Advancements in The Cross-Linking and Morphology of Liquid Crystals
Abstract
:1. Introduction
2. Liquid Crystals
3. Liquid Crystal Epoxies
3.1. Modification of Properties by Changing the Structure
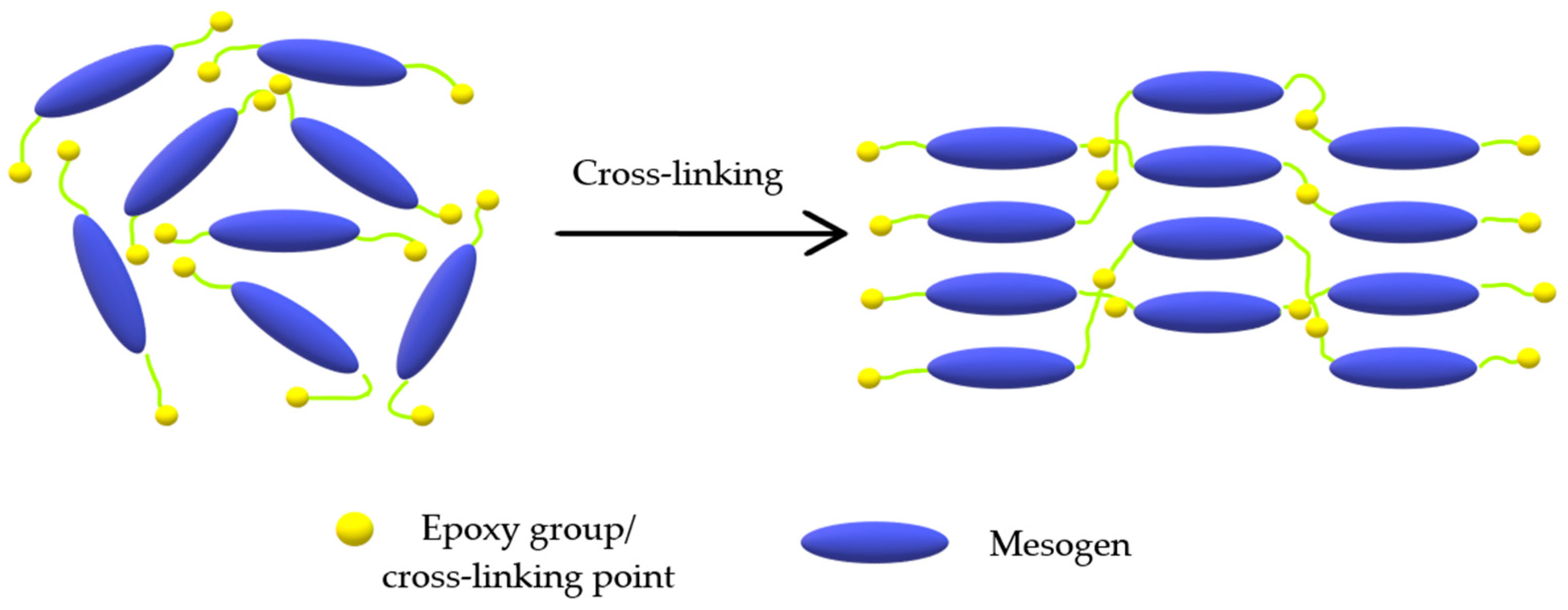
3.2. Liquid Crystal Epoxy Networks
4. Application of Liquid Crystal Polymers
4.1. Non-Linear Optics
4.2. Thermal Conductivity
4.3. Shape Memory Polymers
5. Liquid Crystal Epoxy Composites
5.1. Inorganic Fillers
5.2. Other Composites
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, R.; Ma, L.; Feng, W.; Pan, J.; Wang, Z.; Chen, Z.; Zhang, Y.; Li, C.; Chen, P.; Bisoyi, H.K.; et al. Autonomous Self-Sustained Liquid Crystal Actuators Enabling Active Photonic Applications. Adv. Funct. Mater. 2023, 33, 2301142. [Google Scholar] [CrossRef]
- Sun, J.; Wang, C.; Liu, Y.; Liang, X.; Wang, Z. Liquid Crystal Elastomer Composites for Soft Actuators. Int. J. Smart Nano Mater. 2023, 14, 440–459. [Google Scholar] [CrossRef]
- Maurin, V.; Chang, Y.; Ze, Q.; Leanza, S.; Wang, J.; Zhao, R.R. Liquid Crystal Elastomer–Liquid Metal Composite: Ultrafast, Untethered, and Programmable Actuation by Induction Heating. Adv. Mater. 2023, 2302765. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, G.; Malik, P.; Deep, A. Morphological, Dielectric, Electro-Optic and Photoluminescence Properties of Titanium Oxide Nanoparticles Enriched Polymer Stabilized Cholesteric Liquid Crystal Composites. J. Mol. Liq. 2023, 376, 121406. [Google Scholar] [CrossRef]
- Harada, M.; Ando, J.; Yamaki, M.; Ochi, M. Synthesis, Characterization, and Mechanical Properties of a Novel Terphenyl Liquid Crystalline Epoxy Resin. J. Appl. Polym. Sci. 2015, 132, 41296. [Google Scholar] [CrossRef]
- Zhong, X.; Yang, X.; Ruan, K.; Zhang, J.; Zhang, H.; Gu, J. Discotic Liquid Crystal Epoxy Resins Integrating Intrinsic High Thermal Conductivity and Intrinsic Flame Retardancy. Macromol. Rapid Commun. 2022, 43, 2100580. [Google Scholar] [CrossRef] [PubMed]
- Olamilekan, A.I.; Yeo, H. Thermal Conducting Thermosets Driven by Molecular Structurally Enhanced Mesogen Interactions. ACS Appl. Polym. Mater. 2021, 3, 4147–4155. [Google Scholar] [CrossRef]
- Li, Y.; Ambrogi, V.; Cerruti, P.; Goswami, M.; Yang, Z.; Kessler, M.R.; Rios, O. Functional Liquid Crystalline Epoxy Networks and Composites: From Materials Design to Applications. Int. Mater. Rev. 2022, 67, 201–229. [Google Scholar] [CrossRef]
- Harada, M.; Hirotani, M.; Ochi, M. Synthesis and Improved Mechanical Properties of Twin-Mesogenic Epoxy Thermosets Using Siloxane Spacers with Different Lengths. J. Appl. Polym. Sci. 2019, 136, 47891. [Google Scholar] [CrossRef]
- Lee, M.; Ha, M.Y.; Lee, M.; Kim, J.H.; Kim, S.D.; Kim, I.; Lee, W.B. Aligned Structures of Mesogenic Motifs in Epoxy Resin and Their Thermal Conductivities. Nanoscale Adv. 2022, 4, 1970–1978. [Google Scholar] [CrossRef]
- Uchida, J.; Soberats, B.; Gupta, M.; Kato, T. Advanced Functional Liquid Crystals. Adv. Mater. 2022, 34, 2109063. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Ambrogi, V.; Rios, O.; Xia, M.; He, W.; Yang, Z. Liquid Crystalline Elastomers Based on Click Chemistry. ACS Appl. Mater. Interfaces 2022, 14, 14842–14858. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zhang, F.; Zhao, Y.; Gao, X.; Zhou, W.; Qi, G.; Ding, Y.; Winter, H.H. Rheology of Smectic Liquid Crystalline Elastomers with Dynamic Covalent Bonds. Macromolecules 2023, 56, 7808–7817. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, S. Recent Progress in Dynamic Covalent Chemistries for Liquid Crystal Elastomers. J. Mater. Chem. B 2020, 8, 6610–6623. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, Z.; Wu, F.; Wang, M.; Chen, L. Liquid-Crystal Elastomers Based on Covalent Adaptable Networks: From Molecular Design to Applications. Sci. China Mater. 2023, 66, 3004–3021. [Google Scholar] [CrossRef]
- Utekar, S.; Suriya, V.K.; More, N.; Rao, A. Comprehensive Study of Recycling of Thermosetting Polymer Composites—Driving Force, Challenges and Methods. Compos. B Eng. 2021, 207, 108596. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Burlison, S.R.; Moreno, S.; Minary-Jolandan, M. Additive-Free and Support-Free 3D Printing of Thermosetting Polymers with Isotropic Mechanical Properties. ACS Appl. Mater. Interfaces 2021, 13, 5529–5538. [Google Scholar] [CrossRef] [PubMed]
- Shundo, A.; Aoki, M.; Yamamoto, S.; Tanaka, K. Cross-Linking Effect on Segmental Dynamics of Well-Defined Epoxy Resins. Macromolecules 2021, 54, 5950–5956. [Google Scholar] [CrossRef]
- Di-wu, J.; Zhou, W.; Wang, Y.; Li, Y.; Lv, Y.; Zhang, Y.; Zhang, N.; Chen, Q. Synergetic Improvements of Intrinsic Thermal Conductivity and Breakdown Strength in Liquid Crystal Epoxy Resin for High Voltage Applications. J. Polym. Res. 2024, 31, 72. [Google Scholar] [CrossRef]
- Shiota, A.; Ober, C.K. Synthesis and Curing of Novel LC Twin Epoxy Monomers for Liquid Crystal Thermosets. J. Polym. Sci. A Polym. Chem. 1996, 34, 1291–1303. [Google Scholar] [CrossRef]
- Barclay, G.G.; Ober, C.K.; Papathomas, K.I.; Wang, D.W. Liquid Crystalline Epoxy Thermosets Based on Dihydroxymethylstilbene: Synthesis and Characterization. J. Polym. Sci. A Polym. Chem. 1992, 30, 1831–1843. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Y.; Kong, F.; Peng, W.; Wang, Y.; Yuan, M.; Han, X.; Liu, X.; Li, B. Advances in Liquid Crystal Ep Molecular Structures, Thermal Conductivity, and Promising Applications in Thermal Management. Energy Environ. Mater. 2024, e12698. [Google Scholar] [CrossRef]
- Ruan, K.; Zhong, X.; Shi, X.; Dang, J.; Gu, J. Liquid Crystal Epoxy Resins with High Intrinsic Thermal Conductivities and Their Composites: A Mini-Review. Mater. Today Phys. 2021, 20, 100456. [Google Scholar] [CrossRef]
- Kawamoto, S.; Fujiwara, H.; Nishimura, S. Hydrogen Characteristics and Ordered Structure of Mono-Mesogen Type Liquid-Crystalline Epoxy Polymer. Int. J. Hydrogen Energy 2016, 41, 7500–7510. [Google Scholar] [CrossRef]
- Hong, Y.; Goh, M. Advances in Liquid Crystalline Epoxy Resins for High Thermal Conductivity. Polymers 2021, 13, 1302. [Google Scholar] [CrossRef] [PubMed]
- Kisiel, M.; Mossety-Leszczak, B. The Effect of Nonterminal Liquid Crystalline Epoxy Resin Structure and Curing Agents on the Glass Transition of Polymer Networks. Polymers 2024, 16, 857. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Okamoto, N.; Ochi, M. Influence of the Introduction of Flexible Alkyl Chains on the Thermal Behavior and Mechanical Properties of Mesogenic Epoxy Thermosets. J. Appl. Polym. Sci. 2016, 133, 44244. [Google Scholar] [CrossRef]
- Lin, Z.; Cong, Y.; Zhang, B.; Huang, H. Synthesis and Characterisation of a Novel Y-Shaped Liquid Crystalline Epoxy and Its Effect on Isotropic Epoxy Resin. Liq. Cryst. 2019, 46, 1467–1477. [Google Scholar] [CrossRef]
- Zhu, S.; Li, W.; Lu, B.; Chen, W.; Zhang, W.; Niu, X.; Chen, X.; An, Z. Evaluation of Mesomorphic and Thermal Stabilities for Terminal Epoxy Liquid Crystals. J. Mol. Liq. 2020, 317, 113955. [Google Scholar] [CrossRef]
- Harada, M.; Yokoyama, Y.; Ochi, M. Synthesis of Multifunctional Liquid Crystalline Epoxy Resin Embedded Cyclic-Siloxane Chain with Tg-Less Behavior and Enhanced Toughness. High Perform. Polym. 2021, 33, 3–11. [Google Scholar] [CrossRef]
- Trinh, T.E.; Yeo, H. Enhancement of Thermal Conducting Properties in Epoxy Thermoset Systems Using an Aligned Liquid-Crystalline Mesophase. Mater. Adv. 2024, 5, 1702–1714. [Google Scholar] [CrossRef]
- Carfagna, C.; Amendola, E.; Giamberini, M. Liquid Crystalline Epoxy Based Polymers. Prog. Polym. Sci. 1997, 22, 1607–1647. [Google Scholar] [CrossRef]
- Harada, M.; Yamaguchi, K. Fracture Toughness of Highly Ordered Liquid Crystalline Epoxy Thermosets Achieved by Optimized Composition of Curing Agents. J. Appl. Polym. Sci. 2021, 138, 53–56. [Google Scholar] [CrossRef]
- Harada, M.; Matsumoto, T. Thermal Conductivity and Orientation Structure of Liquid Crystalline Epoxy Thermosets Prepared by Latent Curing Catalyst. Crystals 2024, 14, 47. [Google Scholar] [CrossRef]
- Olamilekan, A.I.; Yeo, H. Curing Behavior of 4,4′-Diglycidyloxybiphenyl with p-Phenylene Diamine Derivatives. Macromol. Res. 2020, 28, 960–967. [Google Scholar] [CrossRef]
- Wu, W.; Li, Z.; Zhang, B. Rational Design and Synthesis of a Novel LCE Material and Its Curing Behaviours. Liq. Cryst. 2021, 48, 1723–1732. [Google Scholar] [CrossRef]
- Zhang, J.; Dang, L.; Zhang, F.; Zhang, K.; Kong, Q.; Gu, J. Effect of the Structure of Epoxy Monomers and Curing Agents: Toward Making Intrinsically Highly Thermally Conductive and Low-Dielectric Epoxy Resins. JACS Au 2023, 3, 3424–3435. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H. Curing Kinetics of Liquid Crystalline 4,4′-Diglycidyloxybiphenyl Epoxy with Various Diamines. Polymer 2019, 168, 209–217. [Google Scholar] [CrossRef]
- Hoekstra, D.C.; Schenning, A.P.H.J.; Debije, M.G. Epoxide and Oxetane Based Liquid Crystals for Advanced Functional Materials. Soft Matter 2020, 16, 5106–5119. [Google Scholar] [CrossRef] [PubMed]
- Jilani, W.; Fourati, N.; Zerrouki, C.; Faugeras, P.A.; Guinault, A.; Zerrouki, R.; Guermazi, H. Exploring the Structural Properties and Enhancement of Opto-Electrical Investigations for the Synthesized Epoxy Based Polymers with Local Nanoscale Structures. Mater. Res. Express 2020, 7, 35305. [Google Scholar] [CrossRef]
- Amendola, E.; Carfagna, C.; Giamberini, M.; Pisaniello, G. Curing Reactions of a Liquid Crystalline Epoxy Resin Based on the Diglycidyl Ether of 4,4′-dihydroxy-α-methylstilbene. Macromol. Chem. Phys. 1995, 196, 1577–1591. [Google Scholar] [CrossRef]
- Lin, Q.; Yee, A.F.; Earls, J.D.; Hefner, R.E.; Sue, H.J. Phase Transformations of a Liquid Crystalline Epoxy during Curing. Polymer 1994, 35, 2679–2682. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z.; Wang, Y.; Zhao, Y.; Shang, L. Cholesteric Cellulose Liquid Crystals with Multifunctional Structural Colors. Adv. Funct. Mater. 2022, 32, 2107242. [Google Scholar] [CrossRef]
- Wani, O.M.; Schenning, A.P.H.J.; Priimagi, A. A Bifacial Colour-Tunable System: Via Combination of a Cholesteric Liquid Crystal Network and Hydrogel. J. Mater. Chem. C Mater. 2020, 8, 10191–10196. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Li, Y.; Liu, W.; Li, H.; Yang, Y. Colorfully Patterned Epoxy Resin Films with a Cholesteric Structure Prepared through a Photopolymerization Approach. ACS Appl. Polym. Mater. 2022, 5, 193–200. [Google Scholar] [CrossRef]
- Zhang, H.; Li, F.; Zhang, R.; Deng, Z.; Ma, Y.; He, Z.; Fang, F.; Miao, Z.; Shen, W. Fabrication of Mesogenic Epoxy Microsphere-Filled Negative-Dielectric-Anisotropy Cholesteric Liquid Crystals for Bistable Devices. J. Mol. Liq. 2023, 384, 122255. [Google Scholar] [CrossRef]
- Higgins, D.A. Probing the Mesoscopic Chemical and Physical Properties of Polymer-Dispersed Liquid Crystals. Adv. Mater. 2000, 12, 251–264. [Google Scholar] [CrossRef]
- Zhao, Y.; He, Z.; Ban, Q.; Li, K.; Ding, S.; Tian, S.; Ren, H.; Zhu, M.; Ma, Q.; Miao, Z. Study on the Electro-Optical Properties of Epoxy Resin Based Polymer Dispersed Liquid Crystal Films. Mol. Cryst. Liq. Cryst. 2021, 722, 1–7. [Google Scholar] [CrossRef]
- Li, Y.; Gong, C.; Li, C.; Ruan, K.; Liu, C.; Liu, H.; Gu, J. Liquid Crystalline Texture and Hydrogen Bond on the Thermal Conductivities of Intrinsic Thermal Conductive Polymer Films. J. Mater. Sci. Technol. 2021, 82, 250–256. [Google Scholar] [CrossRef]
- Wu, K.; Sun, J.J.; Meng, F.; Fan, M.; Kong, X.; Cai, M.; Zhao, T.; Yang, C.; Xin, Y.; Xing, J.; et al. Highly Flexible and Electrically Controlled Grating Enabled by Polymer Dispersed Liquid Crystal. J. Mol. Liq. 2022, 353, 118664. [Google Scholar] [CrossRef]
- Lin, H.; Zhao, Y.; Jiao, X.; Gao, H.; Guo, Z.; Wang, D.; Luan, Y.; Wang, L. Preparation and Application of Polymer-Dispersed Liquid Crystal Film with Step-Driven Display Capability. Molecules 2024, 29, 1109. [Google Scholar] [CrossRef]
- AcademicJayoti, D.; Malik, P.; Singh, A. Analysis of Morphological Behaviour and Electro-Optical Properties of Silica Nanoparticles Doped Polymer Dispersed Liquid Crystal Composites. J. Mol. Liq. 2017, 225, 456–461. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, C.; Zhou, L.; Fang, H.; Huang, J.; Ma, H.; Zhang, Y.; Yang, J.; Zhang, L.Y.; Song, P.; et al. Effect of a Polymercaptan Material on the Electro-Optical Properties of Polymer-Dispersed Liquid Crystal Films. Molecules 2017, 22, 43. [Google Scholar] [CrossRef] [PubMed]
- Ellahi, M.; Taimur, S.; Baloch, N.; Bhayo, A.M.; Sarwar, A.; Qadar, F. Study of Polymer-Dispersed Liquid Crystal (PDLC) Thin Film Technology for Smart Electronic Devices. J. Electron. Mater. 2024, 53, 1094–1104. [Google Scholar] [CrossRef]
- Jilani, W.; Bouzidi, A.; Elleuch, S.; Guermazi, H. Synthesis, Morphology, Vibrational, and Physical Characterization of DGEBA Epoxy Doped Liquid Crystal Organic Polymeric Systems (EDLCPSs) for High-Performance Embedded Capacitors. Opt. Quantum Electron. 2024, 56, 617. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, S.; Saeed, M.H.; Zhou, L.; Gao, H.; Huang, J.; Zhang, L.; Yang, H.; Xiao, J.; Gao, Y. Effects of the Methacrylate Monomers with Different End Groups on the Morphologies, Electro-Optical and Mechanical Properties of Polymer Dispersed Liquid Crystals Composite Films. Liq. Cryst. 2021, 48, 722–734. [Google Scholar] [CrossRef]
- Jilani, W.; Bouzidi, A.; Hamdi, R.; Al-Dossari, M.; Yahia, I.S.; Guermazi, H. Experimental Investigations of DGEBA Epoxy Resin Dispersed Liquid Crystal Polymer Systems for the Development of New Electronic Devices. J. Mol. Liq. 2024, 395, 123923. [Google Scholar] [CrossRef]
- Saeed, M.H.; Zhang, S.; Cao, Y.; Zhou, L.; Hu, J.; Muhammad, I.; Xiao, J.; Zhang, L.; Yang, H. Recent Advances in the Polymer Dispersed Liquid Crystal Composite and Its Applications. Molecules 2020, 25, 5510. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Hu, W.; Zhou, L.; Zhang, H.; Wang, L.; Zhang, C.; Sun, J.; Zhang, L.; Yuan, X.; Zhu, S. The Electro-Optical Properties and Adhesion Strength of Epoxy- Polymercaptan-Based Polymer Dispersed Liquid Crystal Films. Crystals 2021, 11, 576. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, J.; Ren, Y.; Sun, C.; Hu, W.; Yang, H.; Yang, D.K. Programmable Patterned Dynamic Scattering of Liquid Crystals/Epoxy-Based Polymer Composite Film Sandwiched between Two Fully Covered Electrodes. Adv. Opt. Mater. 2023, 11, 2300537. [Google Scholar] [CrossRef]
- Harada, M.; Hamaura, N.; Ochi, M.; Agari, Y. Thermal Conductivity of Liquid Crystalline Epoxy/BN Filler Composites Having Ordered Network Structure. Compos. B Eng. 2013, 55, 306–313. [Google Scholar] [CrossRef]
- Guo, H.; Lu, M.; Liang, L.; Wu, K.; Ma, D.; Xue, W. Liquid Crystalline Epoxies with Lateral Substituents Showing a Low Dielectric Constant and High Thermal Conductivity. J. Electron. Mater. 2017, 46, 982–991. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, J.; Yang, D.; Zhang, J.; Guo, Y.; Zhong, X.; Kong, J.; Gu, J. High-Efficiency Improvement of Thermal Conductivities for Epoxy Composites from Synthesized Liquid Crystal Epoxy Followed by Doping BN Fillers. Compos. B Eng. 2020, 185, 107784. [Google Scholar] [CrossRef]
- Akatsuka, M.; Takezawa, Y. Study of High Thermal Conductive Epoxy Resins Containing Controlled High-Order Structures. J. Appl. Polym. Sci. 2003, 89, 2464–2467. [Google Scholar] [CrossRef]
- Hossain, M.M.; Olamilekan, A.I.; Jeong, H.O.; Lim, H.; Kim, Y.K.; Cho, H.; Jeong, H.D.; Islam, M.A.; Goh, M.; You, N.H.; et al. Diacetylene-Containing Dual-Functional Liquid Crystal Epoxy Resin: Strategic Phase Control for Topochemical Polymerization of Diacetylenes and Thermal Conductivity Enhancement. Macromolecules 2022, 55, 4402–4410. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Z.; Wang, S.; Gu, J. Low Dielectric Constant and Highly Intrinsic Thermal Conductivity Fluorine-containing Epoxy Resins with Ordered Liquid Crystal Structures. SusMat 2023, 3, 877–893. [Google Scholar] [CrossRef]
- Yang, X.; Zhong, X.; Zhang, J.; Gu, J. Intrinsic High Thermal Conductive Liquid Crystal Epoxy Film Simultaneously Combining with Excellent Intrinsic Self-Healing Performance. J. Mater. Sci. Technol. 2021, 68, 209–215. [Google Scholar] [CrossRef]
- Lv, G.; Shen, C.; Shan, N.; Jensen, E.; Li, X.; Evans, C.M.; Cahill, D.G. Odd–Even Effect on the Thermal Conductivity of Liquid Crystalline Epoxy Resins. Proc. Natl. Acad. Sci. USA 2022, 119, e2211151119. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xue, Z.; Li, G.; Wang, Y.; Fu, X.; Zhong, W. UV-Curable Epoxy with “Soft” Segments for 3D-Printable Shape-Memory Materials. J. Mater. Sci. 2018, 53, 12650–12661. [Google Scholar] [CrossRef]
- Ge, Q.; Sakhaei, A.H.; Lee, H.; Dunn, C.K.; Fang, N.X. Multimaterial 4D Printing with Tailorable Shape Memory Polymers. Sci. Rep. 2016, 6, 31110. [Google Scholar] [CrossRef]
- Lee, A.Y.; An, J.; Chua, C.K. Two-Way 4D Printing: A Review on the Reversibility of 3D-Printed Shape Memory Materials. Engineering 2017, 3, 663–674. [Google Scholar] [CrossRef]
- Tosto, C.; Saitta, L.; Latteri, A.; Cicala, G. Epoxy-Based Blend Formulation for Dual Curing in Liquid Crystal Display 3D Printing: A Study on Thermomechanical Properties Variation for Enhanced Printability. Polymers 2024, 16, 358. [Google Scholar] [CrossRef] [PubMed]
- Tosto, C.; Pergolizzi, E.; Blanco, I.; Patti, A.; Holt, P.; Karmel, S.; Cicala, G. Epoxy Based Blends for Additive Manufacturing by Liquid Crystal Display (LCD) Printing: The Effect of Blending and Dual Curing on Daylight Curable Resins. Polymers 2020, 12, 1594. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Chen, Y.; Hu, M.; Qin, S.; Liu, P. 4D Printing of Shape Memory Polymer via Liquid Crystal Display (LCD) Stereolithographic 3D Printing. Mater. Res. Express 2020, 7, 105305. [Google Scholar] [CrossRef]
- AbdulKarim-Talaq, M.; Hassan, K.T.; Hameed, D.A. Improvement of Thermal Conductivity of Novel Asymmetric Dimeric Coumarin Liquid Crystal by Doping with Boron Nitride and Aluminium Oxide Nanoparticles. Mater. Chem. Phys. 2023, 297, 127367. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, C.; Songfeng, E.; Xie, L.; Geng, R.; Lin, C.T.; Li, L.; Yao, Y. Enhanced Thermal Conductivity of Polyurethane Composites via Engineering Small/Large Sizes Interconnected Boron Nitride Nanosheets. Compos. Sci. Technol. 2019, 170, 93–100. [Google Scholar] [CrossRef]
- Feng, Z.; Liu, J.; Chen, X. Liquid Crystal Epoxy Composites Based on Functionalized Boron Nitride: Synthesis and Thermal Properties. Polym. Eng. Sci. 2023, 63, 932–942. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Huang, X.; Gong, C.; Shi, Q.; Zhou, W.; Cheng, Q. Flexible and Thermally Conductive Epoxy-Dispersed Liquid Crystal Composites Filled with Functionalized Boron Nitride Nanosheets. Polym. Compos. 2023, 44, 6581–6592. [Google Scholar] [CrossRef]
- Wu, L.C.; Huang, Y.W.; Yeh, Y.M.; Lin, C.H. Characteristic and Synthesis of High-Temperature Resistant Liquid Crystal Epoxy Resin Containing Boron Nitride Composite. Polymers 2022, 14, 1252. [Google Scholar] [CrossRef]
- Chen, C.; Xue, Y.; Li, X.; Wen, Y.; Liu, J.; Xue, Z.; Shi, D.; Zhou, X.; Xie, X.; Mai, Y.W. High-Performance Epoxy/Binary Spherical Alumina Composite as Underfill Material for Electronic Packaging. Compos. Part A Appl. Sci. Manuf. 2019, 118, 67–74. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, G.; Wu, K.; Shi, J.; Liang, L.; Lu, M. Biphenyl Liquid Crystal Epoxy Containing Flexible Chain: Synthesis and Thermal Properties. J. Appl. Polym. Sci. 2020, 137, e49143. [Google Scholar] [CrossRef]
- Tian, F.; Cao, J.; Ma, W. Enhanced Thermal Conductivity and Rheological Performance of Epoxy and Liquid Crystal Epoxy Composites with Filled Al 2 O 3 Compound. Polym. Test. 2023, 120, 107940. [Google Scholar] [CrossRef]
- Sanchez, W.A.L.; Huang, C.Y.; Chen, J.X.; Soong, Y.C.; Chan, Y.N.; Chiou, K.C.; Lee, T.M.; Cheng, C.C.; Chiu, C.W. Enhanced Thermal Conductivity of Epoxy Composites Filled with Al2o3/Boron Nitride Hybrids for Underfill Encapsulation Materials. Polymers 2021, 13, 147. [Google Scholar] [CrossRef]
- Ahmad, F.; Jeon, Y.J.; Jamil, M. Characteristics of Extra Polymer Layer Doped with (BaTiO3) Nanoparticles: Polymer Dispersed Liquid Crystal Display. Optoelectron. Adv. Mater. Rapid Commun. 2018, 12, 491–494. [Google Scholar]
- Rao Darla, M.; Hegde, S.; Varghese, S. Effect of BaTiO3 Nanoparticle on Electro-Optical Properties of Polymer Dispersed Liquid Crystal Displays. J. Cryst. Process Technol. 2014, 04, 60–63. [Google Scholar] [CrossRef]
- Shim, H.; Lyu, H.K.; Allabergenov, B.; Garbovskiy, Y.; Glushchenko, A.; Choi, B. Enhancement of Frequency Modulation Response Time for Polymer-Dispersed Liquid Crystal. Liq. Cryst. 2016, 43, 1390–1396. [Google Scholar] [CrossRef]
- Kumari, A.; Sinha, A. Role of BaTiO3 Nanoparticles on Electro-Optic Performance of Epoxy-Resin-Based PDLC Devices. Liq. Cryst. 2021, 48, 23–34. [Google Scholar] [CrossRef]
- Burger, N.; Laachachi, A.; Ferriol, M.; Lutz, M.; Toniazzo, V.; Ruch, D. Review of Thermal Conductivity in Composites: Mechanisms, Parameters and Theory. Prog. Polym. Sci. 2016, 61, 1–28. [Google Scholar] [CrossRef]
- Nan, C.W.; Shen, Y.; Ma, J. Physical Properties of Composites near Percolation. Annu. Rev. Mater. Res. 2010, 40, 131–151. [Google Scholar] [CrossRef]
- Nassira, H.; Sánchez-Ferrer, A.; Adamcik, J.; Handschin, S.; Mahdavi, H.; Taheri Qazvini, N.; Mezzenga, R. Gelatin–Graphene Nanocomposites with Ultralow Electrical Percolation Threshold. Adv. Mater. 2016, 28, 6914–6920. [Google Scholar] [CrossRef]
- Han, N.M.; Wang, Z.; Shen, X.; Wu, Y.; Liu, X.; Zheng, Q.; Kim, T.H.; Yang, J.; Kim, J.K. Graphene Size-Dependent Multifunctional Properties of Unidirectional Graphene Aerogel/Epoxy Nanocomposites. ACS Appl. Mater. Interfaces 2018, 10, 6580–6592. [Google Scholar] [CrossRef]
- Nistal, A.; Garcia, E.; Pérez-Coll, D.; Prieto, C.; Belmonte, M.; Osendi, M.I.; Miranzo, P. Low Percolation Threshold in Highly Conducting Graphene Nanoplatelets/Glass Composite Coatings. Carbon 2018, 139, 556–563. [Google Scholar] [CrossRef]
- Włodarska, M.; Mossety-Leszczak, B.; Bąk, G.W.; Kisiel, M.; Dłużniewski, M.; Okrasa, L. Epoxy Matrix with Triaromatic Mesogenic Unit in Dielectric Spectroscopy Observation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 194, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wang, T.; Li, X.; Zhang, Y.; Yu, H. NIR-Vis-UV Light-Responsive Actuator Films of Polymer-Dispersed Liquid Crystal/Graphene Oxide Nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 27494–27501. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Cong, Y.H.; Zhang, B.Y.; Zhang, L.; Huang, H.Z. Comparison of Two Kinds of Liquid Crystalline Monomers with Different Mesogenic Units Grafted Graphene Oxide on Thermal and Mechanical Properties of Epoxy Nanocomposite Materials. Liq. Cryst. 2021, 48, 1671–1684. [Google Scholar] [CrossRef]
- Hu, B.; Cong, Y.H.; Zhang, B.Y.; Zhang, L.; Shen, Y.; Huang, H. zhou Enhancement of Thermal and Mechanical Performances of Epoxy Nanocomposite Materials Based on Graphene Oxide Grafted by Liquid Crystalline Monomer with Schiff Base. J. Mater. Sci. 2020, 55, 3712–3727. [Google Scholar] [CrossRef]
- Padmajan Sasikala, S.; Lim, J.; Kim, I.H.; Jung, H.J.; Yun, T.; Han, T.H.; Kim, S.O. Graphene Oxide Liquid Crystals: A Frontier 2D Soft Material for Graphene-Based Functional Materials. Chem. Soc. Rev. 2018, 47, 6013–6045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Li, S.; Liu, J.; Dong, M.; Li, Y.; Lu, N.; Lei, S.; Tang, J.; Fan, J.; et al. Effect of Graphene Liquid Crystal on Dielectric Properties of Polydimethylsiloxane Nanocomposites. Compos. Part B Eng. 2019; 176, 107338. [Google Scholar] [CrossRef]
- Solodov, A.N.; Balkaev, D.A.; Shayimova, J.R.; Vakhitov, I.R.; Gataullina, R.M.; Sukhov, A.V.; Burilova, E.A.; Amirova, L.M.; Zhuravleva, Y.I.; Amirov, R.R. Tribological Properties of an Epoxy Polymer Containing a Magnetically Oriented Graphene Oxide/Iron Oxide Nanoparticle Composite. Diam. Relat. Mater. 2023, 138, 110211. [Google Scholar] [CrossRef]
- Solodov, A.N.; Balkaev, D.; Amirov, R.; Gataullina, R.; Nurtdinova, L.; Yusupov, R.; Kharintsev, S.S.; Dimiev, A.M. Polymer Composites with Magnetically Tunable Optical Anisotropy. ACS Appl. Polym. Mater. 2023, 5, 6338–6345. [Google Scholar] [CrossRef]
- Bao, T.; Wang, Z.; Zhao, Y.; Wang, Y.; Yi, X. Friction and Mechanical Properties of Amino-Treated Graphene-Filled Epoxy Composites: Modification Conditions and Filler Content. RSC Adv. 2020, 10, 26646–26657. [Google Scholar] [CrossRef]
- Wu, H.; Liu, C.; Cheng, L.; Yu, Y.; Zhao, H.; Wang, L. Enhancing the Mechanical and Tribological Properties of Epoxy Composites: Via Incorporation of Reactive Bio-Based Epoxy Functionalized Graphene Oxide. RSC Adv. 2020, 10, 40148–40156. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, H.; Wen, J.; Chen, J.F.; Mao, Z.; Zhu, P.; Sun, R.; Wu, W.; Peng, J. In-Situ Growth of Diamond/Ag as Hybrid Filler for Enhancing Thermal Conductivity of Liquid Crystal Epoxy. Diam. Relat. Mater. 2024, 141, 110659. [Google Scholar] [CrossRef]
- Khan, M.; Khurram, A.A.; Tiehu, L.; Zhao, T.K.; Xiong, C.; Ali, Z.; Ali, N.; Ullah, A. Reinforcement Effect of Acid Modified Nanodiamond in Epoxy Matrix for Enhanced Mechanical and Electromagnetic Properties. Diam. Relat. Mater. 2017, 78, 58–66. [Google Scholar] [CrossRef]
- Khan, M.; Hamid, A.; Tiehu, L.; Zada, A.; Attique, F.; Ahmad, N.; Ullah, A.; Hayat, A.; Mahmood, I.; Hussain, A.; et al. Surface Optimization of Detonation Nanodiamonds for the Enhanced Mechanical Properties of Polymer/Nanodiamond Composites. Diam. Relat. Mater. 2020, 107, 107897. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, M.; Park, S.J. Implication of Thermally Conductive Nanodiamond-Interspersed Graphite Nanoplatelet Hybrids in Thermoset Composites with Superior Thermal Management Capability. Sci. Rep. 2019, 9, 2893. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Liu, X.; Zhang, W.; Zeng, J.; Liu, J.; Chen, B.; Lin, J.; Tan, L.; Liang, L. Functionalized Mesoporous Silica Liquid Crystal Epoxy Resin Composite: An Ideal Low-Dielectric Hydrophobic Material. J. Mater. Sci. 2022, 57, 1156–1173. [Google Scholar] [CrossRef]
- Qin, T.F.; Wang, H.; He, J.; Qu, Q.Q.; Da, Y.S.; Tian, X.Y. Amino Multi-Walled Carbon Nanotubes Further Improve the Thermal Conductivity of Boron Nitride/Liquid Crystal Epoxy Resin Composites. Express Polym. Lett. 2020, 14, 1169–1179. [Google Scholar] [CrossRef]
- Tian, K.; Yang, S.; Niu, J.; Wang, H. Enhanced Thermal Conductivity and Mechanical Toughness of the Epoxy Resin by Incorporation of Mesogens without Nanofillers. IEEE Access 2021, 9, 31575–31580. [Google Scholar] [CrossRef]
- Chen, G.; Hu, J.; Xu, J.; Sun, J.; Xiao, J.; Zhang, L.; Wang, X.; Hu, W.; Yang, H. Liquid Crystalline Composite Stabilized by Epoxy Polymer with Boscage-Like Morphology for Energy-Efficient Smart Windows with High Stability. Macromol. Mater. Eng. 2022, 307, 2100991. [Google Scholar] [CrossRef]
- Li, H.; Zhang, B.; Yu, K.; Yuan, C.; Zhou, C.; Dunn, M.L.; Qi, H.J.; Shi, Q.; Wei, Q.H.; Liu, J.; et al. Influence of Treating Parameters on Thermomechanical Properties of Recycled Epoxy-Acid Vitrimers. Soft Matter 2020, 16, 1668–1677. [Google Scholar] [CrossRef]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent Organic Networks with Glass-like Fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef]
- Trinh, T.E.; Ku, K.; Yeo, H. Reprocessable and Chemically Recyclable Hard Vitrimers Based on Liquid-Crystalline Epoxides. Adv. Mater. 2023, 35, 2209912. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zając, W.; Kisiel, M.; Mossety-Leszczak, B. Advancements in The Cross-Linking and Morphology of Liquid Crystals. Crystals 2024, 14, 440. https://doi.org/10.3390/cryst14050440
Zając W, Kisiel M, Mossety-Leszczak B. Advancements in The Cross-Linking and Morphology of Liquid Crystals. Crystals. 2024; 14(5):440. https://doi.org/10.3390/cryst14050440
Chicago/Turabian StyleZając, Weronika, Maciej Kisiel, and Beata Mossety-Leszczak. 2024. "Advancements in The Cross-Linking and Morphology of Liquid Crystals" Crystals 14, no. 5: 440. https://doi.org/10.3390/cryst14050440





