R.; et al. Disulfiram inhibits neutrophil extracellular trap formation and protects rodents from acute lung injury and SARS-CoV-2 infection. JCI Insight 2022, 7, e157342. [Google Scholar] [CrossRef]
- Zhu, C.; Shi, S.; Jiang, P.; Huang, X.; Zhao, J.; Jin, Y.; Shen, Y.; Zhou, X.; Liu, H.; Cai, J. Curcumin Alleviates Hepatic Ischemia-Reperfusion Injury by Inhibiting Neutrophil Extracellular Traps Formation. J. Investig. Surg. 2023, 36, 2164813. [Google Scholar] [CrossRef]
- Mendonça, L.M.; Dos Santos, G.C.; Antonucci, G.A.; Dos Santos, A.C.; Bianchi Mde, L.; Antunes, L.M. Evaluation of the cytotoxicity and genotoxicity of curcumin in PC12 cells. Mutat. Res. 2009, 675, 29–34. [Google Scholar] [CrossRef]
- Totani, L.; Amore, C.; Piccoli, A.; Dell’Elba, G.; Di Santo, A.; Plebani, R.; Pecce, R.; Martelli, N.; Rossi, A.; Ranucci, S.; et al. Type-4 Phosphodiesterase (PDE4) Blockade Reduces NETosis in Cystic Fibrosis. Front. Pharmacol. 2021, 12, 702677. [Google Scholar] [CrossRef]
- Totani, L.; Piccoli, A.; Dell’Elba, G.; Concetta, A.; Di Santo, A.; Martelli, N.; Federico, L.; Pamuklar, Z.; Smyth, S.S.; Evangelista, V. Phosphodiesterase type 4 blockade prevents platelet-mediated neutrophil recruitment at the site of vascular injury. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1689–1696. [Google Scholar] [CrossRef]
- Dastgheib, M.; Shetab-Boushehri, S.V.; Baeeri, M.; Gholami, M.; Karimi, M.Y.; Hosseini, A. Rolipram and pentoxifylline combination ameliorates experimental diabetic neuropathy through inhibition of oxidative stress and inflammatory pathways in the dorsal root ganglion neurons. Metab. Brain Dis. 2022, 37, 2615–2627. [Google Scholar] [CrossRef]
- Silverberg, J.I.; French, L.E.; Warren, R.B.; Strober, B.; Kjøller, K.; Sommer, M.O.A.; Andres, P.; Felding, J.; Weiss, A.; Tutkunkardas, D.; et al. Pharmacology of orismilast, a potent and selective PDE4 inhibitor. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 721–729. [Google Scholar] [CrossRef]
- Rizvi, Z.A.; Babele, P.; Sadhu, S.; Madan, U.; Tripathy, M.R.; Goswami, S.; Mani, S.; Kumar, S.; Awasthi, A.; Dikshit, M. Prophylactic treatment of Glycyrrhiza glabra mitigates COVID-19 pathology through inhibition of pro-inflammatory cytokines in the hamster model and NETosis. Front. Immunol. 2022, 13, 945583. [Google Scholar] [CrossRef]
- Healy, L.D.; Puy, C.; Fernández, J.A.; Mitrugno, A.; Keshari, R.S.; Taku, N.A.; Chu, T.T.; Xu, X.; Gruber, A.; Lupu, F.; et al. Activated protein C inhibits neutrophil extracellular trap formation in vitro and activation in vivo. J. Biol. Chem. 2017, 92, 8616–8629. [Google Scholar] [CrossRef]
- von Dadelszen, P.; Magee, L.A.; Benton, S.J.; Hu, Y.; Ansermino, J.M.; Carleton, B.; Carter, C.; Douglas, M.J.; Janssen, P.A.; Lee, S.K.; et al. Activated protein C as disease-modifying therapy in antenatal preeclampsia: An open-label, single arm safety and efficacy trial. Pregnancy Hypertens. 2018, 13, 121–126. [Google Scholar] [CrossRef]
- Huckriede, J.B.; Beurskens, D.M.H.; Wildhagen, K.C.C.A.; Reutelingsperger, C.P.M.; Wichapong, K.; Nicolaes, G.A.F. Design and characterization of novel activated protein C variants for the proteolysis of cytotoxic extracellular histone H3. J. Thromb. Haemost. 2023, 21, 3557–3567. [Google Scholar] [CrossRef] [PubMed]
- Xigris 2 Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/xigris-epar-product-information_en.pdf (accessed on 16 January 2024).
- Baldo, B.A. Enzymes approved for human therapy: Indications, mechanisms and adverse effects. BioDrugs 2015, 29, 31–55. [Google Scholar] [CrossRef]
- Jarrahi, A.; Khodadadi, H.; Moore, N.S.; Lu, Y.; Awad, M.E.; Salles, E.L.; Vaibhav, K.; Baban, B.; Dhandapani, K.M. Recombinant human DNase-I improves acute respiratory distress syndrome via neutrophil extracellular trap degradation. J. Thromb. Haemostasis 2023, 21, 2473–2484. [Google Scholar] [CrossRef]
- Okur, H.K.; Yalcin, K.; Tastan, C.; Demir, S.; Yurtsever, B.; Karakus, G.S.; Kancagi, D.D.; Abanuz, S.; Seyis, U.; Zengin, R.; et al. Preliminary report of in vitro and in vivo effectiveness of dornase alfa on SARS-CoV-2 infection. New Microbes New Infect. 2020, 37, 100756. [Google Scholar] [CrossRef]
- Vural, P.; Akgül, C.; Canbaz, M. Urinary PGE2 and PGF2alpha levels and renal functions in preeclampsia. Gynecol. Obs. Investig. 1998, 45, 237–241. [Google Scholar] [CrossRef]
- Shishikura, K.; Horiuchi, T.; Sakata, N.; Trinh, D.A.; Shirakawa, R.; Kimura, T.; Asada, Y.; Horiuchi, H. Prostaglandin E2 inhibits neutrophil extracellular trap formation through production of cyclic AMP. Br. J. Pharmacol. 2016, 173, 319–331. [Google Scholar] [CrossRef]
- Domingo-Gonzalez, R.; Martínez-Colón, G.J.; Smith, A.J.; Smith, C.K.; Ballinger, M.N.; Xia, M.; Murray, S.; Kaplan, M.J.; Yanik, G.A.; Moore, B.B. Inhibition of Neutrophil Extracellular Trap Formation after Stem Cell Transplant by Prostaglandin E2. Am. J. Respir. Crit. Care Med. 2016, 193, 186–197. [Google Scholar] [CrossRef]
- Baan, R.A.; Stewart, B.W.; Straif, K. (Eds.) Tumour Site Concordance and Mechanisms of Carcinogenesis; International Agency for Research on Cancer: Lyon, France, 2019. [Google Scholar]
- Basyreva, L.Y.; Voinova, E.V.; Gusev, A.A.; Mikhalchik, E.V.; Kuskov, A.N.; Goryachaya, A.V.; Gusev, S.A.; Shtilman, M.I.; Velonia, K.; Tsatsakis, A.M. Fluorouracil neutrophil extracellular traps formation inhibited by polymer nanoparticle shielding. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110382. [Google Scholar] [CrossRef]
- Walsh, S.W.; Strauss, J.F. The Road to Low-Dose Aspirin Therapy for the Prevention of Preeclampsia Began with the Placenta. Int. J. Mol. Sci. 2021, 22, 6985. [Google Scholar] [CrossRef]
- Walsh, S.W.; Al Dulaimi, M.; Strauss, J.F. Aspirin Inhibits the Inflammatory Response of Protease-Activated Receptor 1 in Pregnancy Neutrophils: Implications for Treating Women with Preeclampsia. Int. J. Mol. Sci. 2022, 23, 13218. [Google Scholar] [CrossRef] [PubMed]
- Christian, F.; Smith, E.L.; Carmody, R.J. The Regulation of NF-κβ Subunits by Phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef]
- Gil-Villa, A.M.; Norling, L.V.; Serhan, C.N.; Cordero, D.; Rojas, M.; Cadavid, A. Aspirin triggered-lipoxin A4 reduces the adhesion of human polymorphonuclear neutrophils to endothelial cells initiated by preeclamptic plasma. Prostaglandins Leukot. Essent. Fat. Acids 2012, 87, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Atallah, A.; Lecarpentier, E.; Goffinet, F.; Doret-Dion, M.; Gaucherand, P.; Tsatsaris, V. Aspirin for Prevention of Preeclampsia. Drugs 2017, 77, 1819–1831. [Google Scholar] [CrossRef]
- Scott, S.M.; Rose, S.R. Use of Glucocorticoids for the Fetus and Preterm Infant. Clin. Perinatol. 2018, 45, 93–102. [Google Scholar] [CrossRef]
- Haram, K.; Svendsen, E.; Abildgaard, U. The HELLP syndrome: Clinical issues and management. A Review. BMC Pregnancy Childbirth 2009, 9, 8. [Google Scholar] [CrossRef]
- Tica, O.S.; Tica, A.A.; Cojocaru, D.; Tica, I.; Petcu, C.L.; Cojocaru, V.; Alexandru, D.O.; Tica, V.I. Maternal Steroids on Fetal Doppler Indices, in Growth-Restricted Fetuses with Abnormal Umbilical Flow from Pregnancies Complicated with Early-Onset Severe Preeclampsia. Diagnostics 2023, 13, 428. [Google Scholar] [CrossRef]
- Whitelaw, A.; Thoresen, M. Antenatal steroids and the developing brain. Arch. Dis. Child.-Fetal Neonatal Ed. 2000, 83, F154–F157. [Google Scholar] [CrossRef]
- Peng, Y.; Hong, H.; Gao, N.; Wan, A.; Ma, Y. Bioinformatics methods in biomarkers of preeclampsia and associated potential drug applications. BMC Genom. 2022, 23, 711. [Google Scholar] [CrossRef] [PubMed]
- Causevic, M.; Mohaupt, M. 11beta-Hydroxysteroid dehydrogenase type 2 in pregnancy and preeclampsia. Mol. Asp. Med. 2007, 28, 220–226. [Google Scholar] [CrossRef]
- Kisanga, E.P.; Tang, Z.; Guller, S.; Whirledge, S. Glucocorticoid signaling regulates cell invasion and migration in the human first-trimester trophoblast cell line Sw.71. Am. J. Reprod. Immunol. 2018, 80, e12974. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, H.; Zeng, J.; Miao, X.; Huang, W.; Chen, H.; Huang, Y.; Li, Y.; Ye, D. Glucocorticoid exposure in early placentation induces preeclampsia in rats via interfering trophoblast development. Gen. Comp. Endocrinol. 2016, 225, 61–70. [Google Scholar] [CrossRef]
- Long, J.; Huang, Y.; Wang, G.; Tang, Z.; Shan, Y.; Shen, S.; Ni, X. Mitochondrial ROS Accumulation Contributes to Maternal Hypertension and Impaired Remodeling of Spiral Artery but Not IUGR in a Rat PE Model Caused by Maternal Glucocorticoid Exposure. Antioxidants 2023, 12, 987. [Google Scholar] [CrossRef]
- Wan, T.; Zhao, Y.; Fan, F.; Hu, R.; Jin, X. Dexamethasone Inhibits S. aureus-Induced Neutrophil Extracellular Pathogen-Killing Mechanism, Possibly through Toll-Like Receptor Regulation. Front. Immunol. 2017, 8, 60. [Google Scholar] [CrossRef]
- Fuchisawa, A.; van Eeden, S.; Magee, L.A.; Whalen, B.; Leung, P.C.; Russell, J.A.; Walley, K.R.; von Dadelszen, P. Neutrophil apoptosis in preeclampsia, do steroids confound the relationship? J. Obstet. Gynaecol. Res. 2004, 30, 342–348. [Google Scholar] [CrossRef]
- Tosti, G.; Barberio, A.; Tartaglione, L.; Rizzi, A.; Di Leo, M.; Viti, L.; Sirico, A.; De Carolis, S.; Pontecorvi, A.; Lanzone, A.; et al. Lights and shadows on the use of metformin in pregnancy: From the preconception phase to breastfeeding and beyond. Front. Endocrinol. 2023, 14, 1176623. [Google Scholar] [CrossRef]
- Nashif, S.K.; Mahr, R.M.; Jena, S.; Jo, S.; Nelson, A.B.; Sadowski, D.; Crawford, P.A.; Puchalska, P.; Alejandro, E.U.; Gearhart, M.D.; et al. Metformin impairs trophoblast metabolism and differentiation in a dose-dependent manner. Front. Cell Dev. Biol. 2023, 11, 1167097. [Google Scholar] [CrossRef]
- He, L.; Wu, X.; Zhan, F.; Li, X.; Wu, J. Protective role of metformin in preeclampsia via the regulation of NF-κβ/sFlt-1 and Nrf2/HO-1 signaling pathways by activating AMPK. Placenta 2023, 143, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Dahma, G.; Reddy, G.; Craina, M.; Dumitru, C.; Popescu, A.; Stelea, L.; Neamtu, R.; Gluhovschi, A.; Nitu, R.; Maghiari, A.L.; et al. The Effects of Vitamin D Supplementation before 20 Weeks of Gestation on Preeclampsia: A Systematic Review. J. Pers. Med. 2023, 13, 996. [Google Scholar] [CrossRef]
- AlSubai, A.; Baqai, M.H.; Agha, H.; Shankarlal, N.; Javaid, S.S.; Jesrani, E.K.; Golani, S.; Akram, A.; Qureshi, F.; Ahmed, S.; et al. Vitamin D and preeclampsia: A systematic review and meta-analysis. SAGE Open Med. 2023, 11, 20503121231212093. [Google Scholar] [CrossRef]
- Tian, X.; Zheng, L.; Ma, J.; Xu, Y.; Zhang, Y.; Pi, Y. Inhibition of LAMP3 mediates the protective effect of vitamin D against hypoxia/reoxygenation in trophoblast cells. Braz. J. Med. Biol. Res. 2023, 56, e12816. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández González, L.L.; Pérez-Campos Mayoral, L.; Hernández-Huerta, M.T.; Mayoral Andrade, G.; Martínez Cruz, M.; Ramos-Martínez, E.; Pérez-Campos Mayoral, E.; Cruz Hernández, V.; Antonio García, I.; Matias-Cervantes, C.A.; et al. Targeting Neutrophil Extracellular Trap Formation: Exploring Promising Pharmacological Strategies for the Treatment of Preeclampsia. Pharmaceuticals 2024, 17, 605. https://doi.org/10.3390/ph17050605
Hernández González LL, Pérez-Campos Mayoral L, Hernández-Huerta MT, Mayoral Andrade G, Martínez Cruz M, Ramos-Martínez E, Pérez-Campos Mayoral E, Cruz Hernández V, Antonio García I, Matias-Cervantes CA, et al. Targeting Neutrophil Extracellular Trap Formation: Exploring Promising Pharmacological Strategies for the Treatment of Preeclampsia. Pharmaceuticals. 2024; 17(5):605. https://doi.org/10.3390/ph17050605
Chicago/Turabian StyleHernández González, Leticia Lorena, Laura Pérez-Campos Mayoral, María Teresa Hernández-Huerta, Gabriel Mayoral Andrade, Margarito Martínez Cruz, Edgar Ramos-Martínez, Eduardo Pérez-Campos Mayoral, Víctor Cruz Hernández, Ismael Antonio García, Carlos Alberto Matias-Cervantes, and et al. 2024. "Targeting Neutrophil Extracellular Trap Formation: Exploring Promising Pharmacological Strategies for the Treatment of Preeclampsia" Pharmaceuticals 17, no. 5: 605. https://doi.org/10.3390/ph17050605









