Numerical Simulation of the Density Effect on the Macroscopic Transport Process of Tracer in the Ruhrstahl–Heraeus (RH) Vacuum Degasser
Abstract
:1. Introduction
2. Experimental Principle
2.1. Physical Model
2.2. Numerical Model
2.2.1. Turbulence Model
2.2.2. Tracer Transport Model
2.2.3. Mesh and Boundary Conditions
2.3. Studied Cases
3. Results
3.1. Model Verification
3.2. Flow Pattern
3.3. Transport Process of Salt Tracer
3.4. Comparison of Tracer Transport Processes with Different Densities
3.4.1. Comparison of Tracer Transport Processes with Different Densities When the Lifting Gas Flow Rate Is 40 L/min
3.4.2. Comparison of Different Tracer Transport Processes When the Lifting Gas Flow Rate Is 20 L/min
3.4.3. Comparison of Mixing Time
4. Conclusions
- Under both gas lifting flow rates, the transport rate of the NaCl solution tracer into the ladle is faster, and its transport rate at the bottom of the ladle is also faster than that of the passive scalar tracer. In the area near the up snorkel at the top of the ladle, the transport rate of the NaCl solution tracer remains faster. In the area near the down snorkel at the top of the ladle, the transport rate of the passive scalar is faster.
- The transport of the tracer to the top region of the ladle involves two stages: (1) When the tracer is carried to the bottom of the ladle with the recirculation, at this point, because the density of the salt tracer is higher than that of the passive scalar, density and gravity promote the transport of the tracer, making the transport rate of the salt tracer faster. (2) As the tracer continues to diffuse upwards to the top of the ladle, density and gravity hinder the transport of the tracer, slowing down the transport rate of the salt tracer, with density and gravity playing different roles in the two stages. Due to the different flow field structures inside the ladle, the degree of promotion and obstruction of density and gravity is also different. In the large circulation area on the left side of the ladle, the promoting effect is greater than the hindering effect. Therefore, in the left area at the top of the ladle, the concentration of salt tracer increases faster than that of the passive scalar. In the small circulation flow area on the right side of the ladle, the hindering effect is greater than the promoting effect, resulting in a slower increase in the concentration of salt tracer in the top right area of the ladle compared to the passive scalar. In the middle area of the ladle, the promoting and hindering effects are the same, and the concentration growth rate of the salt tracer is the same as that of the passive scalar.
- For both gas lifting flow rate cases, the mixing time at the top of the ladle is generally higher than that at the bottom. When comparing the mixing time at the top and bottom of the ladle, the difference in the passive scalar case is 1.3% and 11.8% for 40 L/min and 20 L/min flow rate schemes, respectively. In addition, the difference of the NaCl solution case (300 mL) increases to 21% and 21.2% for the two flow rate schemes. In the case of 600 mL NaCl solution, this difference increases to 19.7% and 22.6% for the two schemes.
- When comparing the mixing time between the passive scalar and NaCl solution tracer, at the bottom of the ladle, the difference in mixing time between the passive scalar case and the NaCl solution case is not significant, within the difference range between 1 and 5%. However, at the top of the ladle, the mixing time for the NaCl solution case is significantly longer than that for the passive scalar case, with the difference range between 3 and 14.7%. The increase in the dosage of tracer increases the difference to 17.4–41.1%. This indicates that density and gravity delay the mixing of substances in the top area of the ladle. In the industrial refining process, when adding denser alloys, attention should be paid to the mixing of substances in the top area of the ladle.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.F.; Peng, K.Y.; Ren, Y. Kinetic Analysis for Steel Desulfurization during Ruhrstahl–Heraeus Process. Steel Res. Int. 2023, 95, 2300399. [Google Scholar] [CrossRef]
- She, C.J.; Peng, K.Y.; Sun, Y.; Ren, Y.; Zhang, L.F. Kinetic Model of Desulfurization during RH Refining Process. Metall. Mater. Trans. B 2024, 55, 92–104. [Google Scholar] [CrossRef]
- Wang, H.J.; Xu, R.; Ling, H.T.; Zhong, W.; Chang, L.Z.; Qiu, S.T. Numerical simulation of fluid flow and alloy melting in RH process for electrical steels. J. Iron Steel Res. Int. 2022, 29, 1423–1433. [Google Scholar] [CrossRef]
- Liu, C.; Peng, K.Y.; Wang, Q.; Li, G.Q.; Wang, J.J.; Zhang, L.F. CFD Investigation of Melting Behaviors of Two Alloy Particles during Multiphase Vacuum Refining Process. Metall. Mater. Trans. B 2023, 54, 2174–2187. [Google Scholar] [CrossRef]
- Kim, T.S.; Yang, J.; Park, J.H. Effect of Physicochemical Properties of Slag on the Removal Rate of Alumina Inclusions in the Ruhrstahl–Heraeus (RH) Refining Conditions. Metall. Mater. Trans. B 2022, 53, 2523–2533. [Google Scholar] [CrossRef]
- Zhong, H.J.; Jiang, M.; Wang, Z.Y.; Zhen, X.G.; Zhao, H.M.; Li, T.G.; Wang, X.H. Formation and Evolution of Inclusions in AH36 Steel during LF–RH–CC Process: The Influences of Ca-Treatment, Reoxidation, and Solidification. Metall. Mater. Trans. B 2023, 54, 593–601. [Google Scholar] [CrossRef]
- Peng, K.Y.; Wang, J.J.; Li, Q.L.; Liu, C.; Zhang, L.F. Multiphase Simulation on the Collision, Transport, and Removal of Non-metallic Inclusions in the Molten Steel during RH Refining. Metall. Mater. Trans. B 2023, 54, 928–943. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.H.; He, Y.; Zhou, C.H.; Yuan, B.H.; Zhang, M.; Barati, M. Study of Dispersed Micro-Bubbles and Improved Inclusion Removal in Ruhrstahl–Heraeus (RH) Refining with Argon Injection through Down Leg. Metall. Mater. Trans. B 2023, 54, 2347–2359. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.X.; Ren, Q.; Zhang, L.F. Effect of Ruhrstahl Heraeus Refining Time on Inclusions and Rolling Contact Fatigue Life of a High-Carbon Chromium Bearing Steel. Steel Res. Int. 2023, 94, 2200977. [Google Scholar] [CrossRef]
- Ramírez-López, A. Analysis of Mixing Efficiency in a Stirred Reactor Using Computational Fluid Dynamics. Symmetry 2024, 16, 237. [Google Scholar] [CrossRef]
- He, Q.; Yao, T.L.; Liu, L.; Li, X.C.; Ni, B.; Li, L.F. An experimental study on RH vacuum chamber with a weir. J. Iron Steel Res. Int. 2023, 30, 1929–1938. [Google Scholar] [CrossRef]
- Kato, Y.; Nakato, H.; Fujii, T.; Ohmiya, S.; Takatori, S. Fluid flow in ladle and its effect on decarburization rate in RH degasser. ISIJ Int. 1993, 33, 1088–1094. [Google Scholar] [CrossRef]
- Yoshitomi, K.; Nagase, M.; Uddin, M.A.; Kato, Y. Fluid Mixing in Ladle of RH Degasser Induced by Down Flow. ISIJ Int. 2016, 56, 1119–1123. [Google Scholar] [CrossRef]
- Zhang, L.F.; Li, F. Investigation on the Fluid Flow and Mixing Phenomena in a Ruhrstahl-Heraeus (RH) Steel Degasser Using Physical Modeling. JOM 2014, 66, 1227–1240. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.F.; Li, F.; Peng, K.Y.; Liu, F.G.; Liu, Z.T.; Zhao, Y.Y.; Yang, W.; Zhang, J. Water Modeling on Circulating Flow and Mixing Time in a Ruhrstahl–Heraeus Vacuum Degasser. Steel Res. Int. 2021, 92, 2000608. [Google Scholar] [CrossRef]
- Wang, R.D.; Jin, Y.; Cui, H. The Flow Behavior of Molten Steel in an RH Degasser Under Different Ladle Bottom Stirring Processes. Metall. Mater. Trans. B 2022, 53, 342–351. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.H.; He, Y.; Zhou, C.H.; Yuan, B.H.; Mclean, A. Bubble formation by argon injection through the down-leg snorkel with Ruhrstahl-Heraeus (RH) circulating flow. J. Mater. Process. Technol. 2022, 306, 117647. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Ma, P.; Dong, J.H.; Liu, M.; Liu, Y.G.; Lai, C.B. Effects of Different RH Degasser Nozzle Layouts on the Circulating Flow Rate. Materials 2022, 15, 8476. [Google Scholar] [CrossRef]
- Liu, Z.; Lou, W.; Zhu, M.Y. Numerical Analysis of Fluid Flow and Powder Transport Characteristics of RH-Degasser Ladle Bottom Powder Injection (RH-PBI) Process. Metall. Mater. Trans. B 2024, 55, 418–430. [Google Scholar] [CrossRef]
- Chen, G.J.; He, S.P. Volume of fluid simulation of single argon bubble dynamics in liquid steel under RH vacuum conditions. J. Iron Steel Res. Int. 2024, 31, 828–837. [Google Scholar] [CrossRef]
- Dong, W.L.; Xu, A.J.; Liu, B.S.; Zhou, H.C.; Ji, C.X.; Wang, S.J.; Li, H.B.; Wang, T.H. Mechanism and Model of Nitrogen Absorption of Molten Steel during N2 Injection Process in RH Vacuum. Metall. Mater. Trans. B 2024, 55, 72–82. [Google Scholar] [CrossRef]
- Geng, D.Q.; Zhang, X.; Liu, X.A.; Wang, P.; Liu, H.T.; Chen, H.M.; Dai, C.M.; Lei, H.; He, J.C. Simulation on Flow Field and Mixing Phenomenon in Single Snorkel Vacuum Degasser. Steel Res. Int. 2015, 86, 724–731. [Google Scholar] [CrossRef]
- Dong, J.F.; Feng, C.; Zhu, R.; Wei, G.S.; Jiang, J.J.; Chen, S.Z. Simulation and Application of Ruhrstahl–Heraeus (RH) Reactor with Bottom-Blowing. Metall. Mater. Trans. B 2021, 52, 2127–2138. [Google Scholar] [CrossRef]
- Dong, J.F.; Feng, C.; Zhu, R.; Wei, G.S.; Xia, T.; Zhang, Q.N. Analysis of fluid flow characteristics of RH reactor with different bottom-blowing arrangements. Ironmak. Steelmak. 2023, 50, 325–335. [Google Scholar] [CrossRef]
- Wang, J.H.; Ni, P.Y.; Chen, C.; Ersson, M.; Li, Y. Effect of gas blowing nozzle angle on multiphase flow and mass transfer during RH refining process. Int. J. Miner. Metall. Mater. 2023, 30, 844–856. [Google Scholar] [CrossRef]
- Chen, G.J.; Wang, Q.Q.; He, S.P. Computational Fluid Dynamics Modeling of Argon-Steel (-Slag) Multiphase Flow in an Ruhrstahl-Heraeus Degasser: A Review of Past Numerical Studies. Steel Res. Int. 2023, 94, 2200298. [Google Scholar] [CrossRef]
- Ling, H.T.; Zhang, L.F. Investigation on the Fluid Flow and Decarburization Process in the RH Process. Metall. Mater. Trans. B 2018, 49B, 2709–2721. [Google Scholar] [CrossRef]
- Liu, Z.; Lou, W.T.; Zhu, M.Y. Powder transport behavior in RH degasser with powder injection through up snorkel: A transient numerical model. J. Iron Steel Res. Int. 2023, 30, 1156–1170. [Google Scholar] [CrossRef]
- Silva, A.M.B.; Peixoto, J.J.M.; da Silva, C.A. Numerical and Physical Modeling of Steel Desulfurization on a Modified RH Degasser. Metall. Mater. Trans. B 2023, 54, 2651–2669. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.F.; Sun, Y.; Yang, W. Large Eddy Simulation on the Transient Decarburization of the Molten Steel during RH Refining Process. Metall. Mater. Trans. B 2022, 53, 670–681. [Google Scholar] [CrossRef]
- Peng, K.Y.; Liu, C.; Zhang, L.F.; Sun, Y. Numerical Simulation of Decarburization Reaction with Oxygen Blowing during RH Refining Process. Metall. Mater. Trans. B 2022, 53, 2004–2017. [Google Scholar] [CrossRef]
- Peng, K.Y.; Sun, Y.; Peng, X.X.; Chen, W.; Zhang, L.F. Numerical Simulation on the Desulfurization of the Molten Steel during RH Vacuum Refining Process by CaO Powder Injection. Metall. Mater. Trans. B 2023, 54, 438–449. [Google Scholar] [CrossRef]
- Wang, J.H.; Ni, P.Y.; Zhou, X.B.; Liu, Q.L.; Ersson, M.; Li, Y. Study on Multiphase Flow Characteristics during RH Refining Process Affected by Nonradial Arrangement of Gas-Blowing Nozzle. Steel Res. Int. 2023, 94, 2300200. [Google Scholar] [CrossRef]
- Dai, W.X.; Cheng, G.G.; Zhang, G.L.; Huo, Z.D.; Lv, P.X.; Qiu, Y.L.; Zhu, W.F. Investigation of Circulation Flow and Slag-Metal Behavior in an Industrial Single Snorkel Refining Furnace (SSRF): Application to Desulfurization. Metall. Mater. Trans. B 2020, 51, 611–627. [Google Scholar] [CrossRef]
- Zhang, W.F.; Wang, G.B.; Zhang, Y.L.; Cheng, G.G.; Zhang, G.L. A mathematical model for dehydrogenation of molten steel in the Vacuum degasser. Vacuum 2023, 213, 112149. [Google Scholar] [CrossRef]
- Wang, K.P.; Wang, Y.; Xu, J.F.; Xie, W.; Chen, T.J.; Jiang, M. Influence of Temperature Drop from 1773 K (1500 °C) to 1743 K (1470 °C) on Inclusion of High Carbon Chromium Bearing Steel Melts Treated by RH Vacuum Degassing. Metall. Mater. Trans. B 2022, 53, 3370–3375. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, G.G.; Sun, H.B.; Hou, Z.B.; Wang, X.C.; Zhang, J.Q. Effects of salt tracer amount, concentration and kind on the fluid flow behavior in a hydrodynamic model of continuous casting tundish. Steel Res. Int. 2012, 83, 1141–1151. [Google Scholar] [CrossRef]
- Chen, C.; Jonsson, L.T.I.; Tilliander, A.; Cheng, G.G.; Jönsson, P.G. A mathematical modeling study of tracer mixing in a continuous casting tundish. Metall. Mater. Trans. B 2015, 46, 169–190. [Google Scholar] [CrossRef]
- Ding, C.Y.; Lei, H.; Niu, H.; Zhang, H.; Yang, B.; Zhao, Y. Effects of Salt Tracer Volume and Concentration on Residence Time Distribution Curves for Characterization of Liquid Steel Behavior in Metallurgical Tundish. Metals 2021, 11, 430. [Google Scholar] [CrossRef]
- Ding, C.Y.; Lei, H.; Niu, H.; Zhang, H.; Yang, B.; Li, Q. Effects of Tracer Solute Buoyancy on Flow Behavior in a Single-Strand Tundish. Metall. Mater. Trans. B 2021, 52, 3788–3804. [Google Scholar] [CrossRef]
- Chen, C.; Rui, Q.X.; Cheng, G.G. Effect of salt tracer amount on the mixing time measurement in a hydrodynamic model of gas-stirred ladle system. Steel Res. Int. 2013, 84, 900–907. [Google Scholar] [CrossRef]
- Gómez, A.S.; Conejo, A.N.; Zenit, R. Effect of Separation Angle and Nozzle Radial Position on Mixing Time in Ladles with Two Nozzles. J. Appl. Fluid Mech. 2018, 11, 11–20. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Chen, C.; Lin, W.M.; Yu, Y.C.; Dianyu, E.; Wang, S.B. Numerical Simulation of Tracers Transport Process in Water Model of a Vacuum Refining Unit: Single Snorkel Refining Furnace. Steel Res. Int. 2020, 91, 2000022. [Google Scholar] [CrossRef]
- Ouyang, X.; Lin, W.M.; Luo, Y.Z.; Zhang, Y.X.; Fan, J.P.; Chen, C.; Cheng, G.G. Effect of Salt Tracer Dosages on the Mixing Process in the Water Model of a Single Snorkel Refining Furnace. Metals 2022, 12, 1948. [Google Scholar] [CrossRef]
- Katoh, T.; Okamoto, T. Mixing of Molten Steel in a Ladle with RH Reactor by the Water Model Experiment. Denki Seiko 1979, 50, 128–137. [Google Scholar] [CrossRef]
- Wei, J.H.; Yu, N.W.; Fan, Y.Y.; Yang, S.L.; Ma, J.C.; Zhu, D.P. Study on Flow and Mixing Characteristics of Molten Steel in RH and RH-KTB Refining Processes. J. Shanghai Univ. 2002, 2, 167–175. [Google Scholar] [CrossRef]
- Wei, J.H.; Jiang, X.Y.; Wen, L.J.; Li, B. Mass Transfer Characteristics between Molten Steel and Particles under Conditions of RH-PB(IJ) Refining Process. ISIJ Int. 2007, 47, 408–417. [Google Scholar] [CrossRef]
- Chen, H.M. Mathematical and Physical Simulation for Single Snorkel RH Vacuum Refining Process. Master’s Thesis, Shenyang Northeastern University, Liaoning, China, 2014. [Google Scholar]
- Geng, D.Q.; Lei, H.; He, J.C. Numerical Simulation of the Multiphase Flow in the Rheinsahl–Heraeus (RH) System. Metall. Mater. Trans. B 2010, 41B, 234–247. [Google Scholar] [CrossRef]
- Ling, H.T.; Li, F.; Zhang, L.F.; Conejo, A.N. Investigation on the Effect of Nozzle Number on the Recirculation Rate and Mixing Time in the RH Process Using VOF+DPM Model. Metall. Mater. Trans. B 2016, 47, 1950–1961. [Google Scholar] [CrossRef]
- Mukherjee, D.; Shukla, A.K.; Senk, D.G. Cold Model-Based Investigations to Study the Effects of Operational and Nonoperational Parameters on the Ruhrstahl-Heraeus Degassing Process. Metall. Mater. Trans. B 2017, 48, 763–771. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, C.; Ren, Y.; Zhang, L.F. Modeling on the Fluid Flow and Mixing Phenomena in a RH Steel Degasser with Oval Down-Leg Snorkel. Steel Res. Int. 2018, 89, 1800048. [Google Scholar] [CrossRef]
- Schiller, L.; Naumann, Z. A Drag Coefficient Correlation. Z. Ver. Dtsch. Ing. 1935, 77, 318–320. [Google Scholar]
- Auton, T.R. The Lift Force on a Spherical Body in a Rotational Flow. J. Fluid Mech. 1987, 183, 199–218. [Google Scholar] [CrossRef]
- Shih, T.H.; Liou, W.W.; Shabbir, A.; Yang, Z.; Zhu, J. A new k-ϵ eddy viscosity model for high reynolds number turbulent flows. Comput. Fluids 1995, 24, 227–238. [Google Scholar] [CrossRef]
- Rodi, W. Experience with two-layer models combining the k-epsilon model with a one-equation model near the wall. In Proceedings of the 29th Aerospace Sciences Meeting, Reno, NV, USA,, 1–10 January 1991; ARC: Columbus, OH, USA, 1991; p. 216. [Google Scholar] [CrossRef]
- Ilegbusi, O.J.; Iguchi, M.; Nakajima, K.; Sano, M.; Sakamoto, M. Modeling Mean Flow and Turbulence Characteristics in Gas Agitated Bath with Top Layer. Metall. Mater. Trans. B 1998, 29, 211–222. [Google Scholar] [CrossRef]



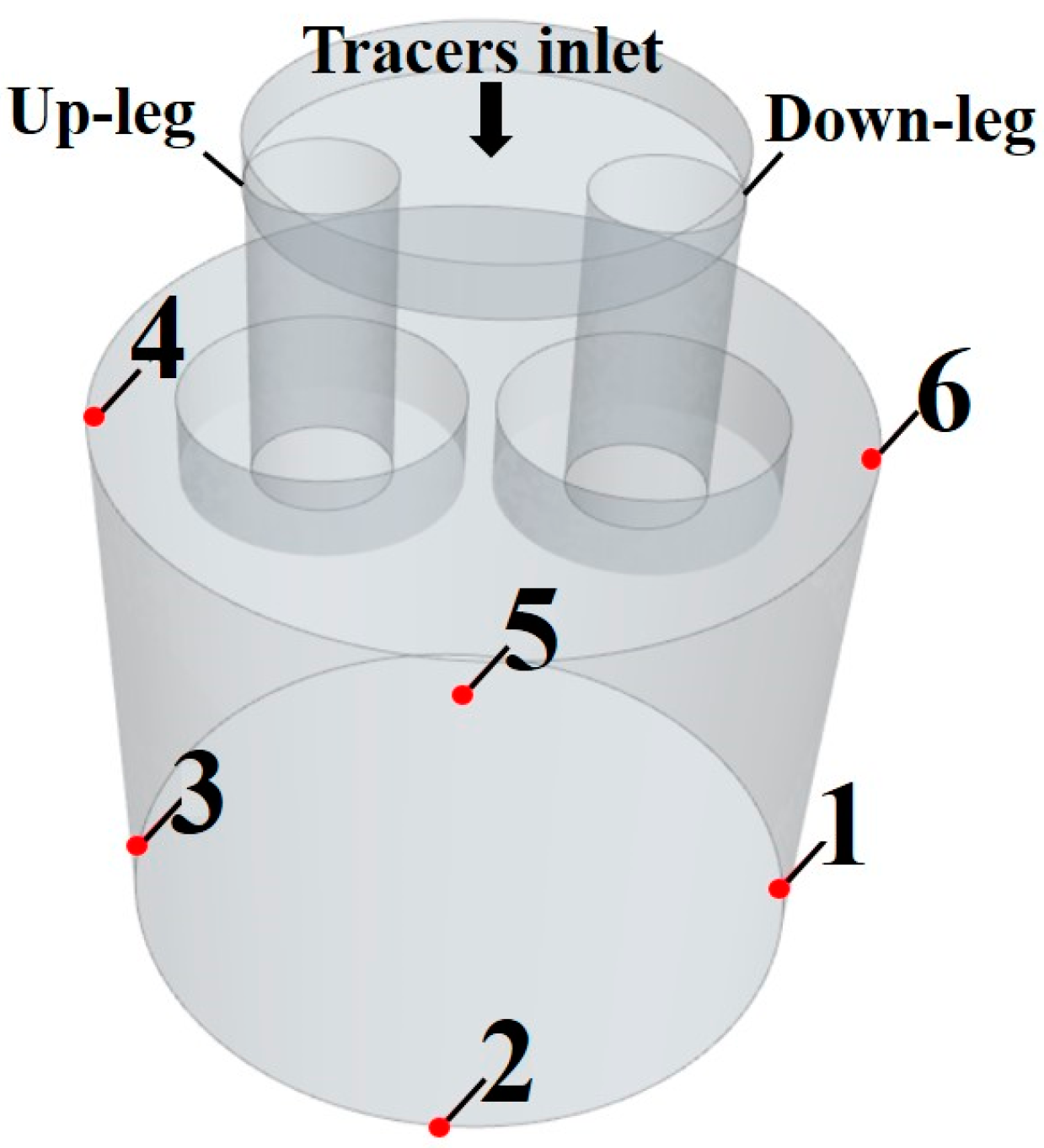


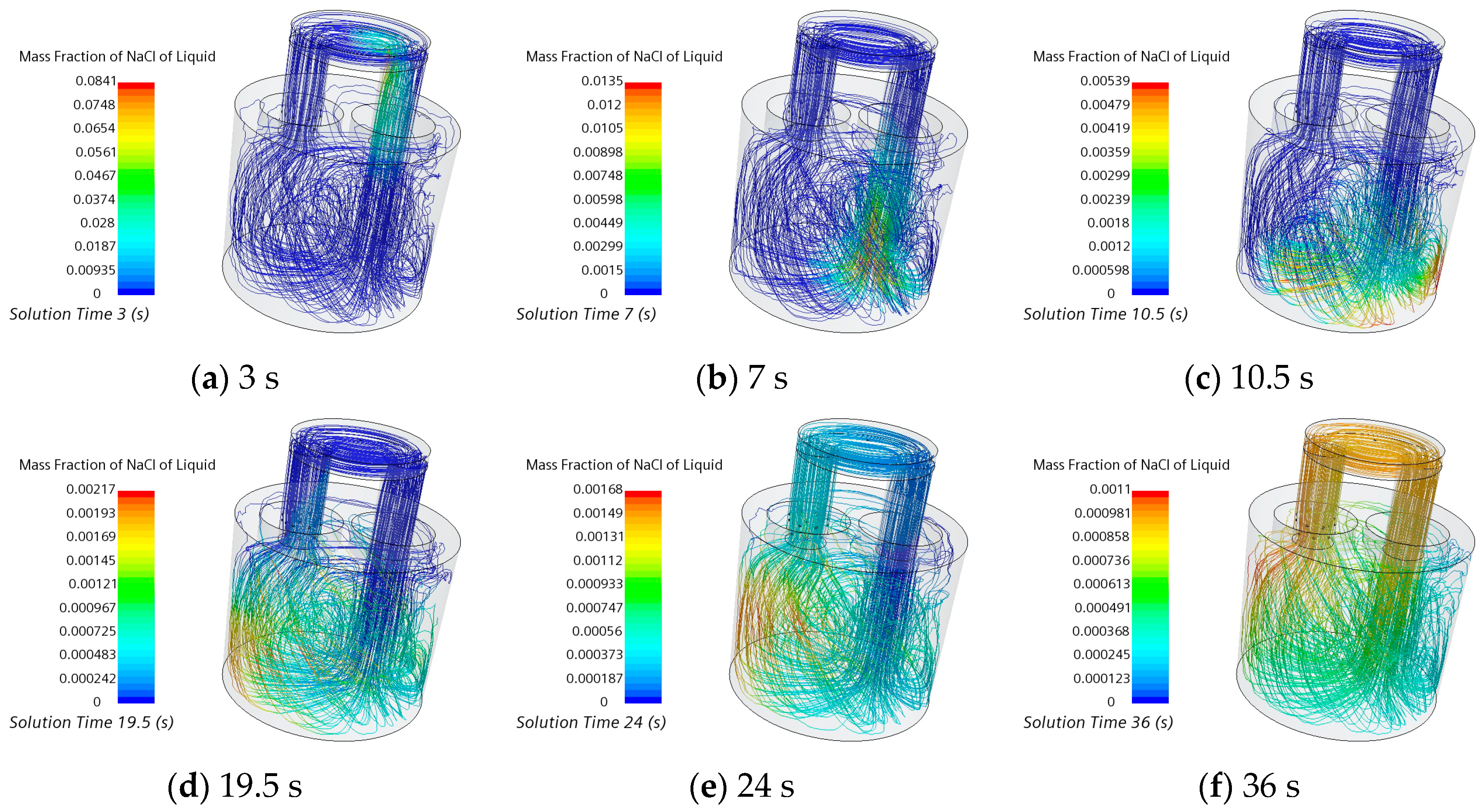






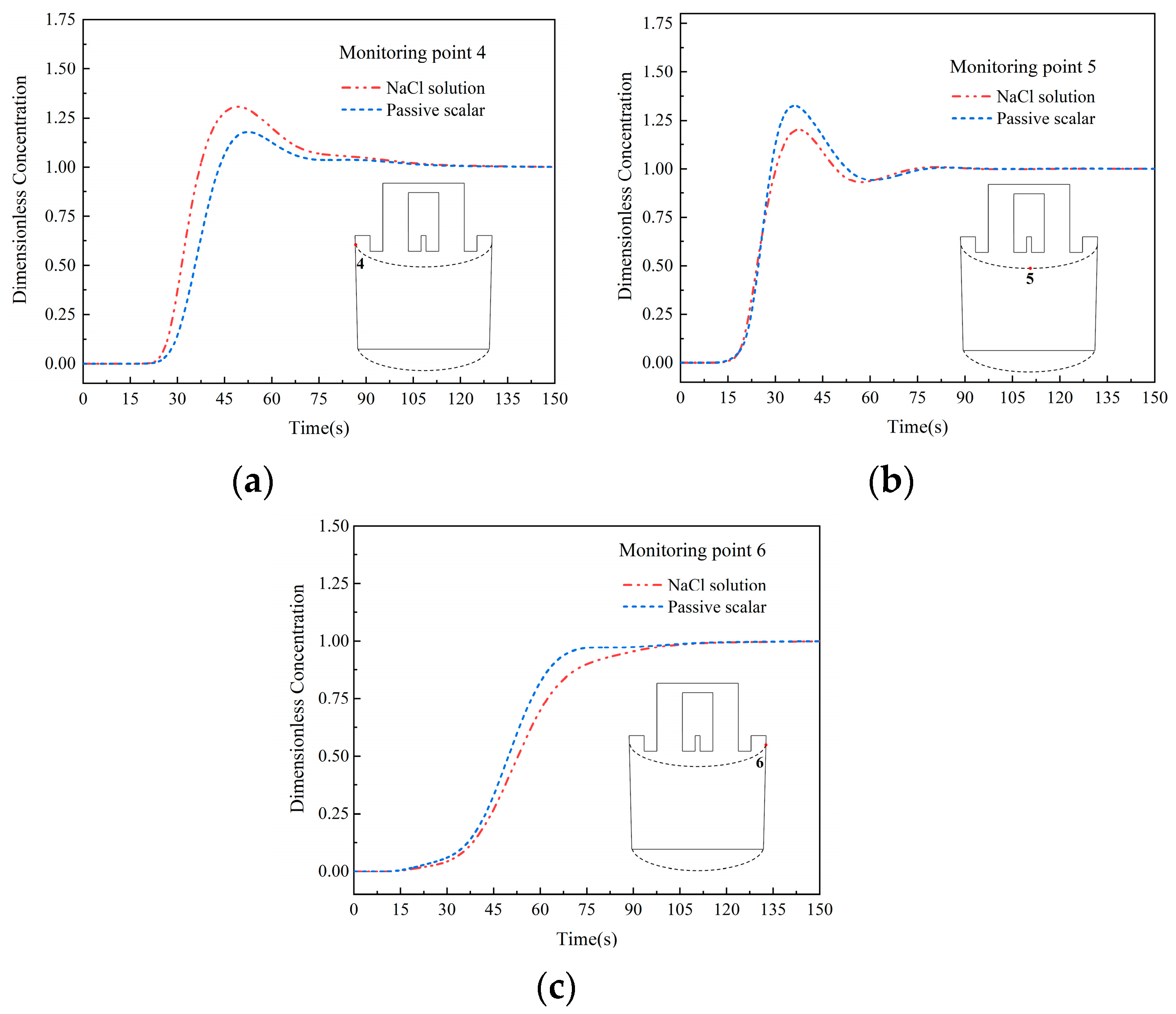










| Investigators | Year | Reactor | Weight | Scale | Tracer | Water Volume/L | Dimensionless Tracer Dosage | ||
|---|---|---|---|---|---|---|---|---|---|
| Kind | Concentration | Dosage/mL | |||||||
| Katoh and Okamoto [45] | 1979 | RH | 340 t | 1:10 | KCl | 30 g/100 cc water | 5 | 61.1 | 0.0818 × 10−3 |
| Wei et al. [46] | 2002 | RH | 90 t | 1:5 | KCl | saturated | 20–30 | 107 | 0.1869 × 10−3– 0.2803 × 10−3 |
| Wei et al. [47] | 2007 | RH-PB (IJ) | 150 t | 1:4 | NaCl | saturated | 10 | 361.1 | 0.02769 × 10−3 |
| Chen et al. [48] | 2014 | SSRF | 150 t | 1:5 | NaCl | saturated | 200 | 158 | 1.265 × 10−3 |
| Geng et al. [49] | 2015 | RH | - | 1:5 | NaCl | saturated | 20 | 741 | 0.027 × 10−3 |
| Ling et al. [50] | 2016 | RH | 210 t | 1:5 | KCl | saturated | 200 | 215 | 0.930 × 10−3 |
| Mukherjee et al. [51] | 2017 | RH | 160–170 t | 1:3 | NaCl | 3 mol/L | 30 | 758–807 | 0.037 × 10−3– 0.039 × 10−3 |
| Luo et al. [52] | 2018 | RH | - | 1:5 | KCl | saturated | 200 | 360 | 0.555 × 10−3 |
| Dai et al. [34] | 2019 | SSRF | 70 t | 1:3 | KCl | saturated | 65 | 332.38 | 0.175 × 10−3 |
| Zhang et al. [43] | 2020 | SSRF | 130 t | 1:5 | KCl | saturated | 150 | 149 | 1.007 × 10−3 |
| Liu et al. [15] | 2021 | RH | - | 1:5 | NaCl | saturated | 200 | >200 | <1 × 10−3 |
| Wang et al. [16] | 2022 | RH | 150 t | 1:4 | KCl | saturated | 200 | 335 | 0.597 × 10−3 |
| Parameters | Industrial Prototype | Water Model |
|---|---|---|
| Inner Diameter of Ladle Top (mm) | 3912 | 978 |
| Inner Diameter of Ladle Bottom (mm) | 3716 | 929 |
| Ladle Height (mm) | 4140 | 1035 |
| Inner Diameter of Vacuum Chamber (mm) | 2298 | 574.5 |
| Outer Diameter of Snorkel (mm) | 1440 | 360 |
| Inner Diameter of Snorkel (mm) | 720 | 180 |
| Length of Snorkel (mm) | 1650 | 412.5 |
| Inner Diameter of Gas Injection Orifices (mm) | 8 | 2 |
| Number of Gas Injection Orifices | 16 | 16 |
| Materials | ρ/(kg·m−3) | μ/(Pa·s) | Working Temperature/K | Lifting Gas Pressure/MPa |
|---|---|---|---|---|
| Air | 1.184 | 1.86 × 10−5 | 298 | 0.6 |
| Argon Gas | 1.782 | 2.22 × 10−5 | 298 | 2.5 |
| Molten Steel | 7000 | 6.1 × 10−3 | 1873 | - |
| Water | 1000 | 0.89 × 10−3 | 298 | - |
| Materials | Cass 1 | Cass 2 | Cass 3 |
|---|---|---|---|
| Tracer Dosage/mL | 300 | 300 | 600 |
| Tracer Kind | NaCl solution | Passive scalar | NaCl solution |
| Point 1 | Point 2 | Point 3 | Point 4 | Point 5 | Point 6 | |
|---|---|---|---|---|---|---|
| Passive scalar (numerical prediction) | 60.9 | 65.26 | 74.95 | 69.65 | 64.78 | 69.25 |
| NaCl solution (numerical prediction) | 59.62 | 64.1 | 74.65 | 88.48 | 62.98 | 88.48 |
| NaCl solution (experimental data) | 83.9 | 79.3 | 81.5 | 101 | 101 | 91 |
| Point 1 | Point 2 | Point 3 | Point 4 | Point 5 | Point 6 | |
|---|---|---|---|---|---|---|
| 300 mL Passive scalar (numerical prediction) | 73.7 | 75.7 | 66.18 | 86.02 | 79.54 | 75.6 |
| 300 mL NaCl solution (numerical prediction) | 71.65 | 74 | 69.5 | 92.05 | 81.82 | 86.68 |
| 600 mL NaCl solution (numerical prediction) | 73.2 | 75.1 | 81.16 | 100.75 | 74 | 106.69 |
| 300 mL NaCl solution (experimental data) | 111 | 102 | 116 | 127 | 122 | 130 |
| 600 mL NaCl solution (experimental data) | 109 | 118 | 108 | 131 | 134 | 143 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Ouyang, X.; Chen, C.; Li, Y.; Wang, T.; Ren, R.; Yang, M.; Zhao, Y.; Xue, L.; Wang, J. Numerical Simulation of the Density Effect on the Macroscopic Transport Process of Tracer in the Ruhrstahl–Heraeus (RH) Vacuum Degasser. Sustainability 2024, 16, 3923. https://doi.org/10.3390/su16103923
Xu Z, Ouyang X, Chen C, Li Y, Wang T, Ren R, Yang M, Zhao Y, Xue L, Wang J. Numerical Simulation of the Density Effect on the Macroscopic Transport Process of Tracer in the Ruhrstahl–Heraeus (RH) Vacuum Degasser. Sustainability. 2024; 16(10):3923. https://doi.org/10.3390/su16103923
Chicago/Turabian StyleXu, Zhibo, Xin Ouyang, Chao Chen, Yihong Li, Tianyang Wang, Ruijie Ren, Mingming Yang, Yansong Zhao, Liqiang Xue, and Jia Wang. 2024. "Numerical Simulation of the Density Effect on the Macroscopic Transport Process of Tracer in the Ruhrstahl–Heraeus (RH) Vacuum Degasser" Sustainability 16, no. 10: 3923. https://doi.org/10.3390/su16103923






