Efficient Electrocatalytic Ammonia Synthesis via Theoretical Screening of Titanate Nanosheet-Supported Single-Atom Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Density Fuctional Theory Calculation
2.2. Chemicals and Material
2.3. Synthesis of TiNS and Ru-TiNS Nanosheets
2.4. Characterizations
2.5. Electrocatalytic Nitrogen Reduction Reaction (NRR) Experiment
3. Results and Discussion
3.1. Theoretical Screening
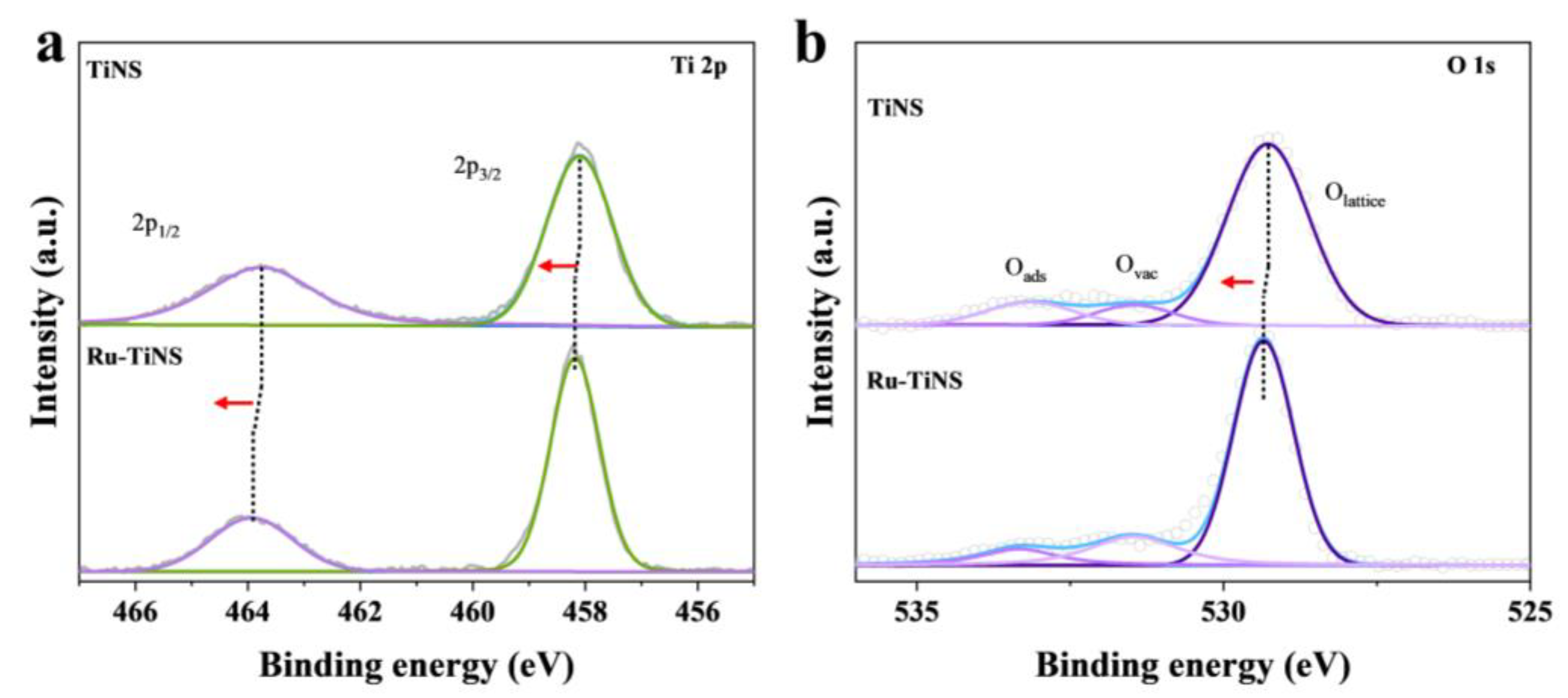
3.2. Characterization of Model Catalyst (Ru-TiNS)
3.3. NRR Performance Test of Ru-TiNS
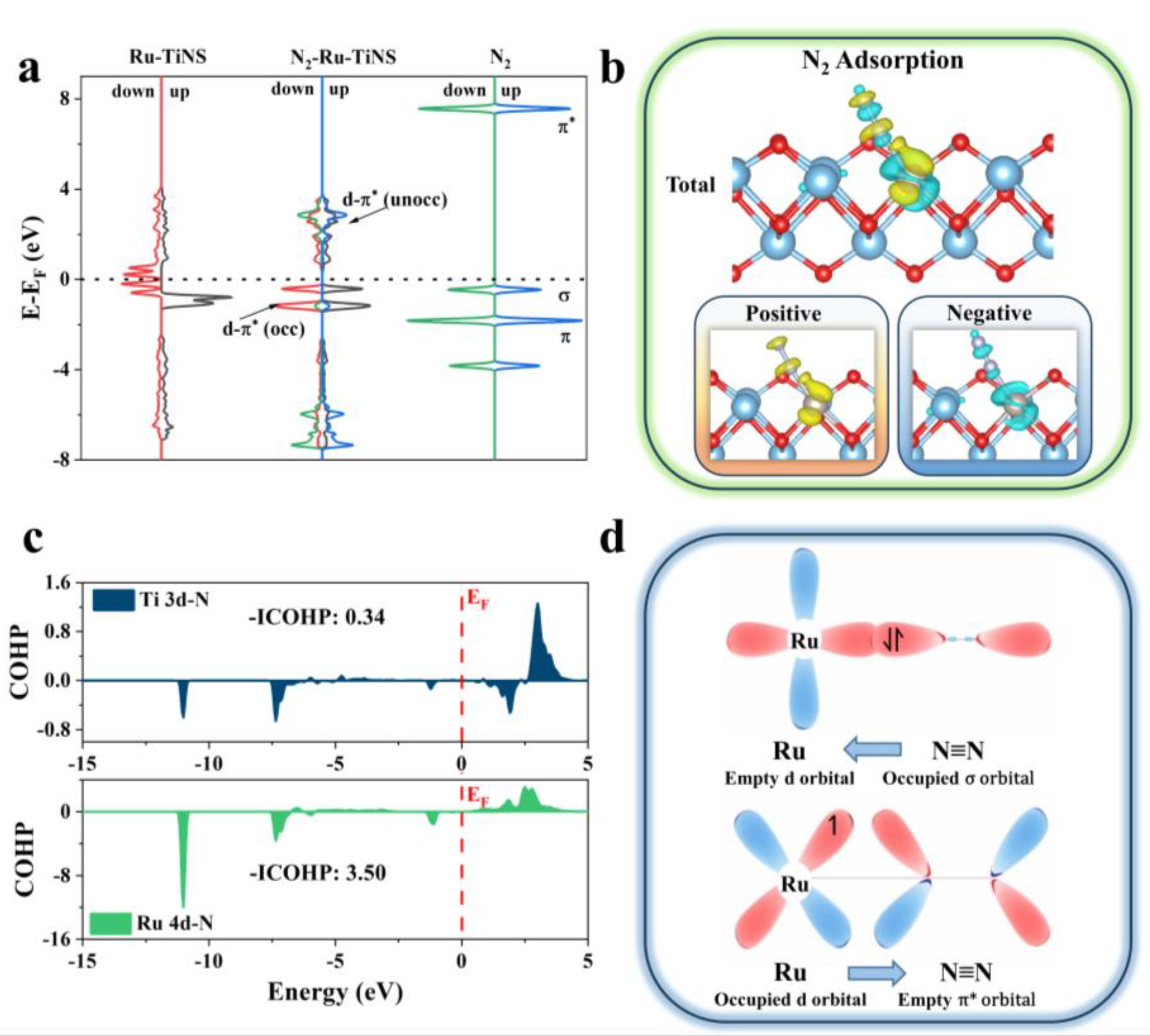
3.4. Interaction between Ru-TiNS and Reactants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dutta, I.; Parsapur, R.K.; Chatterjee, S.; Hengne, A.M.; Tan, D.; Peramaiah, K.; Solling, T.I.; Nielsen, O.J.; Huang, K.-W. The role of fugitive hydrogen emissions in selecting hydrogen carriers. ACS Energy Lett. 2023, 8, 3251–3257. [Google Scholar] [CrossRef]
- Pivovar, B.; Rustagi, N.; Satyapal, S. Hydrogen at scale (H2 @Scale): Key to a clean, economic, and sustainable energy system. Electrochem. Soc. Interface 2018, 27, 47–52. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Stavila, V.; Snider, J.L.; Witman, M.; Bowden, M.E.; Brooks, K.; Tran, B.L.; Autrey, T. Challenges to developing materials for the transport and storage of hydrogen. Nat. Chem. 2022, 14, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Afshan, S.; Hjalmarsson, J. A review of metallic tanks for H2 storage with a view to application in future green shipping. Int. J. Hydrogen Energy 2021, 46, 6151–6179. [Google Scholar] [CrossRef]
- Lu, R.; Rao, D.; Lu, Z.; Qian, J.; Li, F.; Wu, H.; Wang, Y.; Xiao, C.; Deng, K.; Kan, E.; et al. Prominently improved hydrogen purification and dispersive metal binding for hydrogen storage by substitutional doping in porous graphene. J. Phys. Chem. C 2012, 116, 21291–21296. [Google Scholar] [CrossRef]
- Sørensen, R.Z.; Hummelshøj, J.S.; Klerke, A.; Reves, J.B.; Vegge, T.; Nørskov, J.K.; Christensen, C.H. Indirect, Reversible high-density hydrogen storage in compact metal ammine salts. J. Am. Chem. Soc. 2008, 130, 8660–8668. [Google Scholar] [CrossRef] [PubMed]
- Awad, O.I.; Zhou, B.; Harrath, K.; Kadirgama, K. Characteristics of NH3/H2 blend as carbon-free fuels: A review. Int. J. Hydrogen Energy 2023, 48, 38077–38100. [Google Scholar] [CrossRef]
- Guo, J.; Chen, P. Catalyst: NH3 as an energy carrier. Chem 2017, 3, 709–712. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Y.; Zhao, Y.; Zhang, R.; Zhang, J.; Guo, H. NH3 decomposition for H2 generation: Effects of cheap metals and supports on plasma–catalyst synergy. ACS Catal. 2015, 5, 4167–4174. [Google Scholar] [CrossRef]
- Dirtu, D.; Odochian, L.; Pui, A.; Humelnicu, I. Thermal decomposition of ammonia. N2H4-an intermediate reaction product. Open Chem. 2006, 4, 666–673. [Google Scholar] [CrossRef]
- Chang, F.; Gao, W.; Guo, J.; Chen, P. Emerging materials and methods toward ammonia-based energy storage and conversion. Adv. Mater. 2021, 33, 2005721. [Google Scholar] [CrossRef] [PubMed]
- Hollevoet, L.; Jardali, F.; Gorbanev, Y.; Creel, J.; Bogaerts, A.; Martens, J.A. Towards green ammonia synthesis through plasma-driven nitrogen oxidation and catalytic reduction. Angew. Chem. Int. Ed. 2020, 59, 23825–23829. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Khan, M.A.; Mohsin, I.; Wicks, J.; Ip, A.H.; Sumon, K.Z.; Dinh, C.-T.; Sargent, E.H.; Gates, I.D.; Kibria, M.G. Can sustainable ammonia synthesis pathways compete with fossil-fuel based Haber–Bosch processes? Energy Environ. Sci. 2021, 14, 2535–2548. [Google Scholar] [CrossRef]
- Hasan, A.; Dincer, I. Development of an integrated wind and PV system for ammonia and power production for a sustainable community. J. Clean. Prod. 2019, 231, 1515–1525. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, F.; Li, W.; Li, Y.; Liu, C.; Zhao, Z.; Shan, Y.; Ji, Y.; Sun, L. Identification of M-NH2-NH2 intermediate and rate determining step for nitrogen reduction with bioinspired sulfur-bonded few catalyst. Angew. Chem. Int. Ed. 2021, 60, 20331–20341. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, G.; Chen, G.F.; Zhang, H.; Zhang, S.; Wang, H. Comprehensive understanding of the thriving ambient electrochemical nitrogen reduction reaction. Adv. Mater. 2021, 33, 2007650. [Google Scholar] [CrossRef]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B Environ. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Ge, M.; Cai, J.; Iocozzia, J.; Cao, C.; Huang, J.; Zhang, X.; Shen, J.; Wang, S.; Zhang, S.; Zhang, K.-Q.; et al. A review of TiO2 nanostructured catalysts for sustainable H2 generation. Int. J. Hydrogen Energy 2017, 42, 8418–8449. [Google Scholar] [CrossRef]
- Deng, Z.; Li, L.; Ren, Y.; Ma, C.; Liang, J.; Dong, K.; Liu, Q.; Luo, Y.; Li, T.; Tang, B.; et al. Highly efficient two-electron electroreduction of oxygen into hydrogen peroxide over Cu-doped TiO2. Nano Res. 2022, 15, 3880–3885. [Google Scholar] [CrossRef]
- Nong, S.; Dong, W.; Yin, J.; Dong, B.; Lu, Y.; Yuan, X.; Wang, X.; Bu, K.; Chen, M.; Jiang, S.; et al. Well-dispersed ruthenium in mesoporous crystal TiO2 as an advanced electrocatalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2018, 140, 5719–5727. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Sun, H.; Liao, J.; Li, Q.; Xue, Z.; Xue, F.; Liu, F.; Wu, M.; Gao, T.; Teng, L. Study on the catalytic performance of Pd/TiO2 electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 6441–6447. [Google Scholar] [CrossRef]
- Yang, L.; Wang, C.; Li, Y.; Ge, W.; Tang, L.; Shen, J.; Zhu, Y.; Li, C. Frustrated Lewis Pairs on Zr single atoms supported N-doped TiO2−x catalysts for electrochemical nitrate reduction to ammonia. Adv. Funct. Mater. 2024, 2401094. [Google Scholar] [CrossRef]
- Pan, L.; Wang, J.; Lu, F.; Liu, Q.; Gao, Y.; Wang, Y.; Jiang, J.; Sun, C.; Wang, J.; Wang, X. Single-atom or dual-atom in TiO2 nanosheet: Which is the better choice for electrocatalytic urea synthesis? Angew. Chem. Int. Ed. 2023, 62, e202216835. [Google Scholar] [CrossRef]
- Yang, X.; Wang, R.; Wang, S.; Song, C.; Lu, S.; Fang, L.; Yin, F.; Liu, H. Sequential active-site switches in integrated Cu/Fe-TiO2 for efficient electroreduction from nitrate into ammonia. Appl. Catal. B Environ. 2023, 325, 122360. [Google Scholar] [CrossRef]
- Lv, J.; Tian, Z.; Dai, K.; Ye, Y.; Liang, C. Interface and defect engineer of titanium dioxide supported palladium or platinum for tuning the activity and selectivity of electrocatalytic nitrogen reduction reaction. J. Colloid Interf. Sci. 2019, 553, 126–135. [Google Scholar] [CrossRef]
- Song, G.; Gao, R.; Zhao, Z.; Zhang, Y.; Tan, H.; Li, H.; Wang, D.; Sun, Z.; Feng, M. High-spin state Fe(III) doped TiO2 for electrocatalytic nitrogen fixation induced by surface F modification. Appl. Catal. B Environ. 2022, 301, 120809. [Google Scholar] [CrossRef]
- Yang, P.; Guo, H.; Wu, H.; Zhang, F.; Liu, J.; Li, M.; Yang, Y.; Cao, Y.; Yang, G.; Zhou, Y. Boosting charge-transfer in tuned Au nanoparticles on defect-rich TiO2 nanosheets for enhancing nitrogen electroreduction to ammonia production. J. Colloid Interf. Sci. 2023, 636, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhang, L.; Song, J.; Wen, S.; Cheng, Z. Phosphorus cation substitution in TiO2 nanorods toward enhanced N2 electroreduction. Appl. Surf. Sci. 2020, 523, 146517. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes forab initiototal-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Nelson, R.; Ertural, C.; George, J.; Deringer, V.L.; Hautier, G.; Dronskowski, R. LOBSTER: Local orbital projections, atomic charges, and chemical-bonding analysis from projector-augmented-wave-based density-functional theory. J. Comput. Chem. 2020, 41, 1931–1940. [Google Scholar] [CrossRef]
- Maintz, S.; Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. LOBSTER: A tool to extract chemical bonding from plane-wave based DFT. J. Comput. Chem. 2016, 37, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. Crystal orbital hamilton population (COHP) analysis as projected from plane-wave basis sets. J. Phys. Chem. A 2011, 115, 5461–5466. [Google Scholar] [CrossRef] [PubMed]
- Dronskowski, R.; Bloechl, P.E. Crystal orbital Hamilton populations (COHP): Energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 2002, 97, 8617–8624. [Google Scholar] [CrossRef]
- Li, L.; Tang, C.; Yao, D.; Zheng, Y.; Qiao, S.-Z. Electrochemical nitrogen reduction: Identification and elimination of contamination in electrolyte. ACS Energy Lett. 2019, 4, 2111–2116. [Google Scholar] [CrossRef]
- Lu, F.; Wang, J.; Gao, Y.; Wang, Y.; Wang, X. Engineering Pt-Cu diatomics electrocatalysts enables highly efficient urea synthesis. AIChE J. 2024, e18403. [Google Scholar] [CrossRef]
- Tayyebi, E.; Abghoui, Y.; Skúlason, E. Elucidating the mechanism of electrochemical N2 reduction at the Ru(0001) electrode. ACS Catal. 2019, 9, 11137–11145. [Google Scholar] [CrossRef]
- Feng, X.; Liu, J.; Chen, L.; Kong, Y.; Zhang, Z.; Zhang, Z.; Wang, D.; Liu, W.; Li, S.; Tong, L.; et al. Hydrogen radical-induced electrocatalytic N2 reduction at a low potential. J. Am. Chem. Soc. 2023, 145, 10259–10267. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Lu, F.; Zhang, F.; Liu, S.; Zhou, B.; Gao, D.; Wang, X.; Yao, J. Regulating charge transfer of lattice oxygen in single-atom-doped titania for hydrogen evolution. Angew. Chem. Int. Ed. 2020, 59, 15855–15859. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, R.; Liu, Q.; Yang, Y.; Wang, X. Enhancing electrocatalytic ammonia synthesis through theoretical design of cluster catalysts supported on TiO2 surface. Mater. Today Energy 2024, 39, 101466. [Google Scholar] [CrossRef]
- Sasaki, T.; Watanabe, M. Osmotic swelling to exfoliation. Exceptionally high degrees of hydration of a layered titanate. J. Am. Chem. Soc. 1998, 120, 4682–4689. [Google Scholar] [CrossRef]
- Sasaki, T.; Watanabe, M.; Hashizume, H.; Yamada, H.; Nakazawa, H. Macromolecule-like aspects for a colloidal suspension of an exfoliated titanate. Pairwise association of nanosheets and dynamic reassembling process initiated from it. J. Am. Chem. Soc. 1996, 118, 8329–8335. [Google Scholar] [CrossRef]
- Morgan, D.J. Resolving ruthenium: XPS studies of common ruthenium materials. Surf. Interface Anal. 2015, 47, 1072–1079. [Google Scholar] [CrossRef]
- Citrin, P.H.; Ginsbeg, A.P. X-ray photoemission from the Creutz-Taube mixed valence complex: A reassessment. J. Am. Chem. Soc. 2002, 103, 3673–3679. [Google Scholar] [CrossRef]
- Tian, M.; Jiang, Z.; Chen, C.; Kosari, M.; Li, X.; Jian, Y.; Huang, Y.; Zhang, J.; Li, L.; Shi, J.-W.; et al. Engineering Ru/MnCo3Ox for 1,2-dichloroethane benign destruction by strengthening C–Cl cleavage and chlorine desorption: Decisive role of H2O and Reaction Mechanism. ACS Catal. 2022, 12, 8776–8792. [Google Scholar] [CrossRef]
- Li, J.; Yi, D.; Zhan, F.; Zhou, B.; Gao, D.; Guo, D.; Liu, S.; Wang, X.; Yao, J. Monolayered Ru1/TiO2 nanosheet enables efficient visible-light-driven hydrogen evolution. Appl. Catal. B Environ. 2020, 271, 118925. [Google Scholar] [CrossRef]
- Sakamoto, K.; Hayashi, F.; Sato, K.; Hirano, M.; Ohtsu, N. XPS spectral analysis for a multiple oxide comprising NiO, TiO2, and NiTiO3. Appl. Surf. Sci. 2020, 526, 146729. [Google Scholar] [CrossRef]
- Chuang, S.H.; Gao, R.H.; Wang, D.Y.; Liu, H.P.; Chen, L.M.; Chiang, M.Y. Synthesis and characterization of ilmenite-type cobalt titanate powder. J. Chin. Chem. Soc. 2013, 57, 932–937. [Google Scholar] [CrossRef]
- Wang, Y.-w.; Yuan, P.-H.; Fan, C.-M.; Wang, Y.; Ding, G.-Y.; Wang, Y.-F. Preparation of zinc titanate nanoparticles and their photocatalytic behaviors in the photodegradation of humic acid in water. Ceram. Int. 2012, 38, 4173–4180. [Google Scholar] [CrossRef]
- Chuang, S.H.; Gao, R.H.; Gao, K.H.; Chiang, M.Y.; Chao, T.S. Formation and structural characterization of cobalt titanate thin films. J. Chin. Chem. Soc. 2013, 57, 1022–1026. [Google Scholar] [CrossRef]
- Xu, W.; Fan, G.; Chen, J.; Li, J.; Zhang, L.; Zhu, S.; Su, X.; Cheng, F.; Chen, J. Nanoporous palladium hydride for electrocatalytic N2 reduction under ambient conditions. Angew. Chem. Int. Ed. Engl. 2020, 59, 3511–3516. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Wang, X.; Zhang, S.; Zhang, L.; Zhang, R.; Wang, K.; Song, S.; Zhang, H. Boosting electrochemical nitrogen fixation via regulating surface electronic structure by CeO2 hybridization. Small 2024, 2310268. [Google Scholar] [CrossRef]
- Mushtaq, M.A.; Kumar, A.; Yasin, G.; Tabish, M.; Arif, M.; Ajmal, S.; Raza, W.; Naseem, S.; Zhao, J.; Li, P.; et al. Multivalent sulfur vacancy-rich NiCo2S4 @MnO2 urchin-like heterostructures for ambient electrochemical N2 reduction to NH3. Small 2024, 3, e2310431. [Google Scholar] [CrossRef]
- Ling, C.; Niu, X.; Li, Q.; Du, A.; Wang, J. Metal-free single atom catalyst for N2 fixation driven by visible light. J. Am. Chem. Soc. 2018, 140, 14161–14168. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Wang, J.; Yang, Y.; Wang, X. Efficient Electrocatalytic Ammonia Synthesis via Theoretical Screening of Titanate Nanosheet-Supported Single-Atom Catalysts. Materials 2024, 17, 2239. https://doi.org/10.3390/ma17102239
Zhao K, Wang J, Yang Y, Wang X. Efficient Electrocatalytic Ammonia Synthesis via Theoretical Screening of Titanate Nanosheet-Supported Single-Atom Catalysts. Materials. 2024; 17(10):2239. https://doi.org/10.3390/ma17102239
Chicago/Turabian StyleZhao, Kaiheng, Jingnan Wang, Yongan Yang, and Xi Wang. 2024. "Efficient Electrocatalytic Ammonia Synthesis via Theoretical Screening of Titanate Nanosheet-Supported Single-Atom Catalysts" Materials 17, no. 10: 2239. https://doi.org/10.3390/ma17102239




