Liquid–Liquid Equilibrium Behavior of Ternary Systems Comprising Biodiesel + Glycerol and Triglyceride + Methanol: Experimental Data and Modeling
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phase Equilibrium of Biodiesel + Methanol + Triglyceride Ternary System
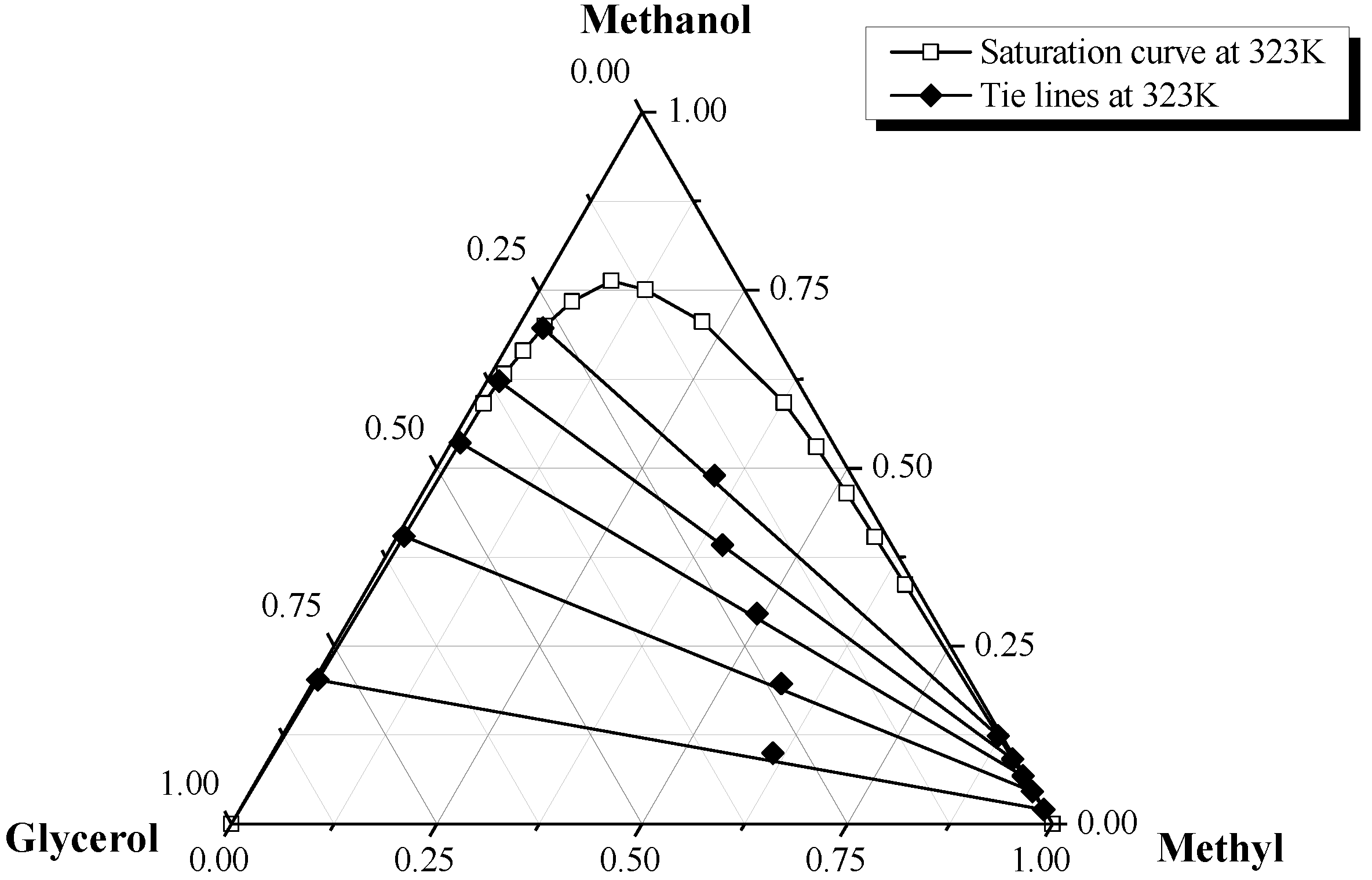
2.2. Phase Equilibrium of Pure Methyl Ester + Methanol + Glycerol Ternary System
2.3. Different Biodiesel + Methanol + Glycerol Ternary Systems
2.4. Analysis of Liquid Phase Equilibrium Data for Waste Oil Biodiesel + Methanol + Glycerol Ternary Liquid
2.5. Liquid Phase Equilibrium Modeling of Waste Oil Biodiesel + Methanol + Glycerol Ternary Liquid
3. Materials and Methods
3.1. Materials and Device
3.2. Saturation Curves of the Glycerol + Methanol + Methyl Ester System
3.3. Tie Lines of the Glycerol + Methanol + Methyl System
3.4. Analysis of Fatty Acid Methyl Esters
3.5. Liquid–Liquid Equilibrium Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kirubakaran, M.; Arul Mozhi Selvan, V. A comprehensive review of low cost biodiesel production from waste chicken fat. Renew. Sustain. Energy Rev. 2018, 82, 390–401. [Google Scholar] [CrossRef]
- Kampars, V.; Kampare, R.; Krumina, A. MgO Catalysts for FAME Synthesis Prepared Using PEG Surfactant during Precipitation and Calcination. Catalysts 2022, 12, 226. [Google Scholar] [CrossRef]
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; Adam, A.A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines—A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, H.; Lin, L. Biodiesel: An Alternative to Conventional Fuel. Energy Procedia 2012, 16, 1874–1885. [Google Scholar] [CrossRef]
- Mathew, G.M.; Raina, D.; Narisetty, V.; Kumar, V.; Saran, S.; Pugazhendi, A.; Sindhu, R.; Pandey, A.; Binod, P. Recent advances in biodiesel production: Challenges and solutions. Sci. Total Environ. 2021, 794, 148751. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.P. Review of process parameters for biodiesel production from different feedstocks. Renew. Sustain. Energy Rev. 2016, 62, 1063–1071. [Google Scholar] [CrossRef]
- Hajjari, M.; Tabatabaei, M.; Aghbashlo, M.; Ghanavati, H. A review on the prospects of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization. Renew. Sustain. Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]
- Ifeanyi-Nze, F.O.; Omiyale, C.O.; Okonkwo, I.U.; Chukwu, C.J.; Nwankwor, C.M.; Onabanjo, A.O.; Adoga, S.O.; Chukwu, J.O.; Chukwurah, K.F.; Ebikemiyen, M.; et al. Biodiesel Synthesis from Waste Vegetable Oil Utilizing Eggshell Ash as an Innovative Heterogenous Catalyst. Arch. adv. Eng. Sci. 2023, 1–18. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, F.; He, W.; Li, G. Research development in purification of glycerol produced from the homogeneous base catalyzed biodiesel. Chem. Ind. Eng. Pro. 2016, 35, 463–471. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Moein, P. Biodiesel synthesis from waste vegetable oil via transesterification reaction in supercritical methanol. J. Supercrit. Fluids 2013, 76, 24–31. [Google Scholar] [CrossRef]
- Jeevahan, J.; Mageshwaran, G.; Joseph, G.B.; Raj, R.B.D.; Kannan, R.T. Various strategies for reducing Nox emissions of biodiesel fuel used in conventional diesel engines: A review. Chem. Eng. Commun. 2017, 204, 1202–1223. [Google Scholar] [CrossRef]
- Chuah, L.F.; Klemeš, J.J.; Yusup, S.; Bokhari, A.; Akbar, M.M. A review of cleaner intensification technologies in biodiesel production. J. Cleaner Prod. 2017, 146, 181–193. [Google Scholar] [CrossRef]
- Basso, R.C.; de Almeida Meirelles, A.J.; Batista, E.A.C. Liquid–liquid equilibrium of pseudoternary systems containing glycerol+ethanol+ethylic biodiesel from crambe oil (Crambe abyssinica) at T/K = (298.2, 318.2, 338.2) and thermodynamic modeling. Fluid Phase Equilib. 2012, 333, 55–62. [Google Scholar] [CrossRef]
- Machado, A.B.; Ardila, Y.C.; de Oliveira, L.H.; Aznar, M.; Wolf Maciel, M.R. Liquid–Liquid Equilibria in Ternary and Quaternary Systems Present in Biodiesel Production from Soybean Oil at (298.2 and 333.2) K. J. Chem. Eng. Data 2012, 57, 1417–1422. [Google Scholar] [CrossRef]
- Mazutti, M.A.; Voll, F.A.P.; Cardozo-Filho, L.; Corazza, M.L.; Lanza, M.; Priamo, W.L.; Oliveira, J.V. Thermophysical properties of biodiesel and related systems: (Liquid+liquid) equilibrium data for soybean biodiesel. J. Chem. Thermodyn. 2013, 58, 83–94. [Google Scholar] [CrossRef]
- Abbott, M.M.; Van Ness, H.C. Thermodynamics of solutions containing reactive species: A guide to fundamentals and applications. Fluid Phase Equilib. 1992, 77, 53–119. [Google Scholar] [CrossRef]
- Xu, X.; Macedo, E.A. New modified Wilson model for electrolyte solutions. Ind. Eng. Chem. Res. 2003, 42, 5702–5707. [Google Scholar] [CrossRef]
- Mesquita, F.M.R.; Evangelista, N.S.; de Sant’Ana, H.B.; de Santiago-Aguiar, R.S. Liquid–Liquid Equilibrium for the Glycerol + Alcohol + Coconut Biodiesel System at Different Temperatures and Atmospheric Pressure. J. Chem. Eng. Data 2012, 57, 3557–3562. [Google Scholar] [CrossRef]
- Maghami, M.; Yousefi Seyf, J.; Sadrameli, S.M.; Haghtalab, A. Liquid–liquid phase equilibrium in ternary mixture of waste fish oil biodiesel–methanol–glycerol: Experimental data and thermodynamic modeling. Fluid Phase Equilib. 2016, 409, 124–130. [Google Scholar] [CrossRef]
- Muhammad, F.; Oliveira, M.B.; Pignat, P.; Jaubert, J.-N.; Pinho, S.P.; Coniglio, L. Phase equilibrium data and modeling of ethylic biodiesel, with application to a non-edible vegetable oil. Fuel 2017, 203, 633–641. [Google Scholar] [CrossRef]
- Noriega, M.A.; Narváez, P.C.; Imbachi, A.D.; Cadavid, J.G.; Habert, A.C. Liquid-liquid equilibrium for biodiesel-glycerol-methanol or ethanol systems using UNIFAC correlated parameters. Energy 2016, 111, 841–849. [Google Scholar] [CrossRef]
- Albuquerque, A.A.; Ng, F.T.T.; Danielski, L.; Stragevitch, L. Phase equilibrium modeling in biodiesel production by reactive distillation. Fuel 2020, 271, 117688. [Google Scholar] [CrossRef]
- Barreau, A.; Brunella, I.; de Hemptinne, J.C.; Coupard, V.; Canet, X.; Rivollet, F. Measurements of Liquid−Liquid Equilibria for a Methanol + Glycerol + Methyl Oleate System and Prediction Using Group Contribution Statistical Associating Fluid Theory. Ind. Eng. Chem. Res. 2010, 49, 5800–5807. [Google Scholar] [CrossRef]
- Doungsri, S.; Sookkumnerd, T.; Wongkoblap, A.; Nuchitprasittichai, A. Equilibrium study for ternary mixtures of biodiesel. IOP Conf. Ser. Mater. Sci. Eng. 2017, 273, 012008. [Google Scholar] [CrossRef]
- Mohadesi, M. Liquid-Liquid Equilibrium for Ternary Systems Containing Biodiesel+Glycerol+Alcohol (Ethanol or Methanol): Thermodynamic Modeling. J. Chem. Pet. Eng. 2020, 54, 285–295. [Google Scholar] [CrossRef]
- Asoodeh, A.; Eslami, F.; Sadrameli, S.M. Liquid–liquid equilibria of systems containing linseed oil biodiesel + methanol + glycerol: Experimental data and thermodynamic modeling. Fuel 2019, 253, 460–473. [Google Scholar] [CrossRef]
- Karmakar, B.; Halder, G. Progress and future of biodiesel synthesis: Advancements in oil extraction and conversion technologies. Energy Convers. Manag. 2019, 182, 307–339. [Google Scholar] [CrossRef]
- Follegatti-Romero, L.A.; Oliveira, M.B.; Batista, F.R.M.; Batista, E.A.C.; Coutinho, J.A.P.; Meirelles, A.J.A. Liquid–liquid equilibria for ternary systems containing ethyl esters, ethanol and glycerol at 323.15 and 353.15 K. Fuel 2012, 94, 386–394. [Google Scholar] [CrossRef]
- Fon Sing, S.; Isdepsky, A.; Borowitzka, M.A.; Moheimani, N.R. Production of biofuels from microalgae. Mitig. Adapt. Strateg. Glob. Change 2013, 18, 47–72. [Google Scholar] [CrossRef]
- Li, Z.; Smith, K.H.; Mumford, K.A.; Wang, Y.; Stevens, G.W. Regression of NRTL parameters from ternary liquid–liquid equilibria using particle swarm optimization and discussions. Fluid Phase Equilib. 2015, 398, 36–45. [Google Scholar] [CrossRef]
- Farajnezhad, A.; Afshar, O.A.; Khansary, M.A.; Shirazian, S.; Ghadiri, M. Correlation of interaction parameters in Wilson, NRTL and UNIQUAC models using theoretical methods. Fluid Phase Equilib. 2016, 417, 181–186. [Google Scholar] [CrossRef]
- Hossain, N.; Bhattacharia, S.K.; Chen, C.-C. Temperature dependence of interaction parameters in electrolyte NRTL model. AlChE J. 2016, 62, 1244–1253. [Google Scholar] [CrossRef]







| Temperature (K) | i–j | UNIQUAC | RSMD (%) | NRTL | RSMD (%) | |||
|---|---|---|---|---|---|---|---|---|
| τij | τji | α | τij | τji | ||||
| 1–2 | 0.389 | 1.235 | 4.98 | 0.2 | −1.054 | 4.341 | 3.95 | |
| 303.15 | 1–3 | 0.146 | 1.176 | 0.2 | 5.754 | 4.736 | ||
| 2–3 | 3.543 | 0.647 | 0.2 | 1.789 | −1.987 | |||
| 1–2 | 0.305 | 1.103 | 0.2 | −3.146 | 4.975 | |||
| 313.15 | 1–3 | 0.102 | 0.968 | 3.56 | 0.2 | 6.459 | 6.341 | 2.76 |
| 2–3 | 3.561 | 0.075 | 0.2 | 2.769 | −0.916 | |||
| 1–2 | 0.891 | 0.755 | 0.2 | −6.785 | 5.246 | |||
| 323.15 | 1–3 | 0.156 | 1.235 | 5.34 | 0.2 | 5.679 | 5.976 | 5.67 |
| 2–3 | 2.316 | 1.479 | 0.2 | 1.598 | −8.137 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Xing, S.; Teng, X.; Liu, R.; Wang, Z.; Lin, B.; Lv, P.; Al-Haimi, A.A.N.M.; Yehia, F.; Luo, W. Liquid–Liquid Equilibrium Behavior of Ternary Systems Comprising Biodiesel + Glycerol and Triglyceride + Methanol: Experimental Data and Modeling. Catalysts 2024, 14, 320. https://doi.org/10.3390/catal14050320
Yang L, Xing S, Teng X, Liu R, Wang Z, Lin B, Lv P, Al-Haimi AANM, Yehia F, Luo W. Liquid–Liquid Equilibrium Behavior of Ternary Systems Comprising Biodiesel + Glycerol and Triglyceride + Methanol: Experimental Data and Modeling. Catalysts. 2024; 14(5):320. https://doi.org/10.3390/catal14050320
Chicago/Turabian StyleYang, Lingmei, Shiyou Xing, Xianbin Teng, Rukuan Liu, Zhongming Wang, Baining Lin, Pengmei Lv, Akram Ali Nasser Mansoor Al-Haimi, Fatma Yehia, and Wen Luo. 2024. "Liquid–Liquid Equilibrium Behavior of Ternary Systems Comprising Biodiesel + Glycerol and Triglyceride + Methanol: Experimental Data and Modeling" Catalysts 14, no. 5: 320. https://doi.org/10.3390/catal14050320





