Latest Advances in Regional Anaesthesia
Abstract
:1. Introduction
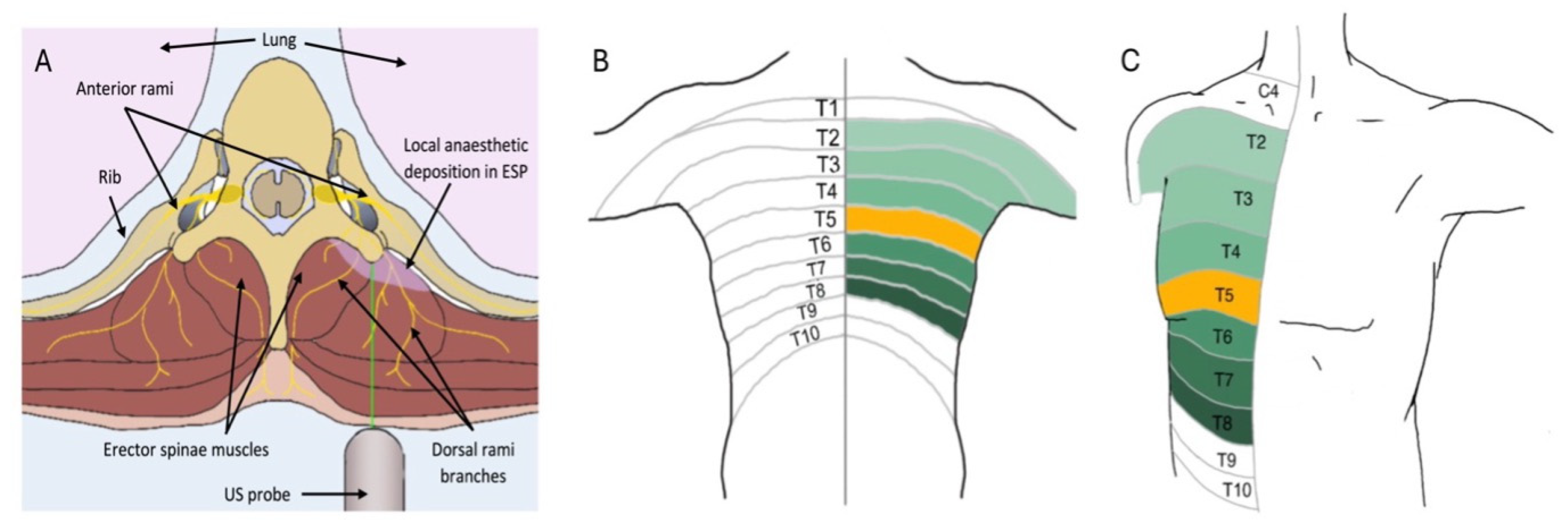
2. Fascial Plane Blocks
3. Neuromodulation Techniques for Acute Pain
4. Pharmacological Advances
5. Regional Anaesthesia outside the Operating Theatre
6. Rebound Pain after Regional Anaesthesia
7. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Mariano, E.R.; Marshall, Z.J.; Urman, R.D.; Kaye, A.D. Ultrasound and its evolution in perioperative regional anesthesia and analgesia. Best Pract. Res. Clin. Anaesthesiol. 2014, 28, 29–39. [Google Scholar] [CrossRef]
- O’Neill, A.; Lirk, P. Multimodal Analgesia. Anesthesiol. Clin. 2022, 40, 455–468. [Google Scholar] [CrossRef]
- Choi, J.J.; Lin, E.; Gadsden, J. Regional anesthesia for trauma outside the operating theatre. Curr. Opin. Anesthesiol. 2013, 26, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Mancel, L.; Van Loon, K.; Lopez, A.M. Role of regional anesthesia in Enhanced Recovery After Surgery (ERAS) protocols. Curr. Opin. Anesthesiol. 2021, 34, 616–625. [Google Scholar] [CrossRef]
- Simpson, J.C.; Bao, X.; Agarwala, A. Pain Management in Enhanced Recovery after Surgery (ERAS) Protocols. Clin. Colon Rectal Surg. 2019, 32, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Admiraal, M.; Marhofer, P.; Hopkins, P.M.; Hollmann, M.W. Peripheral regional anaesthesia and outcomes: A narrative review of the literature from 2013 to 2023. Br. J. Anaesth. 2023, 132, 1082–1096. [Google Scholar] [CrossRef]
- Chin, K.; Mariano, E.; El-Boghdadly, K. Advancing towards the next frontier in regional anaesthesia. Anaesthesia 2021, 76, 3–7. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, Y.; Sandeep, B.; Yang, J. Clinical impact of fascial plane blocks: Defining the research agenda. Br. J. Anaesth. 2023, 131, e180–e183. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.; Versyck, B.; Pawa, A. Ultrasound-guided fascial plane blocks of the chest wall: A state-of-the-art review. Anaesthesia 2021, 76, 110–126. [Google Scholar] [CrossRef]
- Dam, M.; Moriggl, B.; Hansen, C.K.; Hoermann, R.; Bendtsen, T.F.; Børglum, J. The pathway of injectate spread with the transmuscular quadratus lumborum block: A cadaver study. Anesth. Analg. 2017, 125, 303–312. [Google Scholar] [CrossRef]
- Black, N.D.; Stecco, C.; Chan, V.W. Fascial plane blocks: More questions than answers? Anesth. Analg. 2021, 132, 899–905. [Google Scholar] [CrossRef] [PubMed]
- De Cassai, A.; Geraldini, F.; Mariano, E.R.; Kou, A.; Matava, C. Believe the hype? An evaluation of Twitter activity and publication trends related to the erector spinae plane block. J. Clin. Anesth. 2021, 75, 110499. [Google Scholar] [CrossRef] [PubMed]
- Koo, C.-H.; Lee, H.-T.; Na, H.-S.; Ryu, J.-H.; Shin, H.-J. Efficacy of erector spinae plane block for analgesia in thoracic surgery: A systematic review and meta-analysis. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Leong, R.; Tan, E.; Wong, S.; Tan, K.; Liu, C. Efficacy of erector spinae plane block for analgesia in breast surgery: A systematic review and meta-analysis. Anaesthesia 2021, 76, 404–413. [Google Scholar] [CrossRef] [PubMed]
- De Cassai, A.; Bonvicini, D.; Correale, C.; Sandei, L.; Tulgar, S.; Tonetti, T. Erector spinae plane block: A systematic qualitative review. Minerva Anestesiol. 2019, 85, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, W.; Xie, W.; Chen, Z.; Liu, Y. Erector spinae plane block for postoperative analgesia in breast and thoracic surgery: A systematic review and meta-analysis. J. Clin. Anesth. 2020, 66, 109900. [Google Scholar] [CrossRef] [PubMed]
- Forero, M.; Adhikary, S.D.; Lopez, H.; Tsui, C.; Chin, K.J. The erector spinae plane block: A novel analgesic technique in thoracic neuropathic pain. Reg. Anesth. Pain Med. 2016, 41, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-M.; Kim, D.-H.; Jeong, S.-M.; Choi, K.T.; Park, S.; Kwon, H.-J.; Lee, J.-H. Does erector spinae plane block have a visceral analgesic effect?: A randomized controlled trial. Sci. Rep. 2020, 10, 8389. [Google Scholar] [CrossRef]
- Pawa, A.; King, C.; Thang, C.; White, L. Erector spinae plane block: The ultimate ‘plan A’block? Br. J. Anaesth. 2023, 130, 497–502. [Google Scholar] [CrossRef]
- Dautzenberg, K.H.; Zegers, M.J.; Bleeker, C.P.; Tan, E.C.; Vissers, K.C.; van Geffen, G.-J.; van der Wal, S.E. Unpredictable injectate spread of the erector spinae plane block in human cadavers. Anesth. Analg. 2019, 129, e163–e166. [Google Scholar] [CrossRef]
- Jinn, C.K.; Kariem, E.-B. Mechanisms of action of the erector spinae plane (ESP) block: A narrative review. Can. J. Anesth. 2021, 68, 387–408. [Google Scholar]
- Yang, H.M.; Choi, Y.; Kwon, H.J.; O, J.; Cho, T.; Kim, S. Comparison of injectate spread and nerve involvement between retrolaminar and erector spinae plane blocks in the thoracic region: A cadaveric study. Anaesthesia 2018, 73, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, S.D.; Bernard, S.; Lopez, H.; Chin, K.J. Erector spinae plane block versus retrolaminar block: A magnetic resonance imaging and anatomical study. Reg. Anesth. Pain Med. 2018, 43, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Thom, S.; Haber, B.; Sarani, N.; Ottenhoff, J.; Jackson, B.; Rance, L.; Ehrman, R. Regional anesthesia in the emergency department: An overview of common nerve block techniques and recent literature. Curr. Emerg. Hosp. Med. Rep. 2022, 10, 54–66. [Google Scholar] [CrossRef]
- Kietaibl, S.; Ferrandis, R.; Godier, A.; Llau, J.; Lobo, C.; Macfarlane, A.J.; Schlimp, C.J.; Vandermeulen, E.; Volk, T.; von Heymann, C. Regional anaesthesia in patients on antithrombotic drugs: Joint ESAIC/ESRA guidelines. Eur. J. Anaesthesiol. | EJA 2022, 39, 100–132. [Google Scholar] [CrossRef] [PubMed]
- Horlocker, T.T.; Vandermeuelen, E.; Kopp, S.L.; Gogarten, W.; Leffert, L.R.; Benzon, H.T. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines. Obstet. Anesth. Dig. 2019, 39, 28–29. [Google Scholar] [CrossRef]
- Finnerty, D.T.; McMahon, A.; McNamara, J.R.; Hartigan, S.D.; Griffin, M.; Buggy, D.J. Comparing erector spinae plane block with serratus anterior plane block for minimally invasive thoracic surgery: A randomised clinical trial. Br. J. Anaesth. 2020, 125, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, M.; Ciftci, B.; Gölboyu, B.E.; Demiraran, Y.; Bayrak, Y.; Tulgar, S. A randomized trial to compare serratus anterior plane block and erector spinae plane block for pain management following thoracoscopic surgery. Pain Med. 2020, 21, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Finnerty, D.; Ní Eochagáin, A.; Ahmed, M.; Poynton, A.; Butler, J.; Buggy, D. A randomised trial of bilateral erector spinae plane block vs. no block for thoracolumbar decompressive spinal surgery. Anaesthesia 2021, 76, 1499–1503. [Google Scholar] [CrossRef]
- Moorthy, A.; Eochagáin, A.N.; Dempsey, E.; Wall, V.; Marsh, H.; Murphy, T.; Fitzmaurice, G.J.; Naughton, R.A.; Buggy, D.J. Postoperative recovery with continuous erector spinae plane block or video-assisted paravertebral block after minimally invasive thoracic surgery: A prospective, randomised controlled trial. Br. J. Anaesth. 2023, 130, e137–e147. [Google Scholar] [CrossRef]
- Tan, H.S.; Zeng, Y.; Qi, Y.; Sultana, R.; Tan, C.W.; Sia, A.T.; Sng, B.L.; Siddiqui, F.J. Automated mandatory bolus versus basal infusion for maintenance of epidural analgesia in labour. Cochrane Database Syst. Rev. 2023, 5, CD011344. [Google Scholar]
- Eochagain, A.N.; Moorthy, A.; O’Gara, A.; Buggy, D.J. Ultrasound-guided, continuous erector spinae plane (ESP) block in minimally invasive thoracic surgery-comparing programmed intermittent bolus (PIB) vs continuous infusion on quality of recovery and postoperative respiratory function: A double-blinded randomised controlled trial. Trials 2022, 23, 792. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Qiao, Q.; Chen, R.; Xu, Q.; Zhang, Y.; Tian, Y. The effect of ultrasound-guided intercostal nerve block, single-injection erector spinae plane block and multiple-injection paravertebral block on postoperative analgesia in thoracoscopic surgery: A randomized, double-blinded, clinical trial. J. Clin. Anesth. 2020, 59, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Behr, A.U.; Chan, V.W.; Stecco, C. Living versus cadaver fascial plane injection. Reg. Anesth. Pain Med. 2019, 45, 157–158. [Google Scholar] [CrossRef]
- Saadawi, M.; Layera, S.; Aliste, J.; Bravo, D.; Leurcharusmee, P.; Tran, D.Q. Erector spinae plane block: A narrative review with systematic analysis of the evidence pertaining to clinical indications and alternative truncal blocks. J. Clin. Anesth. 2021, 68, 110063. [Google Scholar] [CrossRef]
- Turbitt, L.; Mariano, E.; El-Boghdadly, K. Future directions in regional anaesthesia: Not just for the cognoscenti. Anaesthesia 2020, 75, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Tsui, B.C.; Gupta, R.K. Role of neuromodulation in acute pain settings. Reg. Anesth. Pain Med. 2023, 48, 338–342. [Google Scholar] [CrossRef] [PubMed]
- North, R.B.; Lempka, S.F.; Guan, Y.; Air, E.L.; Poree, L.R.; Shipley, J.; Arle, J.; Rigoard, P.; Thomson, S. Glossary of neurostimulation terminology: A collaborative neuromodulation foundation, institute of neuromodulation, and international neuromodulation society project. Neuromodul. Technol. Neural Interface 2022, 25, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Mekhail, N.; Provenzano, D.; Pope, J.; Krames, E.; Leong, M.; Levy, R.M.; Abejon, D.; Buchser, E.; Burton, A. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: The Neuromodulation Appropriateness Consensus Committee. Neuromodul. Technol. Neural Interface 2014, 17, 515–550. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z. Neuromodulation for pain management. Neural Interface Front. Appl. 2019, 1101, 207–223. [Google Scholar]
- Ilfeld, B.M.; Finneran, J.J., IV. Cryoneurolysis and percutaneous peripheral nerve stimulation to treat acute pain: A narrative review. Anesthesiology 2020, 133, 1127–1149. [Google Scholar] [CrossRef] [PubMed]
- Ilfeld, B.M.; Finneran, J.J.; Gabriel, R.A.; Said, E.T.; Nguyen, P.L.; Abramson, W.B.; Khatibi, B.; Sztain, J.F.; Swisher, M.W.; Jaeger, P. Ultrasound-guided percutaneous peripheral nerve stimulation: Neuromodulation of the suprascapular nerve and brachial plexus for postoperative analgesia following ambulatory rotator cuff repair. A proof-of-concept study. Reg. Anesth. Pain Med. 2019, 44, 310–318. [Google Scholar] [CrossRef]
- Ilfeld, B.M.; Gabriel, R.A.; Said, E.T.; Monahan, A.M.; Sztain, J.F.; Abramson, W.B.; Khatibi, B.; Finneran, J.J.; Jaeger, P.T.; Schwartz, A.K. Ultrasound-guided percutaneous peripheral nerve stimulation: Neuromodulation of the sciatic nerve for postoperative analgesia following ambulatory foot surgery, a proof-of-concept study. Reg. Anesth. Pain Med. 2018, 43, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Ilfeld, B.M.; Grant, S.A. Ultrasound-guided percutaneous peripheral nerve stimulation for postoperative analgesia: Could neurostimulation replace continuous peripheral nerve blocks? Reg. Anesth. Pain Med. 2016, 41, 720–722. [Google Scholar] [CrossRef]
- Ilfeld, B.M.; Plunkett, A.; Vijjeswarapu, A.M.; Hackworth, R.; Dhanjal, S.; Turan, A.; Cohen, S.P.; Eisenach, J.C.; Griffith, S.; Hanling, S. Percutaneous peripheral nerve stimulation (neuromodulation) for postoperative pain: A randomized, sham-controlled pilot study. Anesthesiology 2021, 135, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Ong Sio, L.C.; Hom, B.; Garg, S.; Abd-Elsayed, A. Mechanism of action of peripheral nerve stimulation for chronic pain: A narrative review. Int. J. Mol. Sci. 2023, 24, 4540. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, R.A.; Swisher, M.W.; Ilfeld, B.M. Percutaneous peripheral nerve stimulation for acute postoperative pain. Pain Manag. 2019, 9, 347–354. [Google Scholar] [CrossRef]
- Melzack, R.; Wall, P.D. Pain mechanisms: A new theory. Surv. Anesthesiol. 1967, 11, 89–90. [Google Scholar] [CrossRef]
- Mendell, L.M. Constructing and deconstructing the gate theory of pain. Pain® 2014, 155, 210–216. [Google Scholar] [CrossRef]
- Lin, T.; Gargya, A.; Singh, H.; Sivanesan, E.; Gulati, A. Mechanism of Peripheral Nerve Stimulation in Chronic Pain. Pain Med. 2020, 21 (Suppl. S1), S6–S12. [Google Scholar] [CrossRef]
- Meyer-Frießem, C.H.; Wiegand, T.; Eitner, L.; Maier, C.; Mainka, T.; Vollert, J.; Enax-Krumova, E.K. Effects of spinal cord and peripheral nerve stimulation reflected in sensory profiles and endogenous pain modulation. Clin. J. Pain 2019, 35, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Papuć, E.; Rejdak, K. The role of neurostimulation in the treatment of neuropathic pain. Ann. Agric. Environ. Med. 2013, 1, 14–17. [Google Scholar]
- Melzack, R.; Wall, P.D. Pain Mechanisms: A New Theory: A gate control system modulates sensory input from the skin before it evokes pain perception and response. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Ilfeld, B.M.; Gabriel, R.A.; Saulino, M.F.; Chae, J.; Peckham, P.H.; Grant, S.A.; Gilmore, C.A.; Donohue, M.C.; deBock, M.G.; Wongsarnpigoon, A. Infection rates of electrical leads used for percutaneous neurostimulation of the peripheral nervous system. Pain Pract. 2017, 17, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Strand, N.H.; D’Souza, R.; Wie, C.; Covington, S.; Maita, M.; Freeman, J.; Maloney, J. Mechanism of action of peripheral nerve stimulation. Curr. Pain Headache Rep. 2021, 25, 47. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Naidu, R.; Strand, N.; Sparks, D.; Abd-Elsayed, A.; Kalia, H.; Hah, J.M.; Mehta, P.; Sayed, D.; Gulati, A. A review of the bioelectronic implications of stimulation of the peripheral nervous system for chronic pain conditions. Bioelectron. Med. 2020, 6, 9. [Google Scholar] [CrossRef]
- Desai, N.; Kirkham, K.; Albrecht, E. Local anaesthetic adjuncts for peripheral regional anaesthesia: A narrative review. Anaesthesia 2021, 76, 100–109. [Google Scholar] [CrossRef]
- Fredrickson, M.J.; Abeysekera, A.; White, R. Randomized study of the effect of local anesthetic volume and concentration on the duration of peripheral nerve blockade. Reg. Anesth. Pain Med. 2012, 37, 495–501. [Google Scholar] [CrossRef]
- Albrecht, E.; Chin, K. Advances in regional anaesthesia and acute pain management: A narrative review. Anaesthesia 2020, 75, e101–e110. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, A.; Ward, C.T.; Watson, M.; Sanford, J.; Fiza, B.; Moll, V.; Kaye, R.J.; Hall, O.M.; Cornett, E.M.; Urman, R.D. Liposomal bupivacaine and novel local anesthetic formulations. Best Pract. Res. Clin. Anaesthesiol. 2019, 33, 425–432. [Google Scholar] [CrossRef]
- Biosciences, P. Pacira Announces FDA Approval of Expanded Exparel Label to Include Two Additional Nerve Block Indications. 2023. Available online: https://investor.pacira.com/news-releases/news-release-details/pacira-announces-fda-approval-expanded-exparel-label-include-two#:~:text=(NASDAQ%3A%20PCRX)%2C%20the,liposome%20injectable%20suspension)%20label%20to (accessed on 14 March 2024).
- McCann, M.E. Liposomal Bupivacaine: Effective, Cost-effective, or (Just) Costly? Anesthesiology 2021, 134, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Ilfeld, B.M.; Gabriel, R.A.; Eisenach, J.C. Liposomal bupivacaine infiltration for knee arthroplasty: Significant analgesic benefits or just a bunch of fat? Anesthesiology 2018, 129, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Vandepitte, C.; Kuroda, M.; Witvrouw, R.; Anne, L.; Bellemans, J.; Corten, K.; Vanelderen, P.; Mesotten, D.; Leunen, I.; Heylen, M. Addition of liposome bupivacaine to bupivacaine HCl versus bupivacaine HCl alone for interscalene brachial plexus block in patients having major shoulder surgery. Reg. Anesth. Pain Med. 2017, 42, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Brull, R.; Sheehy, B.; Essandoh, M.K.; Stahl, D.L.; Weaver, T.E.; Abdallah, F.W. Perineural liposomal bupivacaine is not superior to nonliposomal bupivacaine for peripheral nerve block analgesia: A systematic review and meta-analysis. Anesthesiology 2021, 134, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Kolodychuk, N.; Krebs, J.C.; Stenberg, R.; Talmage, L.; Meehan, A.; DiNicola, N. Fascia iliaca blocks performed in the emergency department decrease opioid consumption and length of stay in patients with hip fracture. J. Orthop. Trauma 2022, 36, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Scurrah, A.; Shiner, C.; Stevens, J.; Faux, S. Regional nerve blockade for early analgesic management of elderly patients with hip fracture–a narrative review. Anaesthesia 2018, 73, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Faculty of Pain Medicine of the Royal College of Anaesthetists. Core Standards for Pain Management Services in the UK, 2nd ed.; Faculty of Pain Medicine of the Royal College of Anaesthetists: London, UK, 2021; Available online: https://fpm.ac.uk/sites/fpm/files/documents/2022-01/FPM-Core-Standards-Dec-2021_0.pdf (accessed on 19 March 2024).
- Fabbri, A.; Voza, A.; Riccardi, A.; Serra, S.; Iaco, F.D. The pain management of trauma patients in the emergency department. J. Clin. Med. 2023, 12, 3289. [Google Scholar] [CrossRef]
- Macintyre, P.E. The opioid epidemic from the acute care hospital front line. Anaesth. Intensive Care 2022, 50, 29–43. [Google Scholar] [CrossRef]
- Mauck, M.C.; Zhao, Y.; Goetzinger, A.M.; Tungate, A.S.; Spencer, A.B.; Lal, A.; Barton, C.E.; Beaudoin, F.; McLean, S.A. Incidence of persistent opioid use following traumatic injury. Reg. Anesth. Pain Med. 2024, 49, 79–86. [Google Scholar] [CrossRef]
- Martin, T.J.; Eltorai, A.S.; Dunn, R.; Varone, A.; Joyce, M.F.; Kheirbek, T.; Adams, C., Jr.; Daniels, A.H.; Eltorai, A.E. Clinical management of rib fractures and methods for prevention of pulmonary complications: A review. Injury 2019, 50, 1159–1165. [Google Scholar] [CrossRef]
- Bulger, E.M.; Edwards, T.; Klotz, P.; Jurkovich, G.J. Epidural analgesia improves outcome after multiple rib fractures. Surgery 2004, 136, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Coblentz, I.J.; Ilfeld, B.M.; Finneran Iv, J.J. Thoracic Epidural as a Rescue Analgesic in a Patient with a Continuous Erector Spinae Plane Block for Rib Fractures: A Case Report. Am. J. Case Rep. 2023, 24, e938875. [Google Scholar] [CrossRef] [PubMed]
- Malekpour, M.; Hashmi, A.; Dove, J.; Torres, D.; Wild, J. Analgesic choice in management of rib fractures: Paravertebral block or epidural analgesia? Anesth. Analg. 2017, 124, 1906–1911. [Google Scholar] [CrossRef] [PubMed]
- Mladenovic, J.; Erskine, R.N.; Riley, B.; Mitchell, A.; Abi-Fares, C.; Basson, W.; Anstey, C.; White, L. The association between erector spinae plane block timing and reduced rib fracture related respiratory complications: A cohort study. J. Clin. Anesth. 2022, 82, 110940. [Google Scholar] [CrossRef]
- Adhikary, S.; Liu, W.-M.; Fuller, E.; Cruz-Eng, H.; Chin, K.J. The effect of erector spinae plane block on respiratory and analgesic outcomes in multiple rib fractures: A retrospective cohort study. Anaesthesia 2019, 74, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, A.; Perry-Nguyen, D.; Zhou, L.; Meng, H.; Choi, S.; Niazi, A.U. Evaluation of the effect of erector spinae plane block catheter for traumatic rib fractures on patient experience: A quality assurance initiative. Reg. Anesth. Pain Med. 2023; rapm-2023-104549. [Google Scholar] [CrossRef]
- El-Sherbiny, S.M.; Kamal, R.A.; Elhadary, I.H.; Abdallah, M.Y. Erector spinae plane block versus thoracic epidural block as analgesic techniques for chest trauma: A randomized controlled trial. Res. Opin. Anesth. Intensive Care 2022, 9, 220–227. [Google Scholar]
- Hewson, D.W.; Nightingale, J.; Ogollah, R.; Ollivere, B.J.; Costa, M.L.; Craxford, S.; Bates, P.; Bedforth, N.M. Erector Spinae Plane Blocks for the Early Analgesia of Rib Fractures in Trauma (ESPEAR): Protocol for a multicentre pilot randomised controlled trial with feasibility and embedded qualitative assessment. BMJ Open 2022, 12, e062935. [Google Scholar] [CrossRef] [PubMed]
- Rommens, P.M.; Hopf, J.C.; Herteleer, M.; Devlieger, B.; Hofmann, A.; Wagner, D. Isolated pubic ramus fractures are serious adverse events for elderly persons: An observational study on 138 patients with fragility fractures of the pelvis type I (FFP type I). J. Clin. Med. 2020, 9, 2498. [Google Scholar] [CrossRef] [PubMed]
- Studer, P.; Suhm, N.; Zappe, B.; Bless, N.; Jakob, M. Pubic rami fractures in the elderly–a neglected injury? Swiss Med. Wkly. 2013, 143, w13859. [Google Scholar] [CrossRef]
- Pascarella, G.; Costa, F.; Del Buono, R.; Pulitanò, R.; Strumia, A.; Piliego, C.; De Quattro, E.; Cataldo, R.; Agrò, F.; Carassiti, M. Impact of the pericapsular nerve group (PENG) block on postoperative analgesia and functional recovery following total hip arthroplasty: A randomised, observer-masked, controlled trial. Anaesthesia 2021, 76, 1492–1498. [Google Scholar] [CrossRef]
- Lin, D.-Y.; Morrison, C.; Brown, B.; Saies, A.A.; Pawar, R.; Vermeulen, M.; Anderson, S.R.; Lee, T.S.; Doornberg, J.; Kroon, H.M. Pericapsular nerve group (PENG) block provides improved short-term analgesia compared with the femoral nerve block in hip fracture surgery: A single-center double-blinded randomized comparative trial. Reg. Anesth. Pain Med. 2021, 46, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Girón-Arango, L.; Peng, P.W.; Chin, K.J.; Brull, R.; Perlas, A. Pericapsular nerve group (PENG) block for hip fracture. Reg. Anesth. Pain Med. 2018, 43, 859–863. [Google Scholar] [CrossRef]
- Moorthy, A.; Choi, S.; Safa, B.; McHardy, P.G.; Niazi, A.U. Novel use of continuous pericapsular nerve group (PENG) block technique for traumatic superior and inferior pubic rami fractures: A case report. Reg. Anesth. Pain Med. 2023, 48, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Luftig, J.; Dreyfuss, A.; Mantuani, D.; Howell, K.; White, A.; Nagdev, A. A new frontier in pelvic fracture pain control in the ED: Successful use of the pericapsular nerve group (PENG) block. Am. J. Emerg. Med. 2020, 38, 2761.e2765–2761.e2769. [Google Scholar] [CrossRef] [PubMed]
- Henningsen, M.; Sort, R.; Møller, A.; Herling, S. Peripheral nerve block in ankle fracture surgery: A qualitative study of patients’ experiences. Anaesthesia 2018, 73, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Lavand’homme, P. Rebound pain after regional anesthesia in the ambulatory patient. Curr. Opin. Anesthesiol. 2018, 31, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Leyva, F.; Cubillos, J.; Chin, K.J. Managing rebound pain after regional anesthesia. Korean J. Anesthesiol. 2020, 73, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.Y.; Manoli III, A.; Galos, D.K.; Jain, S.; Tejwani, N.C. Continuous popliteal sciatic nerve block versus single injection nerve block for ankle fracture surgery: A prospective randomized comparative trial. J. Orthop. Trauma 2015, 29, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Salviz, E.A.; Xu, D.; Frulla, A.; Kwofie, K.; Shastri, U.; Chen, J.; Shariat, A.N.; Littwin, S.; Lin, E.; Choi, J. Continuous interscalene block in patients having outpatient rotator cuff repair surgery: A prospective randomized trial. Anesth. Analg. 2013, 117, 1485–1492. [Google Scholar] [CrossRef]
- Hauritz, R.W.; Hannig, K.E.; Balocco, A.L.; Peeters, G.; Hadzic, A.; Børglum, J.; Bendtsen, T.F. Peripheral nerve catheters: A critical review of the efficacy. Best Pract. Res. Clin. Anaesthesiol. 2019, 33, 325–339. [Google Scholar] [CrossRef]
- Finneran, J.J., IV; Ilfeld, B.M. Continuous peripheral nerve blocks for analgesia following painful ambulatory surgery: A review with focus on recent developments in infusion technology. Curr. Opin. Anesthesiol. 2023, 36, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Finneran, J.J., IV; Said, E.T.; Curran, B.P.; Swisher, M.W.; Black, J.R.; Gabriel, R.A.; Sztain, J.F.; Abramson, W.B.; Alexander, B.; Donohue, M.C. Basal infusion versus automated boluses and a delayed start timer for “continuous” sciatic nerve blocks after ambulatory foot and ankle surgery: A randomized clinical trial. Anesthesiology 2022, 136, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Finneran, J.J., IV; Baskin, P.; Kent, W.T.; Hentzen, E.R.; Schwartz, A.K.; Ilfeld, B.M. Automated Boluses and Delayed-Start Timers Prolong Perineural Local Anesthetic Infusions and Analgesia Following Ankle and Wrist Orthopedic Surgery: A Case-Control Series. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e933190-1–e933190-6. [Google Scholar] [CrossRef] [PubMed]
- The European Society of Regional Anaesthesia. Advancing Regional Anaesthesia and Pain Therapy in Europe. 2023. Available online: https://esraeurope.org/about/ (accessed on 5 March 2024).
- Lobo, C. Welcome to ESRA International Committee Members 2021 [Issue 07:[ESRA Updates]. Available online: https://esraeurope.org/newsletter/article-posts/welcome-esra-international-committee-members/ (accessed on 6 March 2024).
- Royal College of Anaesthetists. Stage 3 Curriculum. 2021. Available online: https://rcoa.ac.uk/documents/2021-curriculum-learning-syllabus-stage-3/regional-anaesthesia (accessed on 15 March 2024).
- College of Anaesthesiologists of Ireland. Curriculum for the National Specialist Anaesthesiologists Training Programme. 2020. Available online: https://www.anaesthesia.ie/curriculum/?page=50 (accessed on 15 March 2024).
- Luff, D.; Moosa, F.; Sadavarte, N.; Pinnamaneni, N.; Bedforth, N. 136 Which blocks can you do? An assessment of anaesthetic trainee confidence performing common peripheral nerve blocks. Reg. Anesth. Pain Med. 2021, 70, A71. [Google Scholar]
- McKendrick, M.; Yang, S.; McLeod, G. The use of artificial intelligence and robotics in regional anaesthesia. Anaesthesia 2021, 76, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Bowness, J.S.; Burckett-St Laurent, D.; Hernandez, N.; Keane, P.A.; Lobo, C.; Margetts, S.; Moka, E.; Pawa, A.; Rosenblatt, M.; Sleep, N. Assistive artificial intelligence for ultrasound image interpretation in regional anaesthesia: An external validation study. Br. J. Anaesth. 2023, 130, 217–225. [Google Scholar] [CrossRef]
- Bowness, J.; El-Boghdadly, K.; Burckett-St Laurent, D. Artificial intelligence for image interpretation in ultrasound-guided regional anaesthesia. Anaesthesia 2021, 76, 602–607. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallon, F.; Moorthy, A.; Skerritt, C.; Crowe, G.G.; Buggy, D.J. Latest Advances in Regional Anaesthesia. Medicina 2024, 60, 735. https://doi.org/10.3390/medicina60050735
Fallon F, Moorthy A, Skerritt C, Crowe GG, Buggy DJ. Latest Advances in Regional Anaesthesia. Medicina. 2024; 60(5):735. https://doi.org/10.3390/medicina60050735
Chicago/Turabian StyleFallon, Frances, Aneurin Moorthy, Conor Skerritt, Gillian G. Crowe, and Donal J. Buggy. 2024. "Latest Advances in Regional Anaesthesia" Medicina 60, no. 5: 735. https://doi.org/10.3390/medicina60050735




