A Numerical Simulation and Experimental Study of Fluidization Characteristics of a Bubbling Fluidized Bed in Biomass Gasification
Abstract
:1. Introduction
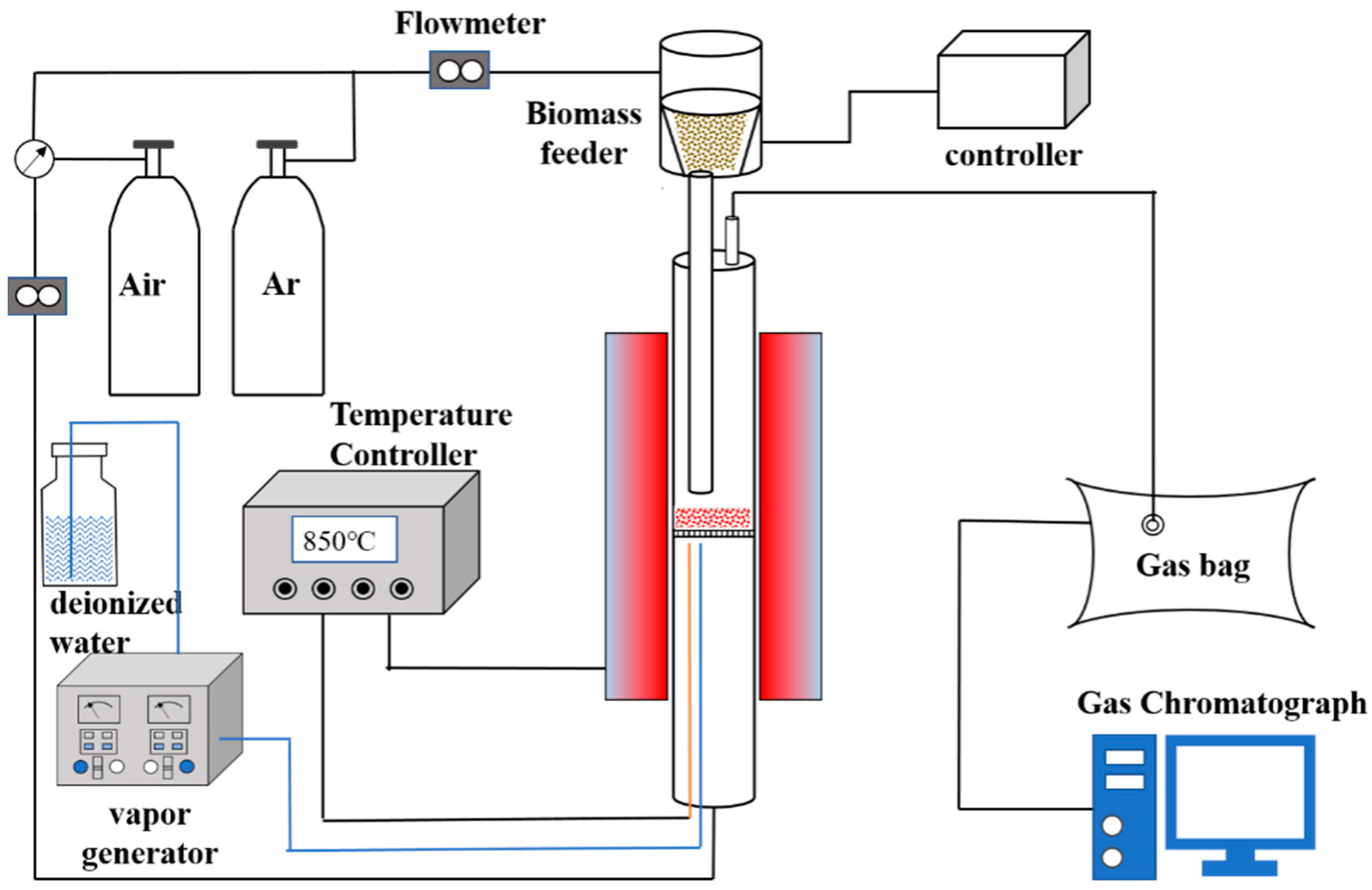
2. Experiment and Simulations
2.1. Materials
2.1.1. Biomass Feedstock
2.1.2. Fluidized Bed Material
2.2. Geometric Modeling and Simulation Conditions
2.3. Experimental Procedure
3. Fluidization Characterization
4. Gasification Properties of Biomass
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liang, S.; Tian, B.L.; Guo, F.Q.; Dong, Y.C.; Du, S.L.; Qian, L. Porous silicon film overcoating biomass char-supported catalysts for improved activity and stability in biomass pyrolysis tar decomposition. Catal. Sci. Technol. 2021, 11, 5938–5951. [Google Scholar] [CrossRef]
- Liang, S.; Guo, F.Q.; Du, S.L.; Tian, B.L.; Dong, Y.C.; Jia, X.P.; Qian, L. Synthesis of Sargassum char-supported Ni-Fe nanoparticles and its application in tar cracking during biomass pyrolysis. Fuel 2020, 275, 117923. [Google Scholar] [CrossRef]
- Guo, F.Q.; Zhan, Y.B.; Jia, X.P.; Zhou, H.M.; Liang, S.; Qian, L. Fabrication of nitrogen-doped hierarchical porous carbons from Sargassum as advanced electrode materials for supercapacitors. New J. Chem. 2021, 45, 15514–15524. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.Q.; Li, Q.H.; Huang, Y.W. Effect of microwave pretreatment on the combustion behavior of lignite/solid waste briquettes. Energy 2018, 149, 730–740. [Google Scholar] [CrossRef]
- Guo, F.Q.; Liang, S.; Jia, X.P.; Peng, K.Y.; Jiang, X.C.; Qian, L. One-step synthesis of biochar-supported potassium-iron catalyst for catalytic cracking of biomass pyrolysis tar. Int. J. Hydrogen Energy 2020, 45, 16398–16408. [Google Scholar] [CrossRef]
- Guo, F.Q.; Liang, S.; Zhao, X.M.; Jia, X.P.; Peng, K.Y.; Jiang, X.C.; Qian, L. Catalytic reforming of biomass pyrolysis tar using the low-cost steel slag as catalyst. Energy 2019, 189, 116161. [Google Scholar] [CrossRef]
- Lin, F.; Xu, M.Z.; Ramasamy, K.K.; Li, Z.L.; Klinger, J.L.; Schaidle, J.A.; Wang, H.M. Catalyst Deactivation and Its Mitigation during Catalytic Conversions of Biomass. ACS Catal. 2022, 12, 13555–13599. [Google Scholar] [CrossRef]
- Yi, B.; Chen, M.; Gao, Y.; Cao, C.; Wei, Q.; Zhang, Z.; Li, L. Investigation on the co-combustion characteristics of multiple biomass and coal under O2/CO2 condition and the interaction between different biomass. J. Environ. Manag. 2023, 325, 116498. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, J.; Tang, C.; Pan, W. Research and application of online monitoring of coal and biomass co-combustion and biomass combustion characteristics based on combustion flame. J. Energy Inst. 2023, 108, 101191. [Google Scholar] [CrossRef]
- Mularski, J.; Li, J. A review on biomass ignition: Fundamental characteristics, measurements, and predictions. Fuel 2023, 340, 127526. [Google Scholar] [CrossRef]
- McCann, M.C.; Carpita, N.C. Biomass recalcitrance: A multi-scale, multi-factor, and conversion-specific property. J. Exp. Bot. 2015, 66, 4109–4118. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Trinh, L.T.P.; Song, Y.; Lee, Y.G.; Bae, H.J. Bioconversion of biomass waste into high value chemicals. Bioresour. Technol. 2020, 298, 122386. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.; Mirkouei, A.; Diaz, L.A. A comprehensive state-of-technology review for upgrading bio-oil to renewable or blended hydrocarbon fuels. Renew. Sustain. Energy Rev. 2020, 118, 109548. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Conversion of biomass to biofuels and life cycle assessment: A review. Environ. Chem. Lett. 2021, 19, 4075–4118. [Google Scholar] [CrossRef]
- Raheem, A.; Wan Azlina, W.; Taufiq Yap, Y.H.; Danquah, M.K.; Harun, R. Thermochemical conversion of microalgal biomass for biofuel production. Renew. Sustain. Energy Rev. 2015, 49, 990–999. [Google Scholar] [CrossRef]
- Qiu, B.B.; Tao, X.D.; Wang, J.H.; Liu, Y.; Li, S.T.; Chu, H.Q. Research progress in the preparation of high-quality liquid fuels and chemicals by catalytic pyrolysis of biomass: A review. Energy Convers. Manag. 2022, 261, 115647. [Google Scholar] [CrossRef]
- Sekar, M.; Mathimani, T.; Alagumalai, A.; Chi, N.T.L.; Duc, P.A.; Bhatia, S.K.; Brindhadevi, K.; Pugazhendhi, A. A review on the pyrolysis of algal biomass for biochar and bio-oil—Bottlenecks and scope. Fuel 2021, 283, 119190. [Google Scholar] [CrossRef]
- Fu, W.M.; Zhang, Y.N.; Cui, L.F.; Liu, H.; Maqsood, T. Experimental microwave-assisted air gasification of biomass in fluidized bed reactor. Bioresour. Technol. 2023, 369, 128378. [Google Scholar] [CrossRef] [PubMed]
- Hanchate, N.; Ramani, S.; Mathpati, C.S.; Dalvi, V.H. Biomass gasification using dual fluidized bed gasification systems: A review. J. Clean. Prod. 2021, 280, 123148. [Google Scholar] [CrossRef]
- Wang, S.; Shen, Y.S. Coarse-grained CFD-DEM modelling of dense gas-solid reacting flow. Int. J. Heat Mass Transfer. 2022, 184, 122302. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels Production by Biomass Gasification: A Review. Energies 2018, 11, 811. [Google Scholar] [CrossRef]
- Karl, J.; Pröll, T. Steam gasification of biomass in dual fluidized bed gasifiers: A review. Renew. Sustain. Energy Rev. 2018, 98, 64–78. [Google Scholar] [CrossRef]
- Tezer, Ö.; Karabağ, N.; Öngen, A.; Çolpan, C.Ö.; Ayol, A. Biomass gasification for sustainable energy production: A review. Int. J. Hydrogen Energy 2022, 47, 15419–15433. [Google Scholar] [CrossRef]
- Blaszczuk, A.; Jagodzik, S. Investigation of Heat Transfer in a Large-Scale External Heat Exchanger with Horizontal Smooth Tube Bundle. Energies 2021, 14, 5553. [Google Scholar] [CrossRef]
- George, J.; Arun, P.; Muraleedharan, C. Experimental investigation on co-gasification of coffee husk and sawdust in a bubbling fluidised bed gasifier. J. Energy Inst. 2019, 92, 1977–1986. [Google Scholar] [CrossRef]
- Nijenhuis, J.; Korbee, R.; Lensselink, J.; Kiel, J.H.A.; van Ommen, J.R. A method for agglomeration detection and control in full-scale biomass fired fluidized beds. Chem. Eng. Sci. 2007, 62, 644–654. [Google Scholar] [CrossRef]
- Mirek, P. Air Distributor Pressure Drop Analysis in a Circulating Fluidized-Bed Boiler for Non-reference Operating Conditions. Chem. Eng. Technol. 2020, 43, 2233–2246. [Google Scholar] [CrossRef]
- Mirek, P.; Klajny, M. Air nozzle design criteria for protection against the backflow of solids in CFB boilers. Appl. Therm. Eng. 2018, 141, 503–515. [Google Scholar] [CrossRef]
- Cardoso, J.; Silva, V.; Eusébio, D.; Brito, P.; Boloy, R.M.; Tarelho, L.; Silveira, J.L. Comparative 2D and 3D analysis on the hydrodynamics behaviour during biomass gasification in a pilot-scale fluidized bed reactor. Renew. Energy 2019, 131, 713–729. [Google Scholar] [CrossRef]
- Armstrong, L.M.; Luo, K.H.; Gu, S. Two-dimensional and three-dimensional computational studies of hydrodynamics in the transition from bubbling to circulating fluidised bed. Chem. Eng. J. 2010, 160, 239–248. [Google Scholar] [CrossRef]
- Bahramian, A. Simultaneous effects of mesh refinement, grid configuration and wall boundary condition on prediction of pressure gradients and velocity profiles of microparticles in a conical fluidized bed. Particuology 2019, 43, 123–136. [Google Scholar] [CrossRef]
- Glicksman, L.R. Scaling relationships for fluidized beds. Chem. Eng. Sci. 1984, 39, 1373–1379. [Google Scholar] [CrossRef]
- Pallarès, D.; Johnsson, F. Macroscopic modelling of fluid dynamics in large-scale circulating fluidized beds. Prog. Energy Combust. Sci. 2006, 32, 539–569. [Google Scholar] [CrossRef]
- Mirek, P.; Ziaja, J. The influence of sampling point on solids suspension density applied in scaling of the hydrodynamics of a supercritical CFB boiler. Chem. Process. Eng. 2011, 32, 391–399. [Google Scholar] [CrossRef]
- Ruivo, L.C.M.; Pio, D.T.; Yaremchenko, A.A.; Tarelho, L.A.C.; Frade, J.R.; Kantarelis, E.; Engvall, K. Iron-based catalyst (Fe2-xNixTiO5 for tar decomposition in biomass gasification. Fuel 2021, 300, 120859. [Google Scholar] [CrossRef]
- Matamba, T.; Iglauer, S.; Keshavarz, A. A progress insight of the formation of hydrogen rich syngas from coal gasification. J. Energy Inst. 2022, 105, 81–102. [Google Scholar] [CrossRef]
- Ruoppolo, G.; Miccio, F.; Miccio, M.; Brachi, P.; Chirone, R. Sewage sludge ashes as a primary catalyst for the abatement of tar in biomass gasification: Bubbling versus spouted-fluidized bed configuration. Can. J. Chem. Eng. 2021, 99, 1706–1714. [Google Scholar] [CrossRef]
- Gokon, N.; Kumaki, S.; Miyaguchi, Y.; Bellan, S.; Kodama, T.; Cho, H. Development of a 5kWth internally circulating fluidized bed reactor containing quartz sand for continuously-fed coal-coke gasification and a beam-down solar concentrating system. Energy 2019, 166, 1–16. [Google Scholar] [CrossRef]
- Gokon, N.; Izawa, T.; Abe, T.; Kodama, T. Steam gasification of coal cokes in an internally circulating fluidized bed of thermal storage material for solar thermochemical processes. Int. J. Hydrogen Energy 2014, 39, 11082–11093. [Google Scholar] [CrossRef]
- Yang, H.T.; Wang, C.; Xu, S.P.; Liu, R. Biomass gasification over hematite in a decoupled dual loop gasifier. Fuel Process. Technol. 2019, 192, 140–146. [Google Scholar] [CrossRef]
- Jiang, C.; Jin, X.Y.; Xu, T.T.; Xiao, B.; Hu, Z.Q.; Wang, X. Biomass chemical looping gasification for syngas production using modified hematite as oxygen carriers. J. Environ. Sci. 2023, 125, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Yuan, S.; Liu, C.; Zhang, T.; Xie, X.; Deng, X.; He, R. A Fe-Ca/SiO2 catalyst for efficient production of light aromatics from catalytic pyrolysis of biomass. Fuel 2020, 279, 118500. [Google Scholar] [CrossRef]
- Tomishige, K.; Miyazawa, T.; Asadullah, M.; Ito, S.I.; Kunimori, K. Syngas production from gasification of biomass over Rh/CeO/SiOcatalyst: Pyrogasification, steam reforming and CO reforming. Bull. Jpn. Pet. Inst. 2003, 46, 322–327. [Google Scholar] [CrossRef]
- Vasanthakumar, P.; Karvembu, R. Unmodified Maghemite from River Sand as a Selective Catalyst for Base-Free Transfer Hydrogenation of Furfural, Levulinic Acid, and o-Vanillin: A Pathway for Sustainable Biomass Conversions. ACS Sustain. Chem. Eng. 2020, 8, 17069–17078. [Google Scholar] [CrossRef]
- Li, M.; Sun, L.; Chen, L.; Feng, H.; Zhao, B.; Yang, S.; Xie, X.; Zhang, X. Syngas production from biomass chemical looping gasification with Fe2O3–CaO oxygen carrier. J. Therm. Anal. Calorim. 2022, 147, 7811–7817. [Google Scholar] [CrossRef]
- Qin, W.; Luo, L.; Chen, S.; Iqbal, T.; Xiao, X.; Dong, C. Efficient strategy of utilizing alkaline liquid waste boosting biomass chemical looping gasification to produce hydrogen. Fuel Process. Technol. 2021, 217, 106818. [Google Scholar] [CrossRef]
- Deng, J.; Gao, S.; Yang, T.; Ma, D.; Luo, X.D.; Liu, H.; Yuan, S.F. Investigating the promotion of Fe-Co catalyst by alkali and alkaline earth metals of inherent metal minerals for biomass pyrolysis. Renew. Energy 2023, 213, 134–147. [Google Scholar] [CrossRef]
- Wei, G.Q.; Yang, M.; Huang, Z.; Bai, H.C.; Chang, G.Z.; He, F.; Yi, Q.; Huang, Y.; Zheng, A.Q.; Zhao, K.; et al. Syngas production from lignite via chemical looping gasification with hematite oxygen carrier enhanced by exogenous metals. Fuel 2022, 321, 124119. [Google Scholar] [CrossRef]
- Lu, Q.X.; Yuan, S.F.; Li, J.F.; Chen, X.; Li, K.; Xie, X.G.; Fu, X.L.; He, Z.Y. Influence of Mg and Co addition on Fe based catalyst for in-situ biomass pyrolysis. J. Anal. Appl. Pyrolysis 2023, 169, 105832. [Google Scholar] [CrossRef]
- Zhang, S.P.; Wang, J.X.; Ye, L.; Li, S.; Su, Y.H.; Zhang, H.Y. Investigation into biochar supported Fe-Mo carbides catalysts for efficient biomass gasification tar cracking. Chem. Eng. J. 2023, 454, 140072. [Google Scholar] [CrossRef]
- Gao, N.B.; Li, A.M.; Quan, C. A novel reforming method for hydrogen production from biomass steam gasification. Bioresour. Technol. 2009, 100, 4271–4277. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.Q.; Peng, K.Y.; Liang, S.; Jia, X.P.; Jiang, X.C.; Qian, L. Evaluation of the catalytic performance of different activated biochar catalysts for removal of tar from biomass pyrolysis. Fuel 2019, 258, 116204. [Google Scholar] [CrossRef]
- Xu, S.S.; Slater, T.J.A.; Huang, H.; Zhou, Y.T.; Jiao, Y.L.; Parlett, C.M.A.; Guan, S.L.; Chansai, S.; Xu, S.J.; Wang, X.R.; et al. Developing silicalite-1 encapsulated Ni nanoparticles as sintering-/coking-resistant catalysts for dry reforming of methane. Chem. Eng. J. 2022, 446, 137439. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, K.S. Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield—A review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Shahbaz, M.; Yusup, S.; Inayat, A.; Patrick, D.O.; Ammar, M. The influence of catalysts in biomass steam gasification and catalytic potential of coal bottom ash in biomass steam gasification: A review. Renew. Sustain. Energy Rev. 2017, 73, 468–476. [Google Scholar] [CrossRef]
- Hejazi, B. Heat integration and waste minimization of biomass steam gasification in a bubbling fluidized bed reactor. Biomass Bioenergy 2022, 159, 106409. [Google Scholar] [CrossRef]
- Hoang, A.T.; Huang, Z.H.; Nizetic, S.; Pandey, A.; Nguyen, X.P.; Luque, R.; Ong, H.C.; Said, Z.; Le, T.H.; Pham, V.V. Characteristics of hydrogen production from steam gasification of plant-originated lignocellulosic biomass and its prospects in Vietnam. Int. J. Hydrogen Energy 2022, 47, 4394–4425. [Google Scholar] [CrossRef]
- Pang, Y.J.; Yang, C.; Wu, Y.T.; Chen, Y.S.; Li, H. Study on counter-flow steam gasification characteristics of biochar with Fe2O3/CaO in-situ catalysis in fixed bed. Appl. Energy 2022, 326, 120046. [Google Scholar] [CrossRef]






| Sample | Ultimate Analysis (wt%, ad a) | Proximate Analysis (wt%, ad a) | ||||||
|---|---|---|---|---|---|---|---|---|
| C | H | O * | N | S | M | V | FC * | |
| Waste woods | 46.15 | 6.81 | 41.81 | 5.12 | 0.11 | 2.01 | 78.33 | 14.79 |
| Sample | Fe | O * | Si | Ca | Al |
|---|---|---|---|---|---|
| Hematite | 64.64 | 31.36 | 2.03 | 1.39 | 0.21 |
| ρ (kg/m3) | d (μm) | H (m) | V (m/s) | |
|---|---|---|---|---|
| A1 | 2600 | 335 | 0.027 | 0.13 |
| A2 | 2600 | 335 | 0.054 | 0.13 |
| A3 | 2600 | 335 | 0.081 | 0.13 |
| A4 | 2600 | 335 | 0.108 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, N.; Zhu, K.; Fang, S.; Deng, L.; Lin, Y.; Huang, Z.; Li, J.; Huang, H. A Numerical Simulation and Experimental Study of Fluidization Characteristics of a Bubbling Fluidized Bed in Biomass Gasification. Energies 2024, 17, 2302. https://doi.org/10.3390/en17102302
Gao N, Zhu K, Fang S, Deng L, Lin Y, Huang Z, Li J, Huang H. A Numerical Simulation and Experimental Study of Fluidization Characteristics of a Bubbling Fluidized Bed in Biomass Gasification. Energies. 2024; 17(10):2302. https://doi.org/10.3390/en17102302
Chicago/Turabian StyleGao, Na, Kang Zhu, Shiwen Fang, Lisheng Deng, Yan Lin, Zhen Huang, Jun Li, and Hongyu Huang. 2024. "A Numerical Simulation and Experimental Study of Fluidization Characteristics of a Bubbling Fluidized Bed in Biomass Gasification" Energies 17, no. 10: 2302. https://doi.org/10.3390/en17102302






