Lactobacillus delbrueckii subsp. bulgaricus Alleviates Acute Injury in Hypoxic Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Animal Experiment Design
2.3. Body Weight and Organ Water Content
2.4. Histological Analysis of Colon, Lung, and Brain Tissues
2.5. Determination of Gene Transcription Levels in Colon Tissue
2.6. Integrity Measurement of Ileal Mechanical Barrier
2.7. Cytokine Measurement
2.8. Antioxidant Capacity Assessment
2.9. Hypoxia-Related Factors Measurement
2.10. Mouse Fecal Microbiota 16S rRNA Amplicon Sequencing
2.11. Mouse Fecal Metabolite Measurement
2.12. Statistical Analysis
3. Results
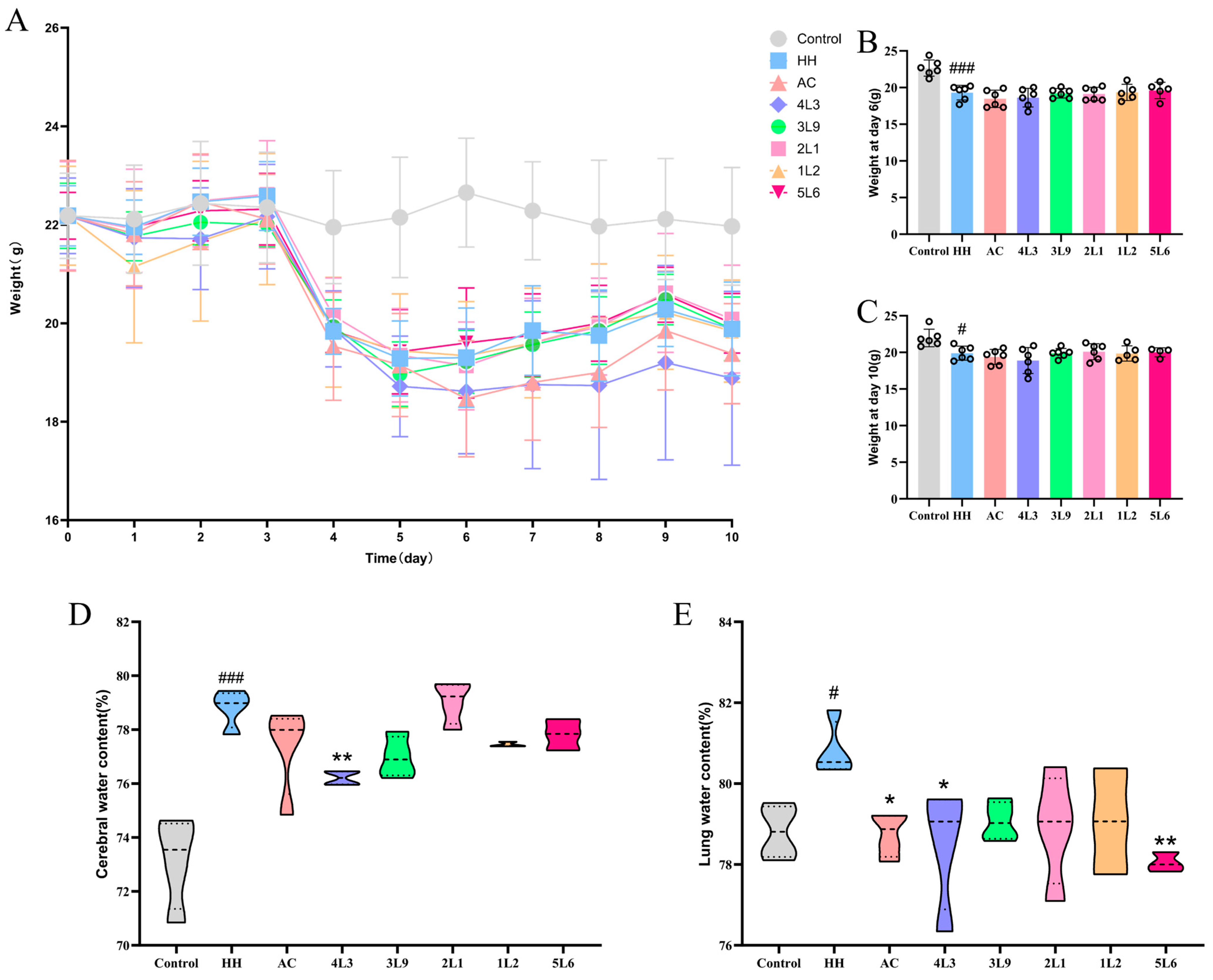
3.1. Lactobacillus delbrueckii subsp. bulgaricus 4L3 Significantly Alleviates Brain and Lung Edema in Mice
3.2. Intervention with Certain Strains of Lactobacillus delbrueckii subsp. bulgaricus Alleviates Specific Tissue Damage in Mice
3.3. Influence of Lactobacillus delbrueckii subsp. bulgaricus on the Transcription Levels of Tight Junction Protein Genes in the Colon
3.4. Effect on Ileal Mechanical Barrier Integrity
3.5. Lactobacillus delbrueckii subsp. bulgaricus Reduces Cytokine Secretion in the Ileum
3.6. Lactobacillus delbrueckii subsp. bulgaricus Alleviates Oxidative Stress in Mice
3.7. Effect of Lactobacillus delbrueckii subsp. bulgaricus Intervention on Serum Hypoxia-Related Factors
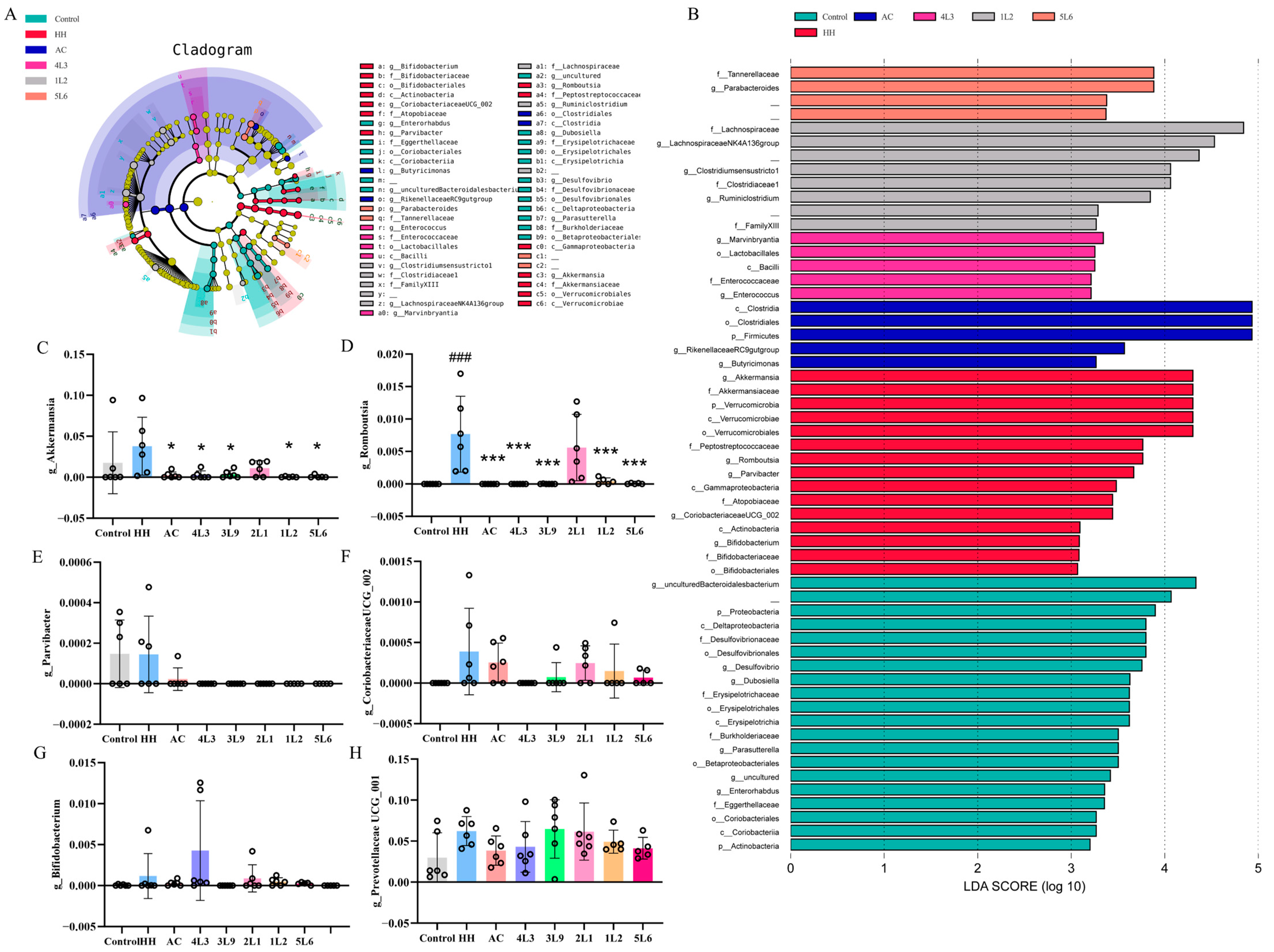
3.8. Impact on Gut Microbiota Composition
3.9. Impact on Fecal Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luks, A.M.; Swenson, E.R.; Bartsch, P. Acute high-altitude sickness. Eur. Respir. Rev. 2017, 26, 16009. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Berryman, C.E.; Young, A.J.; Radcliffe, P.N.; Branck, T.A.; Pantoja-Feliciano, I.G.; Rood, J.C.; Pasiakos, S.M. Associations between the gut microbiota and host responses to high altitude. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G1003–G1015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wu, W.; Deng, Z.; Zheng, X.; Zhang, J.; Deng, S.; Chen, J.; Ma, Q.; Wang, Y.; Yu, X.; et al. High altitude increases the expression of hypoxia-inducible factor 1α and inducible nitric oxide synthase with intest-inal mucosal barrier failure in rats. Int. J. Clin. Exp. Pathol. 2015, 8, 5189. [Google Scholar]
- Khanna, K.; Mishra, K.P.; Chanda, S.; Eslavath, M.R.; Ganju, L.; Kumar, B.; Singh, S.B. Effects of Acute Exposure to Hypobaric Hypoxia on Mucosal Barrier Injury and the Gastrointestinal Immune Axis in Rats. High. Alt. Med. Biol. 2019, 20, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhou, D.J.; Chen, Z.; Zhou, Q.Q.; Wu, K.; Tian, K.; Li, Z.W.; Xiao, Z.L. Establishment and evaluation of an experimental rat model for high-altitude intestinal barrier injury. Exp. Ther. Med. 2017, 13, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Zhang, Y.; Zhou, Y.; Gamah, M.; Yuan, Z.; Liu, J.; Cao, C.; Gao, X.; Zhang, H.; Ren, Y.; et al. Effects of hypoxic exposure on immune responses of intestinal mucosa to Citrobacter colitis in mice. Biomed. Pharmacother. 2020, 129, 110477. [Google Scholar] [CrossRef]
- Frühauf, H.; Erb, A.; Maggiorini, M.; Lutz, T.; Schwizer, W.; Fried, M.; Fox, M.R.; Goetze, O. Unsedated transnasal esophago-gastroduodenoscopy at 4559m-Endoscopic findings in healthy mountaineers after rapid ascent to high altitude. Swiss Med. Wkly. 2010, 183, 5S. [Google Scholar]
- Jia, Z.; Zhao, X.; Liu, X.; Zhao, L.; Jia, Q.; Shi, J.; Xu, X.; Hao, L.; Xu, Z.; Zhong, Q.; et al. Impacts of the Plateau Environment on the Gut Microbiota and Blood Clinical Indexes in Han and Tibetan Individuals. mSystems 2020, 5, 10–128. [Google Scholar] [CrossRef]
- Pan, Z.; Hu, Y.; Huang, Z.; Han, N.; Li, Y.; Zhuang, X.; Yin, J.; Peng, H.; Gao, Q.; Zhang, W.; et al. Alterations in gut microbiota and metabolites associated with altitude-induced cardiac hypertrophy in rats during hypobaric hypoxia challenge. Sci. China Life Sci. 2022, 65, 2093–2113. [Google Scholar] [CrossRef]
- Lan, D.; Ji, W.; Lin, B.; Chen, Y.; Huang, C.; Xiong, X.; Fu, M.; Mipam, T.D.; Ai, Y.I.; Zeng, B.O.; et al. Correlations between gut microbiota community structures of Tibetans and geography. Sci. Rep. 2017, 7, 16982. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, H.; Wei, Q.; Zhao, C.; Yang, X.; Wu, X.; Xia, T.; Liu, G.; Zhang, L.; Gao, Y.; et al. Comparative Analyses of Fecal Microbiota in European Mouflon (Ovis orientalis musimon) and Blue Sheep (Pseudois nayaur) Living at Low or High Altitudes. Front. Microbiol. 2019, 10, 1735. [Google Scholar] [CrossRef] [PubMed]
- Wiley, A.S.; Li, K.; Dan, Z.; Wang, H.; Zhou, Y.; Du, Y.; Ren, Y.I.; Shi, Y.; Nie, Y. Comparative Analysis of Gut Microbiota of Native Tibetan and Han Populations Living at Different Altitudes. PLoS ONE 2016, 11, e0155863. [Google Scholar]
- Suzuki, T.A.; Martins, F.M.; Nachman, M.W. Altitudinal variation of the gut microbiota in wild house mice. Mol. Ecol. 2019, 28, 2378–2390. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, T.; Beasley, D.E.; Heděnec, P.; Xiao, Z.; Zhang, S.; Li, J.; Lin, Q.; Li, X. Diet Diversity Is Associated with Beta but not Alpha Diversity of Pika Gut Microbiota. Front. Microbiol. 2016, 7, 1169. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Sun, Y.; Shao, X.; Zhou, Y.; Yu, Y.; Kuai, X.; Zhou, C. Leaky gut in IBD: Intestinal barrier–gut microbiota interaction. J. Microbiol. Biotechnol. 2022, 32, 825. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Zhang, B.; Qiao, L.; Song, X.; Pi, S.; Chang, J.; Zhang, X.; Zeng, X.; Zhu, L.; Xu, C. Biogenic Selenium Nanoparticles Synthesized by Lactobacillus casei ATCC 393 Alleviate Acute Hypobaric Hypoxia-Induced Intestinal Barrier Dysfunction in C57BL/6 Mice. Biol. Trace Elem. Res. 2023, 201, 4484–4496. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Zhang, X.; Jia, X.; Qin, Y.; Sun, N.; Xin, J.; Zeng, Y.; Jing, B.; Fang, J.; Pan, K.; et al. Lactobacillus johnsonii YH1136 plays a protective role against endogenous pathogenic bacteria induced intestinal dysfunction by reconstructing gut microbiota in mice exposed at high altitude. Front. Immunol. 2022, 13, 1007737. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, Z.; Huang, Z.; Li, Y.; Han, N.; Zhuang, X.; Peng, H.; Gao, Q.; Wang, Q.; Yang Lee, B.J.; et al. Gut Microbiome-Targeted Modulations Regulate Metabolic Profiles and Alleviate Altitude-Related Cardiac Hypertrophy in Rats. Microbiol. Spectr. 2022, 10, e01053-21. [Google Scholar] [CrossRef]
- Khanna, K.; Mishra, K.P.; Chanda, S.; Ganju, L.; Singh, S.B.; Kumar, B. Effect of Synbiotics on Amelioration of Intestinal Inflammation Under Hypobaric Hypoxia. High. Alt. Med. Biol. 2021, 22, 32–44. [Google Scholar] [CrossRef]
- Harrison, M.F.; Anderson, P.J.; Johnson, J.B.; Richert, M.; Miller, A.D.; Johnson, B.D. Acute Mountain Sickness Symptom Severity at the South Pole: The Influence of Self-Selected Prophylaxis with Acetazolamide. PLoS ONE 2016, 11, e0148206. [Google Scholar] [CrossRef]
- Mckenna, Z.J.; Gorini Pereira, F.; Gillum, T.L.; Amorim, F.T.; Deyhle, M.R.; Mermier, C.M. High-altitude exposures and intestinal barrier dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 322, R192–R203. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef] [PubMed]
- Dinmore, A.; Edwards, J.; Menzies, I.; Travis, S.P. Intestinal carbohydrate absorption and permeability at high altitude (5730 m). J. Appl. Physiol. 1994, 76, 1903–1907. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, H.; Duan, J.; Chen, J.; Wang, Q.; Liu, X.; Wang, C. Exploration of acute phase proteins and inflammatory cytokines in early stage diagnosis of acute mountain sickness. High. Alt. Med. Biol. 2018, 19, 170–177. [Google Scholar] [CrossRef]
- Li, X.-Y.; He, C.; Zhu, Y.; Lu, N.H. Role of gut microbiota on intestinal barrier function in acute pancreatitis. World J. Gastroenterol. 2020, 26, 2187. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Ding, M.; Su, J.; Ye, J.; He, X.; Zhang, Y.; Shang, X. Stachyose in combination with L. rhamnosus GG ameliorates acute hypobaric hypoxia-induced intestinal barrier dysfunction through alleviating inflammatory response and oxidative stress. Free Radic. Biol. Med. 2024, 212, 505–519. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, F.; Wang, W.; Sun, C.; Gao, D.; Ma, J.; Hussain, M.A.; Xu, C.; Jiang, Z.; Hou, J.; et al. Study of the alleviation effects of a combination of Lactobacillus rhamnosus and inulin on mice with colitis. Food Funct. 2020, 11, 3823–3837. [Google Scholar] [CrossRef] [PubMed]
- Dotan, I.; Rachmilewitz, D. Probiotics in inflammatory bowel disease: Possible mechanisms of action. Curr. Opin. Gastroenterol. 2005, 21, 426–430. [Google Scholar] [PubMed]
- Jang, S.-E.; Min, S.-W. Lactobacillus sakei S1 Improves Colitis Induced by 2,4,6-Trinitrobenzene Sulfonic Acid by the Inhibition of NF-kappaB Signaling in Mice. J. Microbiol. Biotechnol. 2020, 30, 71–78. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, X.; Zhao, T.; Qiao, M.; Zhao, X.; Zhao, M.; Xu, L.; Zhao, Y.; Wu, L.; Wu, K.; et al. Hypoxia augments LPS-induced inflammation and triggers high altitude cerebral edema in mice. Brain Behav. Immun. 2017, 64, 266–275. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Wu, H.; D’Alessandro, A.; Yegutkin, G.G.; Song, A.; Sun, K.; Li, J.; Cheng, N.Y.; Huang, A.; et al. Beneficial role of erythrocyte adenosine A2B receptor–Mediated AMP-activated Protein Kinase activation in high-altitude hypoxia. Circulation 2016, 134, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Acciarino, A.; Diwakarla, S.; Handreck, J.; Bergola, C.; Sahakian, L.; McQuade, R.M. The role of the gastrointestinal barrier in obesity-associated systemic inflammation. Obes. Rev. 2024, 25, e13673. [Google Scholar] [CrossRef]
- Ali, M.H.; Schlidt, S.A.; Chandel, N.S.; Hynes, K.L.; Schumacker, P.T.; Gewertz, B.L. Endothelial permeability and IL-6 production during hypoxia: Role of ROS in signal transduction. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1999, 277, L1057–L1065. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-Q.; Yang, D.-Z.; Luo, Y.-J.; Li, S.Z.; Liu, F.Y.; Wang, G.S. Over-starvation aggravates intestinal injury and promotes bacterial and endotoxin translocation under high-altitude hypoxic environment. World J. Gastroenterol. WJG 2011, 17, 1584. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Y.; Li, W.; Wang, S.; Zheng, J.; Xu, G.; Li, G.; Shen, X.; Yang, J. Gut microbiota imbalance mediates intestinal barrier damage in high-altitude exposed mice. FEBS J. 2022, 289, 4850–4868. [Google Scholar] [CrossRef] [PubMed]
- Nijiati, Y.; Maimaitiyiming, D.; Yang, T.; Li, H.; Aikemu, A. Research on the improvement of oxidative stress in rats with high-altitude pulmonary hypertension through the participation of irbesartan in regulating intestinal flora. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4540–4553. [Google Scholar]
- Buchwald, W.; Mikolajczak, P.L.; Krajewska-Patan, A.; Dreger, M.; Górska-Paukszta, M.; Szulc, M.; Polcyn, P.; Piorunska-Mikolajczak, A.; Mielcarek, S.; Czerny, B.; et al. Involvement of the different extracts from roots of Salvia miltiorrhiza Bunge on acute hypobaric hypoxia-induced cardiovascular effects in rats--preliminary report. Pol. J. Vet. Sci. 2012, 15, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Maity, C.; Ghosh, K.; Mondal, K.C. Alteration of predominant gastrointestinal flora and oxidative damage of large intestine under simulated hypobaric hypoxia. Z. Für. Gastroenterol. 2014, 52, 180–186. [Google Scholar] [CrossRef]
- Sireswar, S.; Dey, G.; Biswas, S. Influence of fruit-based beverages on efficacy of Lacticaseibacillus rhamnosus GG (Lactobacillus rhamnosus GG) against DSS-induced intestinal inflammation. Food Res. Int. 2021, 149, 110661. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Y.; Zhang, D.; Wu, W.Y.; Kang, X.; Wu, Q.; Wang, P.; Liu, X.; Gao, G.; Zhou, Y.; et al. Hypobaric hypoxia regulates iron metabolism in rats. J. Cell Biochem. 2019, 120, 14076–14087. [Google Scholar] [CrossRef]
- Nourkami-Tutdibi, N.; Küllmer, J.; Monz, D.; Monz, D.; Zemlin, M.; Tutdibi, E. Serum vascular endothelial growth factor is a potential biomarker for acute mountain sickness. Front. Physiol. 2023, 14, 1083808. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-L.; Law, T.C. Chronic hypoxia-and monocrotaline-induced elevation of hypoxia-inducible factor-1α levels and pulmonary hypertension. J. Biomed. Sci. 2004, 11, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Francino, M.P. The gut microbiome and metabolic health. Curr. Nutr. Rep. 2017, 6, 16–23. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Bian, T.; Sun, Y.; Zhang, Z.; Liu, T.; Gao, F.; Wang, Y.; Cao, R.; Xin, E.; et al. Hedysari Radix Praeparata Cum Melle repairs impaired intestinal barrier function and alleviates colitis-associated colorectal cancer via remodeling gut microbiota and metabolism. J. Funct. Foods 2023, 108, 105748. [Google Scholar] [CrossRef]
- Chen, J.; Huang, C.; Wang, J.; Zhou, H.; Lu, Y.; Lou, L.; Zheng, J.; Tian, L.; Wang, X.; Cao, Z.; et al. Dysbiosis of intestinal microbiota and decrease in paneth cell antimicrobial peptide level during acute necrotizing pancreatitis in rats. PLoS ONE 2017, 12, e0176583. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Pan, J.; Chen, H.; Chen, X.; Ye, Z.; Yuan, H.; Sun, H.; Wan, P. Oyster polysaccharides ameliorate intestinal mucositis and improve metabolism in 5-fluorouracil-treated S180 tumour-bearing mice. Carbohydr. Polym. 2021, 256, 117545. [Google Scholar] [CrossRef] [PubMed]
- Fala, A.M.; Oliveira, J.F.; Adamoski, D.; Aricetti, J.A.; Dias, M.M.; Dias, M.V.; Sforça, M.L.; Lopes-de-Oliveira, P.S.; Rocco, S.A.; Caldana, C.; et al. Unsaturated fatty acids as high-affinity ligands of the C-terminal Per-ARNT-Sim domain from the Hypoxia-inducible factor 3α. Sci. Rep. 2015, 5, 12698. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Itoi, T.; Terada, N.; Nakanishi, H.; Taguchi, R.; Hamaoka, K. Change in the membranous lipid composition accelerates lipid peroxidation in young rat hearts subjected to 2 weeks of hypoxia followed by hyperoxia. Circ. J. 2008, 72, 1359–1366. [Google Scholar] [CrossRef]
- Xiong, X.; Tan, B.; Song, M.; Ji, P.; Kim, K.; Yin, Y.; Liu, Y. Nutritional intervention for the intestinal development and health of weaned pigs. Front. Vet. Sci. 2019, 6, 46. [Google Scholar] [CrossRef]
- Schneider, J.; Blazy, I.; Dechaux, M.; Rabier, D.; Mason, N.P.; Richalet, J.P. Response of nitric oxide pathway to L-arginine infusion at the altitude of 4350 m. Eur. Respir. J. 2001, 18, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Yuhong, L.; Zhengzhong, B.; Feng, T.; Quanyu, Y.; Ge, R.L. L-arginine Attenuates Hypobaric Hypoxia-Induced Increase in Ornithine Decarboxylase 1. Wilderness Environ. Med. 2017, 28, 285–290. [Google Scholar] [CrossRef]









| Number in Article | Strain Number | Species | Strain Source |
|---|---|---|---|
| 1L2 | DQHXNS1L21 | Lactobacillus. Delbrueckii subsp. bulgaricus | Dairy products |
| 2L1 | DQHXNS2L1 | Lactobacillus. Delbrueckii subsp. bulgaricus | Dairy products |
| 3L9 | DQHXNS3L9 | Lactobacillus. Delbrueckii subsp. bulgaricus | Dairy products |
| 4L3 | DQHXNS4L3 (CCFM1387) | Lactobacillus. Delbrueckii subsp. bulgaricus | Dairy products |
| 5L6 | DQHXNS5L6 | Lactobacillus. Delbrueckii subsp. bulgaricus | Dairy products |
| Primer Name | Sequence (5′-3′) |
|---|---|
| β-actin | F: CCTTCCAGCAGATGTGGATCA R: CTCAGTAACAGTCCGCCTAGAA |
| Occludin (OCLN) | F: CCCCAATGTTGAAGAGTGGGTTA R: CACACTCAAGGTCAGAGGAATCT |
| ZO-1 (TJP1) | F: CTCAAGTTCCTGAAGCCCGT R: GCAAAAGACCAACCGTCAGG |
| ZO-2 (TJP2) | F: ATGGGAGCAGTACACCGTGA R: GCTGAACGGCAAACGAATGG |
| Claudin-3 (CLDN3) | F: ACCAACTGCGTACAAGACGAG R: CGGGCACCAACGGGTTATAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, K.; Ling, H.; Wang, L.; Tian, P.; Jin, X.; Zhao, J.; Chen, W.; Wang, G.; Bi, Y. Lactobacillus delbrueckii subsp. bulgaricus Alleviates Acute Injury in Hypoxic Mice. Nutrients 2024, 16, 1465. https://doi.org/10.3390/nu16101465
Song K, Ling H, Wang L, Tian P, Jin X, Zhao J, Chen W, Wang G, Bi Y. Lactobacillus delbrueckii subsp. bulgaricus Alleviates Acute Injury in Hypoxic Mice. Nutrients. 2024; 16(10):1465. https://doi.org/10.3390/nu16101465
Chicago/Turabian StyleSong, Ke, Hui Ling, Linlin Wang, Peijun Tian, Xing Jin, Jianxin Zhao, Wei Chen, Gang Wang, and Yujing Bi. 2024. "Lactobacillus delbrueckii subsp. bulgaricus Alleviates Acute Injury in Hypoxic Mice" Nutrients 16, no. 10: 1465. https://doi.org/10.3390/nu16101465





