Highly Mechanical Strength, Flexible and Stretchable Wood-Based Elastomers without Chemical Cross-Linking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Delignification of the Balsa Wood
2.3. Preparation of Super Stretched Wood Based Elastic Materials
2.4. Characterization
2.4.1. Morphology
2.4.2. X-ray Diffraction Measurement
2.4.3. Small-Angle X-ray Scattering Measurement
2.4.4. Fourier Transform Infrared (FTIR) Characterization
2.4.5. Chemical Composition Analysis
2.4.6. Tensile Test
2.4.7. Sensor Performance
3. Results and Discussion
3.1. The Physical Properties of SWE
3.2. The Chemical Properties of SWE
3.3. Strengthening and Toughening of SWE
3.4. The Flexible Deformation Capability of SWE
3.5. The Application in the Field of Flexible Electronics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dakneviciute, V.; Milasiene, D.; Ukvalbergiene, K. Tensile Strength, Elasticity and Cracking Character of Softwood Tissues. Medžiagotyra 2015, 21, 260–264. [Google Scholar]
- Ramage, M.H.; Burridge, H.; Busse-Wicher, M.; Fereday, G.; Reynolds, T.; Shah, D.U.; Wu, G.; Yu, L.; Fleming, P.; Densley-Tingley, D.; et al. The wood from the trees: The use of timber in construction. Renew. Sustain. Energy Rev. 2017, 68, 333–359. [Google Scholar] [CrossRef]
- Shi, X.; Luo, J.; Luo, J.; Li, X.; Han, K.; Li, D.; Cao, X.; Wang, Z.L. Flexible Wood-Based Triboelectric Self-Powered Smart Home System. ACS Nano 2022, 16, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, C.; Bian, S.; Hu, K.; Zheng, K.; Sun, Q. Reversible photo-responsive smart wood with resistant to extreme weather. J. Mater. Sci. 2022, 57, 3337–3347. [Google Scholar] [CrossRef]
- Ugolev, B.N. Wood as a natural smart material. Wood Sci. Technol. 2014, 48, 553–568. [Google Scholar] [CrossRef]
- Takahashi, A.; Yamamoto, N.; Ooka, Y.; Toyohiro, T. Tensile Examination and Strength Evaluation of Latewood in Japanese Cedar. Materials 2022, 15, 2347. [Google Scholar] [CrossRef] [PubMed]
- Longo, R.; Laux, D.; Pagano, S.; Delaunay, T.; Le Clézio, E.; Arnould, O. Elastic characterization of wood by Resonant Ultrasound Spectroscopy (RUS): A comprehensive study. Wood Sci. Technol. 2018, 52, 383–402. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Z.; Chu, C.; Ni, Y.; Neisiany, R.E.; You, Z. Biodegradable Elastomers and Gels for Elastic Electronics. Adv. Sci. 2022, 9, 2105146. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Li, X.C.; Yuan, H.; Liu, D.; Lai, W.Y. Self-Healing Elastic Electronics: Materials Design, Mechanisms, and Applications. Adv. Funct. Mater. 2024. early view. [Google Scholar] [CrossRef]
- Walley, S.M.; Rogers, S.J. Is Wood a Material? Taking the Size Effect Seriously. Materials 2022, 15, 5403. [Google Scholar] [CrossRef]
- Keying, L.; Dong, W.; Lanying, L.; Feng, F.U. Research Progress in Multi-scale Interface Structure and Mechanical Properties of Wood. Trans. China Pulp Pap. 2021, 36, 88–94. [Google Scholar] [CrossRef]
- Shumin, Y.; Xing’E, L.; Lili, S.; Jianfeng, M.A.; Genlin, T.; Zehui, J. The Characteristics and Representation Methods of Lignin for Bamboo. Mater. Rep. 2020, 34, 7177–7182. [Google Scholar] [CrossRef]
- Börcsök, Z.; Pásztory, Z. The role of lignin in wood working processes using elevated temperatures: An abbreviated literature survey. Eur. J. Wood Wood Prod. 2021, 79, 511–526. [Google Scholar] [CrossRef]
- Galos, J.; Das, R.; Sutcliffe, M.P.; Mouritz, A.P. Review of balsa core sandwich composite structures. Mater. Des. 2022, 221, 111013. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, P.; Wang, Z.; Ma, F.; Ji, H.; Li, X. Combined effect of rays and vessels to achieve high strength and toughness in balsa wood. Mater. Lett. 2023, 352, 135137. [Google Scholar] [CrossRef]
- Gao, R.; Huang, Y.; Gan, W.; Xiao, S.; Gao, Y.; Fang, B.; Zhang, X.; Lyu, B.; Huang, R.; Li, J.; et al. Superhydrophobic elastomer with leaf-spring microstructure made from natural wood without any modification chemicals. Chem. Eng. J. 2022, 442, 136338. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, S.; Li, X.; Zou, H.; Zhuo, B.; Ti, P.; Yuan, Q. Optimization and absorption performance of wood sponge. J. Mater. Sci. 2021, 56, 8479–8496. [Google Scholar] [CrossRef]
- Song, D.; Zeng, M.; Min, P.; Jia, X.; Gao, F.; Yu, Z.; Li, X. Electrically conductive and highly compressible anisotropic MXene-wood sponges for multifunctional and integrated wearable devices. J. Mater. Sci. Technol. 2023, 144, 102–110. [Google Scholar] [CrossRef]
- Kuang, X.; Arıcan, M.O.; Zhou, T.; Zhao, X.; Zhang, Y.S. Functional Tough Hydrogels: Design, Processing, and Biomedical Applications. Acc. Mater. Res. 2023, 4, 101–114. [Google Scholar] [CrossRef]
- Li, C.; Wang, C.; Keplinger, C.; Zuo, J.; Jin, L.; Sun, Y.; Zheng, P.; Cao, Y.; Lissel, F.; Linder, C.; et al. A highly stretchable autonomous self-healing elastomer. Nat. Chem. 2016, 8, 618–624. [Google Scholar] [CrossRef]
- Steck, J.; Kim, J.; Yang, J.; Hassan, S.; Suo, Z. Topological adhesion. I. Rapid and strong topohesives. Extrem. Mech. Lett. 2020, 39, 100803. [Google Scholar] [CrossRef]
- Ajdary, R.; Tardy, B.L.; Mattos, B.D.; Bai, L.; Rojas, O.J. Plant Nanomaterials and Inspiration from Nature: Water Interactions and Hierarchically Structured Hydrogels. Adv. Mater. 2021, 33, e2001085. [Google Scholar] [CrossRef]
- Yongyue, Z.; Jiangtao, S.; Zongying, F.; Yun, L. Research Progress of Cellulose Self-Healing Hydrogels. Sci. Silvae Sin. 2024, 60, 128–138. [Google Scholar] [CrossRef]
- Dong, Y.; Pan, N.; Zhu, M.; Tang, M.; Wu, Y.; You, Z.; Zhou, X.; Chen, M. An anti-swelling, strong and flexible wood-based composite hydrogel as strain sensor. Ind. Crops Prod. 2022, 187, 115491. [Google Scholar] [CrossRef]
- Wang, S.; Li, K.; Zhou, Q. High strength and low swelling composite hydrogels from gelatin and delignified wood. Sci. Rep. 2020, 10, 17842. [Google Scholar] [CrossRef]
- Shen, X.; Nie, K.; Zheng, L.; Wang, Z.; Wang, Z.; Li, S.; Jin, C.; Sun, Q. Muscle-inspired capacitive tactile sensors with superior sensitivity in an ultra-wide stress range. J. Mater. Chem. C Mater. Opt. Electron. Devices 2020, 8, 5913–5922. [Google Scholar] [CrossRef]
- Adelnia, H.; Ensandoost, R.; Shebbrin Moonshi, S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Holloway, J.L.; Lowman, A.M.; Palmese, G.R. The role of crystallization and phase separation in the formation of physically cross-linked PVA hydrogels. Soft Matter 2013, 9, 826–833. [Google Scholar] [CrossRef]
- Yan, G.; He, S.; Chen, G.; Ma, S.; Zeng, A.; Chen, B.; Yang, S.; Tang, X.; Sun, Y.; Xu, F.; et al. Highly Flexible and Broad-Range Mechanically Tunable All-Wood Hydrogels with Nanoscale Channels via the Hofmeister Effect for Human Motion Monitoring. Nano-Micro Lett. 2022, 14, 84. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Zhou, T.; Wan, Z.; Yang, Q.; Xu, Z.; Li, D.; Jin, Y. Toward Strong and Tough Wood-Based Hydrogels for Sensors. Biomacromolecules 2021, 22, 5204–5213. [Google Scholar] [CrossRef]
- Borrega, M.; Ahvenainen, P.; Serimaa, R.; Gibson, L. Composition and structure of balsa (Ochroma pyramidale) wood. Wood Sci. Technol. 2015, 49, 403–420. [Google Scholar] [CrossRef]
- Jung, W.; Savithri, D.; Sharma-Shivappa, R.; Kolar, P. Changes in Lignin Chemistry of Switchgrass due to Delignification by Sodium Hydroxide Pretreatment. Energies 2018, 11, 376. [Google Scholar] [CrossRef]
- Tarvo, V.; Lehtimaa, T.; Kuitunen, S.; Alopaeus, V.; Vuorinen, T.; Aittamaa, J. A Model for Chlorine Dioxide Delignification of Chemical Pulp. J. Wood Chem. Technol. 2010, 30, 230–268. [Google Scholar] [CrossRef]
- Kubo, S.; Kadla, J.F. The Formation of Strong Intermolecular Interactions in Immiscible Blends of Poly(vinyl alcohol) (PVA) and Lignin. Biomacromolecules 2003, 4, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Wei, L.; Lin, C.; Ma, Q.; Dai, H.; Zhu, J.Y. Lignin-Containing Cellulose Nanofibril-Reinforced Polyvinyl Alcohol Hydrogels. ACS Sustain. Chem. Eng. 2018, 6, 4821–4828. [Google Scholar] [CrossRef]
- Ram, F.; Garemark, J.; Li, Y.; Pettersson, T.; Berglund, L.A. Functionalized Wood Veneers as Vibration Sensors: Exploring Wood Piezoelectricity and Hierarchical Structure Effects. ACS Nano 2022, 16, 15805–15813. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, Y.; Nishiyama, Y.; Pingali, S.V.; O Neill, H.M.; Zhang, Q.; Berglund, L.A. Small Angle Neutron Scattering Shows Nanoscale PMMA Distribution in Transparent Wood Biocomposites. Nano Lett. 2021, 21, 2883–2890. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tang, Q.; Yang, F.; Xu, L.; Wang, X.; Zhang, J. Mechanical and thermal properties of rice straw cellulose nanofibrils-enhanced polyvinyl alcohol films using freezing-and-thawing cycle method. Cellulose 2019, 26, 3193–3204. [Google Scholar] [CrossRef]
- Kong, W.; Wang, C.; Jia, C.; Kuang, Y.; Pastel, G.; Chen, C.; Chen, G.; He, S.; Huang, H.; Zhang, J.; et al. Muscle-Inspired Highly Anisotropic, Strong, Ion-Conductive Hydrogels. Adv. Mater. 2018, 30, 1801934. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, C.; Yang, W.; Yao, C.; Jing, Y.; Yu, N.; Su, S.; Mahmud, S.; Zhang, X.; Zhu, J. Design of delignified wood-based high-performance composite hydrogel electrolyte with double crosslinking of sodium alginate and PAM for flexible supercapacitors. Ind. Crops Prod. 2024, 210, 118187. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Z.; Wang, S.; Yao, X.; Han, X.; Cao, W.; Pu, J. An anti-freezing and strong wood-derived hydrogel for high-performance electronic skin and wearable sensing. Compos. Part B Eng. 2022, 239, 109954. [Google Scholar] [CrossRef]
- Chen, L.; Wei, X.; Wang, F.; Jian, S.; Yang, W.; Ma, C.; Duan, G.; Jiang, S. In-situ polymerization for mechanical strong composite actuators based on anisotropic wood and thermoresponsive polymer. Chin. Chem. Lett. 2022, 33, 2635–2638. [Google Scholar] [CrossRef]



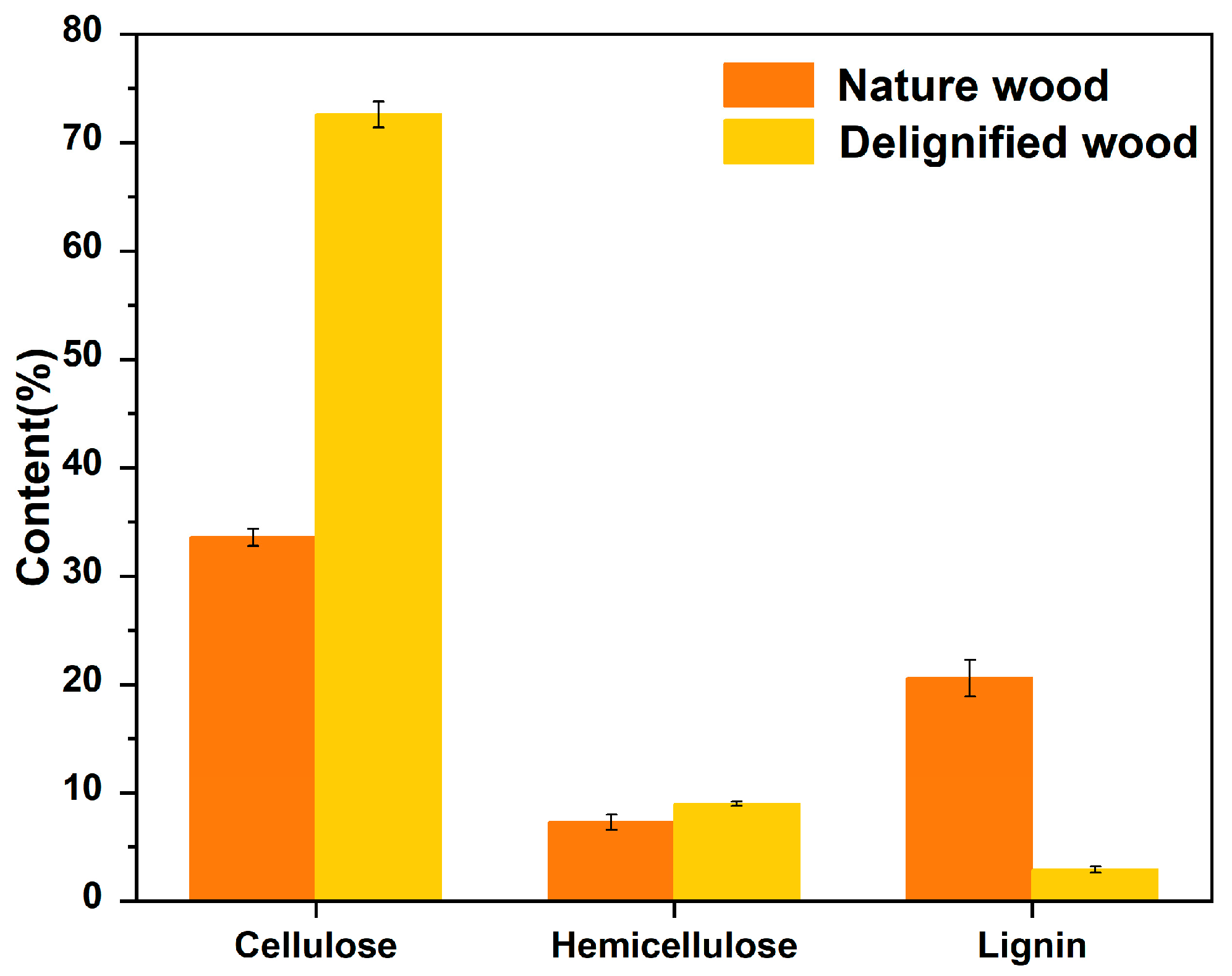






| Name | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|
| Nature wood | 33.6 ± 0.8 | 7.3 ± 0.7 | 20.6 ± 1.7 |
| Delignified wood | 72.6 ± 1.2 | 9.0 ± 0.2 | 2.6 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, J.; Lu, Y.; Shi, J. Highly Mechanical Strength, Flexible and Stretchable Wood-Based Elastomers without Chemical Cross-Linking. Forests 2024, 15, 836. https://doi.org/10.3390/f15050836
Zhang Y, Li J, Lu Y, Shi J. Highly Mechanical Strength, Flexible and Stretchable Wood-Based Elastomers without Chemical Cross-Linking. Forests. 2024; 15(5):836. https://doi.org/10.3390/f15050836
Chicago/Turabian StyleZhang, Yongyue, Jiayao Li, Yun Lu, and Jiangtao Shi. 2024. "Highly Mechanical Strength, Flexible and Stretchable Wood-Based Elastomers without Chemical Cross-Linking" Forests 15, no. 5: 836. https://doi.org/10.3390/f15050836





