Polyethylene Glycol-Modified Cationic Liposome as a Promising Nano Spray for Acute Pneumonia Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Preparation of RBV-PCL
2.3. Characterization of the Physicochemical Properties of Nanomaterial Particles
2.4. Drug Release Analysis
2.5. In Vitro Cytotoxicity Assay
2.6. Immunofluorescence Experiment
2.7. Enzyme-Linked Immunosorbent Assay Experiment
2.8. Mouse Model of AP
2.9. Histopathological Analysis
2.10. Analysis of Inflammatory Factor Levels
2.11. Flow Cytometry Analysis
2.12. Statistical Methods
3. Results
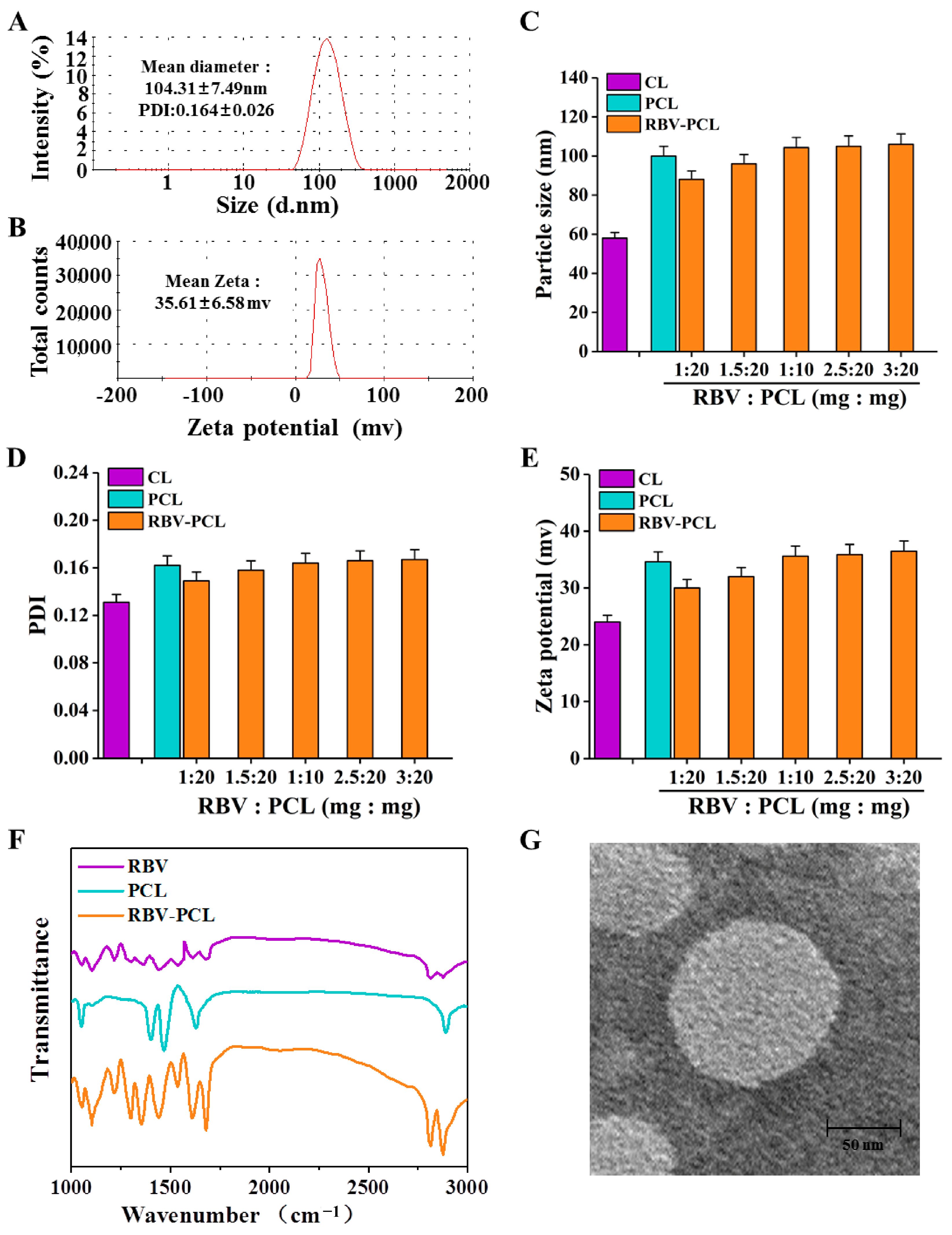
3.1. Synthesis and Characterization of RBV-PCL
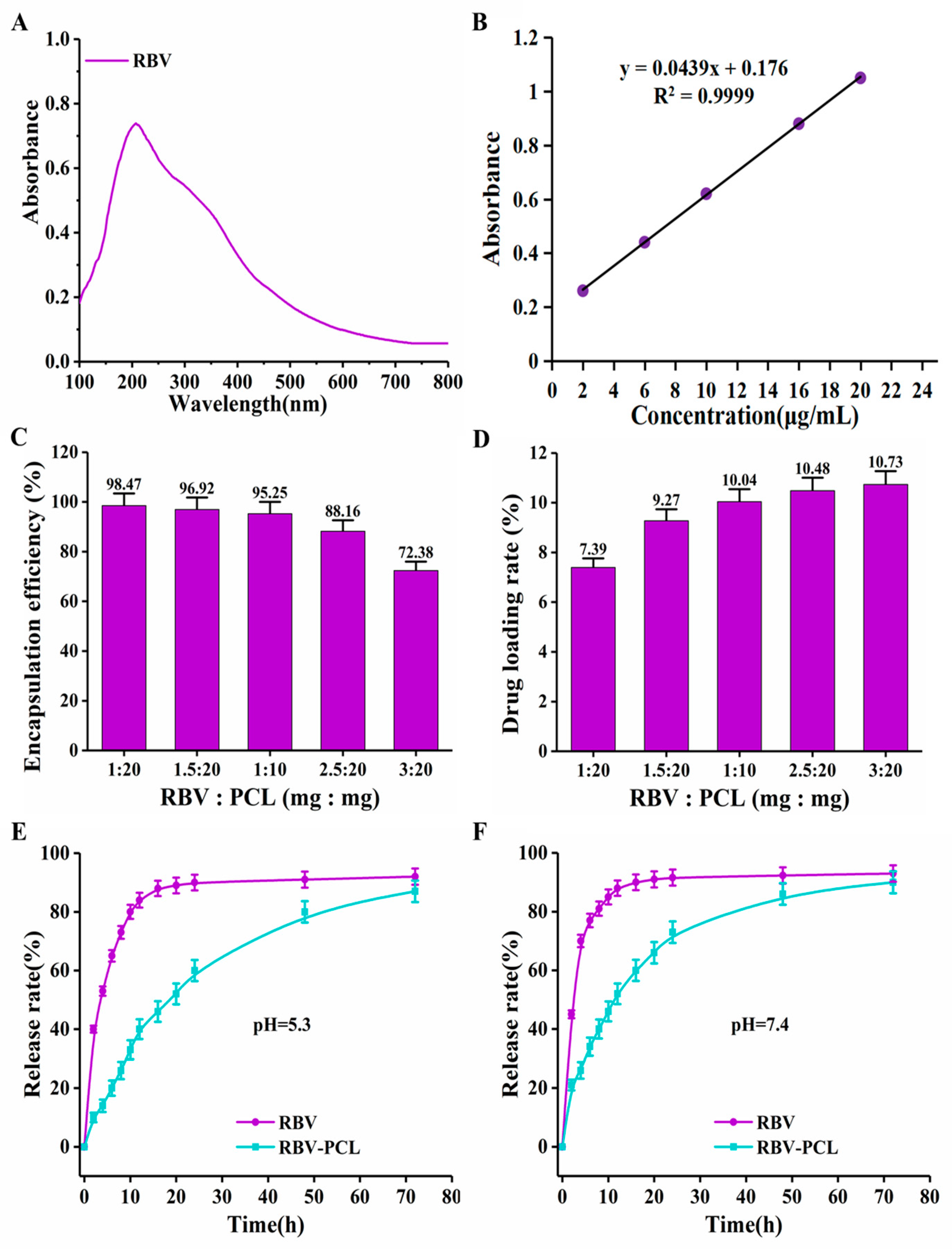
3.2. Drug Loading Efficiency and Drug Release Performance Assay
3.3. RBV-PCL Cytotoxicity, Uptake, and Effect on Secretion of the Inflammatory Factor IL-6 by LPS-Stimulated Cells
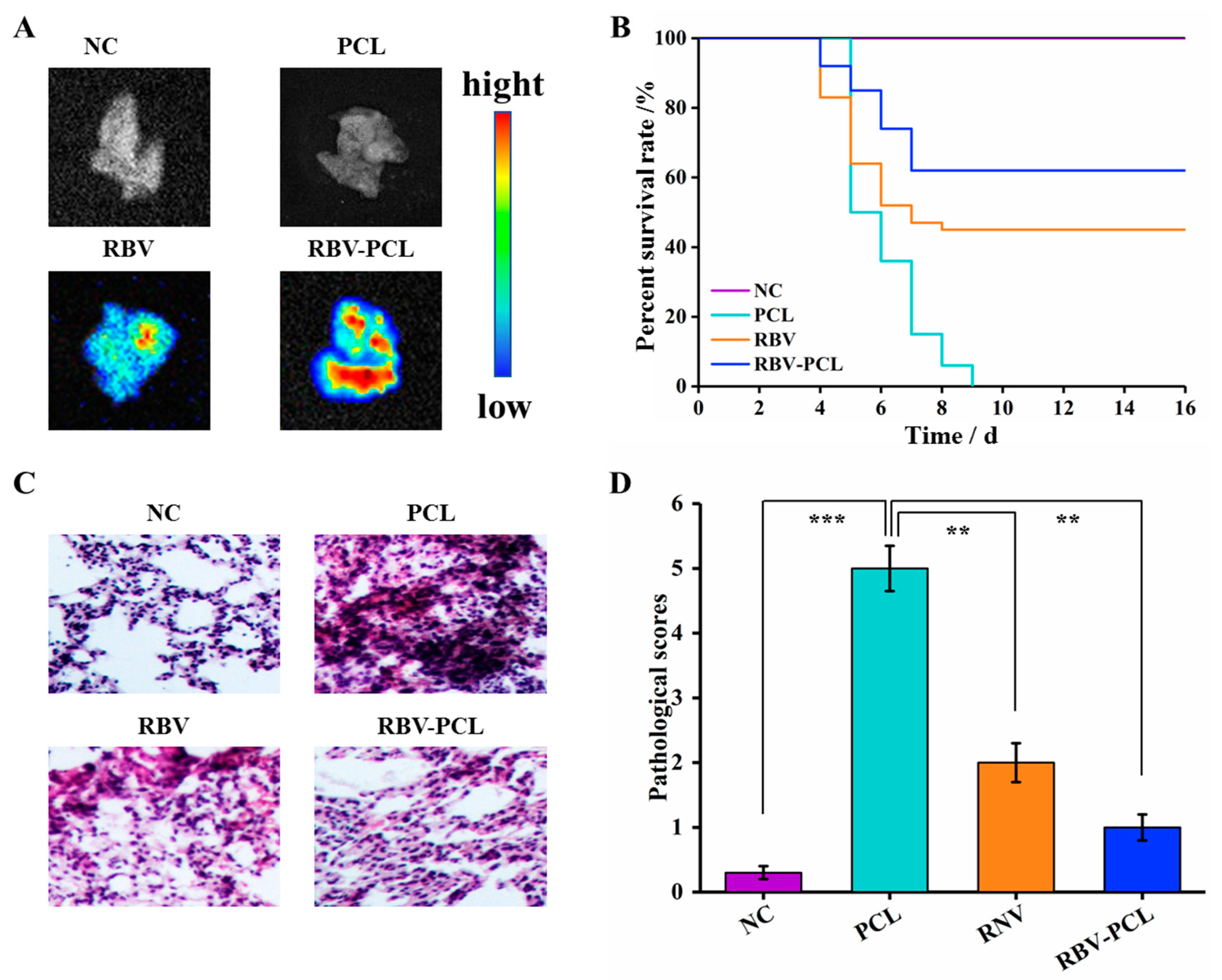
3.4. RBV-PCL Inhibits LPS-Induced Acute Lung Injury in Mice In Vivo
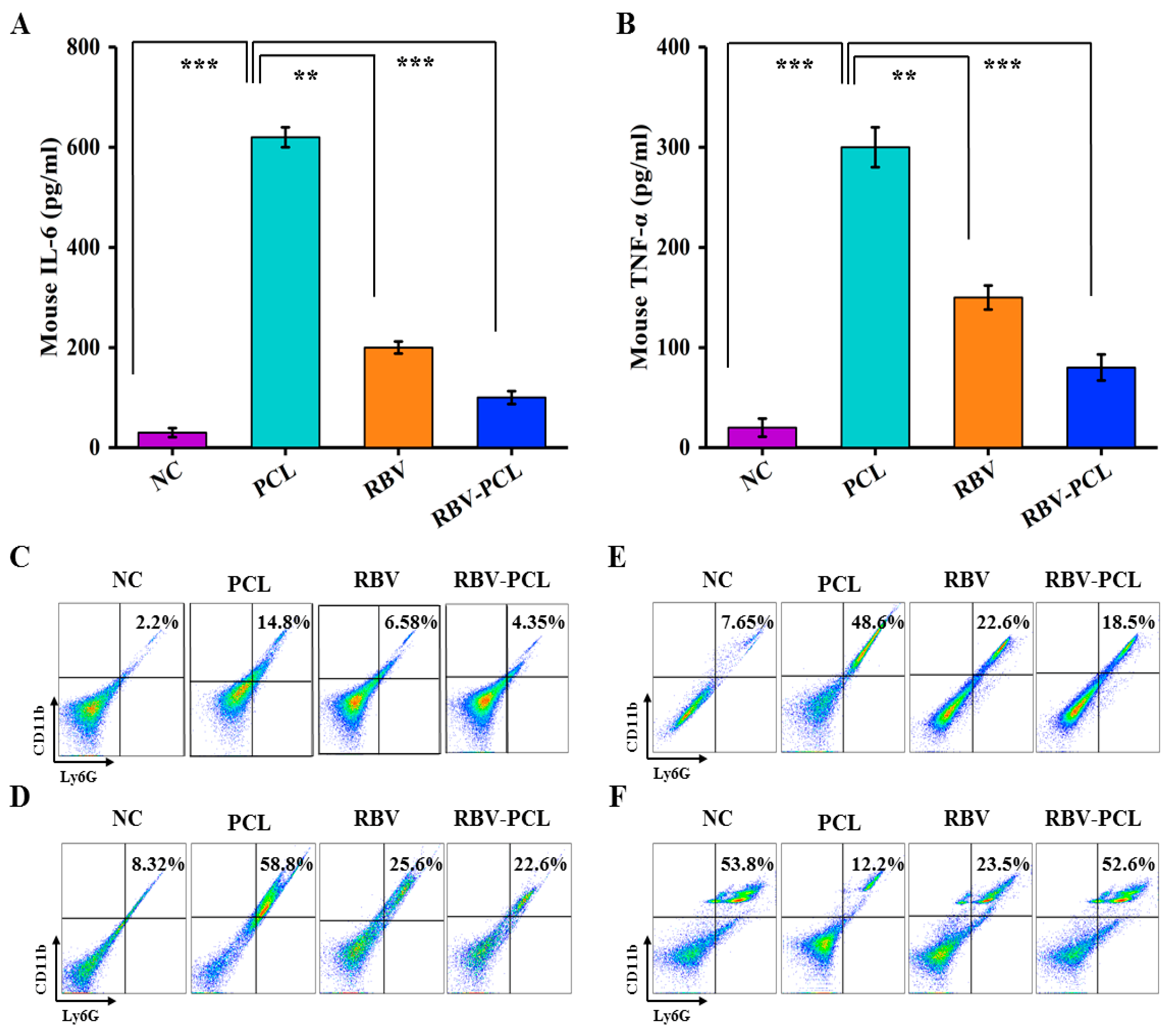
3.5. Effects of RBV-PCL on the Levels of Inflammatory Factors and Infiltration of Neutrophils and Macrophages in LPS-Infected Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gierse, L.C.; Meene, A.; Skorka, S.; Cuypers, F.; Surabhi, S.; Ferrero-Bordera, B.; Kreikemeyer, B.; Becher, D.; Hammerschmidt, S.; Siemens, N.; et al. Impact of Pneumococcal and Viral Pneumonia on the Respiratory and Intestinal Tract Microbiomes of Mice. Microbiol. Spectr. 2023, 11, e0344722. [Google Scholar] [CrossRef] [PubMed]
- Vanderlinden, E.; Marchand, A.; Van Berwaer, R.; van Dam, W.; Arzel, P.; Klaassen, H.; Persoons, L.; Chaltin, P.; Naesens, L. A broad influenza virus inhibitor acting via IMP dehydrogenase and in synergism with ribavirin. Antivir. Res. 2021, 196, 105208. [Google Scholar] [CrossRef] [PubMed]
- Moniuszko, M. Effects of Pegylated Interferon Alpha and Ribavirin (peg IFN-α/RBV) Therapeutic Approach on Regulatory T Cells in HCV-Mono infected and HCV/HIV-Coinfected Patients. Viruses 2021, 13, 1448. [Google Scholar] [CrossRef]
- Martín-Cerezuela, M.; Cuéllar-Monreal, M.J.; Monte-Boquet, E.; Solé-Jover, A.; Poveda-Andrés, J.L. Oral Ribavirin for Treatment of Respiratory Syncytial Virus in Lung Transplantation Recipients. Transplant. Proc. 2021, 53, 2702–2705. [Google Scholar] [CrossRef]
- Fabara, S.P.; Ortiz, J.F.; Smith, D.W.; Parwani, J.; Srikanth, S.; Varghese, T.; Paez, M.; Desai, P.; Tirupathi, R. Crimean-Congo Hemorrhagic Fever Beyond Ribavirin: A Systematic Review. Cureus 2021, 13, e17842. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Philips, L.; Xu, C.; Yeh, L.T. Pharmacokinetics and safety of viramidine, a prodrug of ribavirin, in healthy volunteers. J. Clin. Pharmacol. 2004, 44, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Koog, L.V.D.; Gandek, T.B.; Nagelkerke, A. Liposomes and Extracellular Vesicles as Drug Delivery Systems: A Comparison of Composition, Pharmacokinetics, and Functionalization. Adv. Healthc. Mater. 2021, 11, e2100639. [Google Scholar] [CrossRef]
- Cullis, P.R.; Zhigaltsev, I.V. Morphological Behavior of Liposomes and Lipid Nanoparticles. Langmuir 2023, 39, 3185–3193. [Google Scholar]
- Xiao, Z.; Zhao, S.; Zhang, X.; Wei, G.; Su, Z. Recent advances in peptide engineering of PEG hydrogels: Strategies, functional regulation, and biomedical applications. Macromol. Mater. Eng. 2022, 307, 14. [Google Scholar] [CrossRef]
- Stefano, A.D. Preparation, Characterization, and Biological Evaluation of a Hydrophilic Peptide Loaded on PEG-PLGA Nanoparticles. Pharmaceutics 2022, 14, 1821. [Google Scholar] [CrossRef]
- Hohokabe, M.; Higashi, K.; Yamada, Y.; Fujimoto, T.; Tokumoto, T.; Imamura, H.; Morita, T.; Ueda, K.; Limwikrant, W.; Moribe, K. Modification of liposomes composed of a cationic lipid TMAG and an anionic lipid DSPG with a PEGylated lipid based on the investigation of lipid structures. Colloids Surf. B 2023, 661, 130891. [Google Scholar] [CrossRef]
- Chen, H.J.; Cheng, Y.A.; Chen, Y.T.; Li, C.C.; Huang, B.C.; Hong, S.R.; Chen, I.J.; Ho, K.W.; Chen, C.Y.; Chen, F.M.; et al. Targeting and internalizing PEGylated nanodrugs to enhance the therapeutic efficacy of hematologic malignancies by anti-PEG bispecific antibody (mPEG×CD20). Cancer Nanotechnol. 2023, 14, 78. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Wang, X.; Li, Z.; Chen, Y.J.; Wu, F.B.; Men, K.; Xu, T.; Luo, Y.; Yang, L. Novel antimicrobial peptide–modified azithromycin-loaded liposomes against methicillin-resistant Staphylococcus aureus. Int. J. Nanomed. 2016, 11, 6781–6794. [Google Scholar] [CrossRef]
- Paul, S.; Roy, T.; Bose, A.; Chatterjee, D.; Chowdhury, V.R.; Rana, M.; Das, A. Liposome Mediated Pulmonary Drug Delivery System: An updated Review. Res. J. Pharm. Technol. 2021, 14, 1791–1796. [Google Scholar] [CrossRef]
- Altube, M.J.; Perez, N.; Romero, E.L.; Morilla, M.J.; Higa, L.H.; Perez, A.P. Inhaled lipid nanocarriers for pulmonary delivery of glucocorticoids: Previous strategies, recent advances and key factors description. Int. J. Pharm. 2023, 642, 123146. [Google Scholar] [CrossRef] [PubMed]
- Mainelis, G.; Seshadri, S.; Garbuzenko, O.B.; Han, T.; Wang, Z.; Minko, T. Characterization and application of a nose-only exposure chamber for inhalation delivery of liposomal drugs and nucleic acids to mice. J. Aerosol Med. Pulm. Drug Deliv. 2013, 26, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Ming, C.; Xiujuan, C.; Jiabin, C.; Zhihong, Y. Investigation of the Effect of Paclitaxel Liposome Aerosol Inhalation on Bleomycin-induced Pulmonary Fibrosis in Rats. Chin. J. Mod. Appl. Pharm. 2019, 36, 2149–2153. [Google Scholar]
- Kastantin, M.; Ananthanarayanan, B.; Karmali, P.; Ruoslahti, E.; Tirrell, M. Effect of the lipid chain melting transition on the stability of DSPE-PEG (2000) micelles. Langmuir 2009, 25, 7279–7286. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wu, J.; Lin, X.; Chen, Z.; Lin, L.; Yang, J. Enumeration and Molecular Characterization of Circulating Tumor Cell Using an Epithelial Cell Adhesion Molecule/Vimentin/Epidermal Growth Factor Receptor Joint Capture System in Lung Cancer. Clin. Med. Insights Oncol. 2024, 18, 11795549241231568. [Google Scholar] [CrossRef]
- Liang, X.; Shi, B.; Wang, K.; Fan, M.; Jiao, D.; Ao, J.; Song, N.; Wang, C.; Gu, J.; Li, Z. Development of self-assembling peptide nanovesicle with bilayers for enhanced EGFR-targeted drug and gene delivery. Biomaterials 2016, 82, 194–207. [Google Scholar] [CrossRef]
- Kitamoto, K.; Tanaka, Y.; Kuboyama, T.; Fujiki, Y.; Tomida, K.; Kamimori, T.; Hara, S. Newly diagnosed ANCA-associated vasculitis after COVID-19 infection: A case report. J. Med. Case Rep. 2023, 17, 366. [Google Scholar] [CrossRef]
- Messina, E.; Danise, A.; Ferrari, G.; Andolina, A.; Chiurlo, M.; Razanakolona, M.; Barakat, M.; Israel, R.J.; Castagna, A. Ribavirin Aerosol in the Treatment of SARS-CoV-2: A Case Series. Infect. Dis. Ther. 2021, 10, 2791–2804. [Google Scholar] [CrossRef]
- Wang, K.; Ding, S.; Zeng, L.; Zhou, J.; Cao, Y.; Wu, J.; Lu, L.; Bian, X.; Tian, G. Antisense Oligonucleotides-Laden UiO-66@Au nanohybrid for enhanced radiotherapy against hypoxic tumor by dual-inhibition of carbonic anhydrase IX. Appl. Mater. Today 2021, 25, 101201. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, K.; Ding, S.; Zeng, L.; Miao, J.; Cao, Y.; Zhang, X.; Tian, G.; Bian, X. Anti-VEGFR2-labeled enzyme-immobilized metal-organic frameworks for tumor vasculature targeted catalytic therapy. Acta Biomater. 2022, 141, 364–373. [Google Scholar] [CrossRef]
- Zhao, K.; Guo, T.; Wang, C.; Zhou, Y.; Xiong, T.; Wu, L.; Li, X.; Mittal, P.; Shi, S.; Gref, R.; et al. Glycoside scutellarin enhanced CD-MOF anchoring for laryngeal delivery. Acta Pharm. Sin. B 2020, 10, 1709–1718. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, K.; Pan, Y.; Rao, L.; Luo, G. Engineered Cell Membrane-Derived Nanoparticles in Immune Modulation. Adv. Sci. 2021, 8, e2102330. [Google Scholar] [CrossRef]
- Unal, M.A.; Bitirim, C.V.; Ummak, G.Y.; Bereketoglu, S.; Cevher Zeytin, I.; Besbinar, O.; Gurcan, C.; Aydos, D.; Goksoy, E.; Kocakaya, E.; et al. Ribavirin shows antiviral activity against SARS-CoV-2 and downregulates the activity of TMPRSS2 and the expression of ACE2 In Vitro. Can. J. Physiol. Pharmacol. 2021, 99, 449–460. [Google Scholar] [CrossRef]
- Zhenyuan, X.U. Effect of Budesonide Formoterol Combined with Tiotropium Bromide Atomization Inhalation on Arterial Blood Gas and Pulmonary Function in Patients with Chronic Obstructive Pulmonary Disease. Chin. Foreign Med. Res. 2023, 21, 27–31. [Google Scholar]
- Maurya, P.; Shakya, A.; Naik, R.R. Pulmonary Drug Delivery: Role and Application of Lipid Carriers. Syst. Rev. Pharm. 2021, 11, 1758–1771. [Google Scholar]
- Demirel, E.; Durmaz, Y.Y. PEGylated reduced graphene oxide as nanoplatform for targeted gene and drug delivery. Eur. Polym. J. 2023, 186, 111841. [Google Scholar] [CrossRef]
- Amani, A.; Alizadeh, M.R.; Yaghoubi, H.; Nohtani, M. Novel multi-targeted nanoparticles for targeted co-delivery of nucleic acid and chemotherapeutic agents to breast cancer tissues. Mater. Sci. Eng. C 2021, 118, 111494. [Google Scholar] [CrossRef]
- Patel, H.P.; Chaudhari, P.S.; Gandhi, P.A.; Desai, B.V.; Desai, D.T.; Dedhiya, P.P.; Vyas, B.A.; Maulvi, F.A. Nose to brain delivery of tailored clozapine nanosuspension stabilized using (+)-alpha-tocopherol polyethylene glycol 1000 succinate: Optimization and in vivo pharmacokinetic studies. Int. J. Pharm. 2021, 600, 10. [Google Scholar] [CrossRef]
- Cannito, S.; Bincoletto, V.; Turato, C.; Pontisso, P.; Scupoli, M.T.; Ailuno, G.; Andreana, I.; Stella, B.; Arpicco, S.; Bocca, C. Hyaluronated and PEGylated Liposomes as a Potential Drug-Delivery Strategy to Specifically Target Liver Cancer and Inflammatory Cells. Molecules 2022, 27, 1062. [Google Scholar] [CrossRef]
- Mukherjee, D.; Paul, D.; Sarker, S.; Hasan, M.N.; Ghosh, R.; Prasad, S.E.; Vemula, P.K.; Das, R.; Adhikary, A.; Pal, S.K.; et al. Polyethylene Glycol-Mediated Fusion of Extracellular Vesicles with Cationic Liposomes for the Design of Hybrid Delivery Systems. ACS Appl. Bio Mater. 2021, 4, 8259–8266. [Google Scholar] [CrossRef] [PubMed]
- Togami, K.; Maruta, Y.; Nanbu, M.; Tada, H.; Chono, S. Prolonged distribution of aerosolized PEGylated liposomes in the lungs of mice with bleomycin-induced pulmonary fibrosis. Drug Dev. Ind. Pharm. 2020, 46, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Liu, Y.; Xia, L.; Li, M.; Song, Z.; Hu, H.; Yang, Z.; Wang, L.; Cheng, X.; Wang, M.; et al. A clinical pilot study on the safety and efficacy of aerosol inhalation treatment of IFN-κplus TFF2 in patients with moderate COVID-19. E Clin. Med. 2020, 25, 100478. [Google Scholar]
- Ensign, L.M.; Schneider, C.; Suk, J.S.; Cone, R.; Hanes, J.L. Mucus Penetrating Nanoparticles: Biophysical Tool and Method of Drug and Gene Delivery. Adv. Mater. 2012, 24, 3887–3894. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wang, Y.Y.; Yang, M.; Schneider, C.; Zhong, W.; Pulicare, S.; Choi, W.J.; Mert, O.; Fu, J.; Lai, S.K.; et al. Biodegradable mucus-penetrating nanoparticles composed of diblock copolymers of polyethylene glycol and poly (lactic-co-glycolic acid). Drug Deliv. Transl. Res. 2012, 2, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, M. The Role of Neutrophils and TH17 Cells in the Immunopathology of Severe Asthmax. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2011, 10, 427–441. [Google Scholar] [CrossRef]
- Xiang, S.Y.; Ye, Y.; Yang, Q.; Xu, H.R.; Shen, C.X.; Ma, M.Q.; Jin, S.W.; Mei, H.X.; Zheng, S.X.; Smith, F.G.; et al. RvD1 accelerates the resolution of inflammation by promoting apoptosis of the recruited macrophages via the ALX/FasL-FasR/caspase-3 signaling pathway. Cell Death Discov. 2021, 7, 339. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Chen, D.; Zhang, C.; Lu, L.; Shang, F.; Li, Y. Polyethylene Glycol-Modified Cationic Liposome as a Promising Nano Spray for Acute Pneumonia Treatment. Polymers 2024, 16, 1384. https://doi.org/10.3390/polym16101384
Wang K, Chen D, Zhang C, Lu L, Shang F, Li Y. Polyethylene Glycol-Modified Cationic Liposome as a Promising Nano Spray for Acute Pneumonia Treatment. Polymers. 2024; 16(10):1384. https://doi.org/10.3390/polym16101384
Chicago/Turabian StyleWang, Kai, Dagui Chen, Chenxi Zhang, Lu Lu, Fusheng Shang, and Yinghua Li. 2024. "Polyethylene Glycol-Modified Cationic Liposome as a Promising Nano Spray for Acute Pneumonia Treatment" Polymers 16, no. 10: 1384. https://doi.org/10.3390/polym16101384




