Sodium Carbonate Pulping of Wheat Straw—An Alternative Fiber Source for Various Paper Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials (Collection and Characterization)
2.2. Pulping and Bleaching
2.3. Pulp Characterization
2.4. Pulp Beating, Handsheet Preparation, and Paper Testing
3. Results
3.1. Raw Material Characterization
3.2. Na2CO3 Pulping
3.2.1. Influence of Liquor:Solid Ratio (LSR)
3.2.2. Influence of Pulping Temperature
3.2.3. Influence of Chemical Charge
3.2.4. Paper Properties and Comparisons with Reference Pulps
- Na2CO3 pulp: 3:1 LSR, 12% Na2CO3, 150 °C, 30 min; 72% yield, kappa 75;
- NaOH pulp: 3:1 LSR, 12% NaOH, 150 °C, 30 min; 59% yield, kappa 26.
3.3. NACO Pulping
3.3.1. Influence of Pulping Temperature and Pulping Time
3.3.2. Influence of NaOH Addition and Pulping Time
3.4. Bleaching of NACO Pulps
3.4.1. Comparison of Acid Washing (A Stage) and Q Stage and their Influence on the Subsequent P Stage
3.4.2. Influence of Bleaching Temperature and Bleaching Time
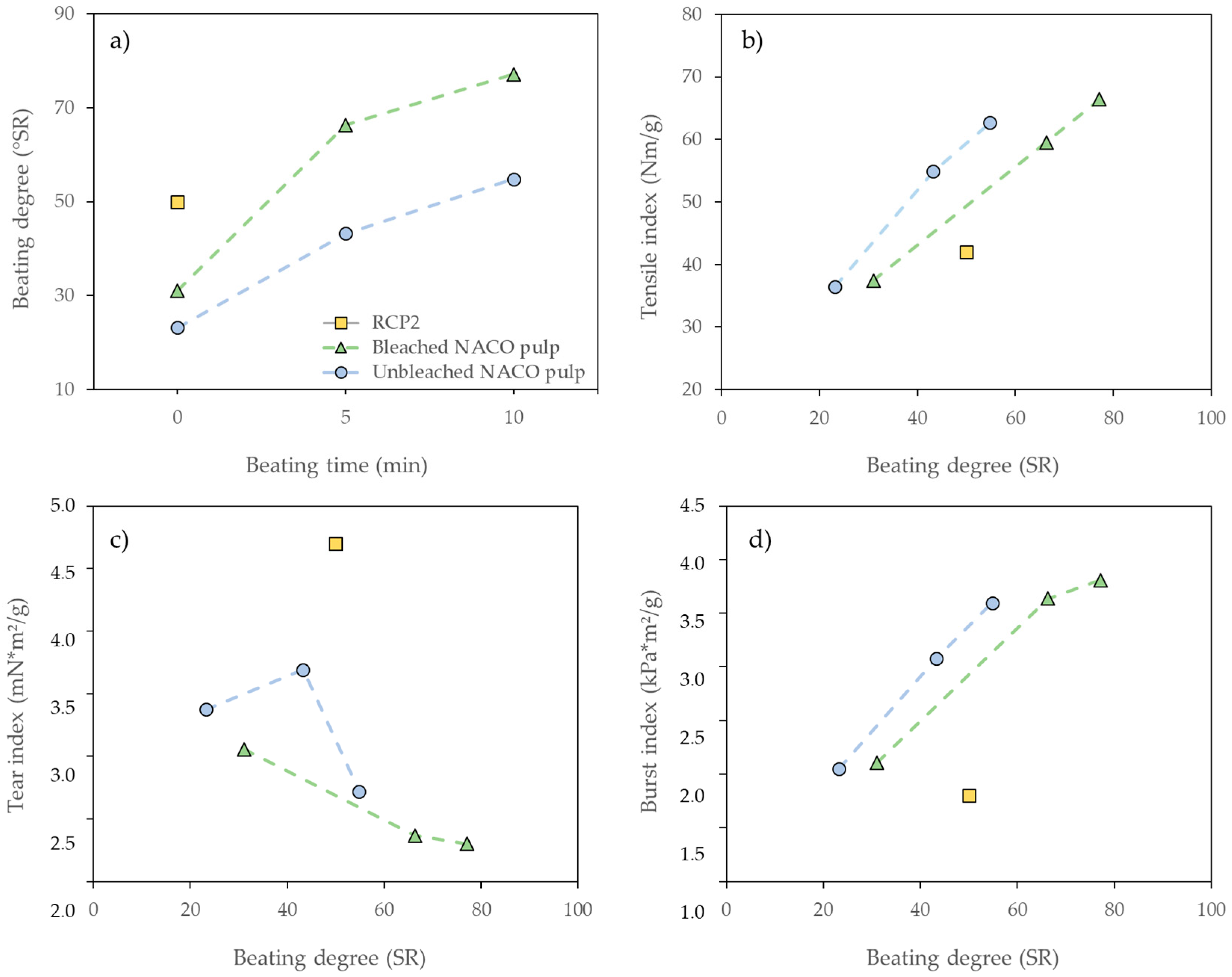
3.4.3. Paper Properties and Comparison with Reference Pulp
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cepi Key Statistics. 2022. Available online: https://www.cepi.org/wp-content/uploads/2023/07/2022-Key-Statistics-FINAL.pdf (accessed on 13 December 2023).
- Billerudkorsnäs. Annual Review. 2013. Available online: https://www.billerud.com/globalassets/billerudkorsnas/investors/financial-reports/2013/209898_bk_arsoversikt_eng.pdf (accessed on 27 December 2023).
- Triantafyllou, V.I.; Akrida-Demertzi, K.; Demertzis, P.G. A study on the migration of organic pollutants from recycled paperboard packaging materials to solid food matrices. Food Chem. 2007, 101, 1759–1768. [Google Scholar] [CrossRef]
- Papier 2023—Ein Leistungsbericht + Ausführliche Statistiken. Available online: https://www.papierindustrie.de/papierindustrie/statistik/papier-2023-herunterladen (accessed on 13 December 2023).
- Tofani, G.; Cornet, I.; Tavernier, S. Multiple linear regression to predict the brightness of waste fibres mixtures before bleaching. Chem. Pap. 2022, 76, 4351–4365. [Google Scholar] [CrossRef]
- German Federal Statistical Office—Index of Wholesale Selling Prices—Recovered Paper and Scrap Metals. Available online: https://www.destatis.de/DE/Themen/Wirtschaft/Preise/Grosshandelspreisindex/Publikationen/Downloads-Grosshandelspreise/grosshandelsverkaufspreise-altpapier-xlsx-5612802.xlsx?__blob=publicationFile (accessed on 13 December 2023).
- DBFZ Resource Data Repository. Available online: https://webapp.dbfz.de/resources/?lang=en (accessed on 13 December 2023).
- Worku, L.A.; Bachheti, A.; Bachheti, R.K.; Rodrigues Reis, C.E.; Chandel, A.K. Agricultural Residues as Raw Materials for Pulp and Paper Production: Overview and Applications on Membrane Fabrication. Membranes 2023, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, P. Chapter 7—Recovery of Chemicals from Nonwood Black Liquor. In Pulp and Paper Industry; Bajpai, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 147–157. [Google Scholar] [CrossRef]
- Salehi, K.; Latibari, A.J.; Kordsachia, O.; Saake, B. The potential of bagasse soda pulp as a strength enhancer for old corrugated pulp. Appita J. Tech. Assoc. Austral. N. Z. Pulp Paper Ind. 2017, 70, 371–377. Available online: https://www.openagrar.de/receive/openagrar_mods_00035540 (accessed on 13 December 2023).
- Puitel, A.C.; Marin, N.; Puiu, P.; Gavrilescu, D. Lignocellulosic Agricultural Residues - A Virgin Fibre Supply Solution for Paper-Based Packaging. Cell Chem. Technol. 2015, 49, 633–639. [Google Scholar]
- Puitel, A.C.; Moisei, N.; Tofanica, B.M.; Gavrilescu, D. Turning Wheat Straw in a Sustainable Raw Material for Paper Industry. Environ. Eng. Manag. J. 2017, 16, 1027–1032. [Google Scholar]
- Marin, N.; Puitel, A.C.; Chesca, A.M.; Gavrilescu, D. Response Surface Modeling of Wheat Straw Pulping using Sodium Carbonate and Sodium Hydroxide Mixtures. Cell Chem. Technol. 2017, 51, 745–753. [Google Scholar]
- Fiala, W.; Danielsson, O.; Ryrberg, K.-G.; Nardi, F. Oxygen Pulping of Non-Wood Plant Fibers According to the NACO Process. Prog. Rep. Non-Wood Plant Fiber Pulping 1983, 14, 77–86. [Google Scholar]
- IPS Engineering. Non Wood Pulping Technology. Available online: https://www.ips-engineering.it/images/PDF/IPS%20_Pulping.pdf (accessed on 13 December 2023).
- Lorenz, D.; Erasmy, N.; Akil, Y.; Saake, B. A new method for the quantification of monosaccharides, uronic acids and oligosaccharides in partially hydrolyzed xylans by HPAEC-UV/VIS. Carbohydr. Polym. 2016, 140, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Larsson, A.; Moldin, A.; Edlund, U. Comparison of lignin distribution, structure, and morphology in wheat straw and wood. Ind. Crops Prod. 2022, 187, 115432. [Google Scholar] [CrossRef]
- Janzon, R.; Schütt, F.; Oldenburg, S.; Fischer, E.; Körner, I.; Saake, B. Steam pretreatment of spruce forest residues: Optimal conditions for biogas production and enzymatic hydrolysis. Carbohydr. Polym. 2014, 100, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, K.; Zhang, L.; Wu, Y. Synchronous silicon removal and viscosity reduction in the soda-oxygen pulping of wheat straw. Cellulose 2021, 28, 9081–9089. [Google Scholar] [CrossRef]
- Castellan, A.; Grelier, S. Part III—Wood and Fibres, Chapter 21: Color and Color Reversion of Cellulosic and Lignocellulosic Fibers. In Lignocellulosic Fibers and Wood Handbook; Belgacem, N., Pizzi, A., Eds.; Scrivener Publishing LCC: Beverly, MA, USA, 2016; pp. 531–551. [Google Scholar] [CrossRef]
- Sheoran, M.; Kaur, N.; Chandra, A.; Gautam, A.K.; Arya, R.K. Pilot-scale soda pulping of wheat straw using continuous pulp digester. J. Indian Chem. Soc. 2020, 97, 403–408. [Google Scholar]
- Lehnen, R. Herstellung von Spezialzellstoffen aus Alternativen Rohstoffen für Hochwertige Anwendungen (HeSpeRoh). Available online: https://www.fnr.de/ftp/pdf/berichte/22000517.pdf (accessed on 13 December 2023).
- Salehi, K.; Kordsachia, O.; Patt, R. Comparison of MEA/AQ, soda and soda/AQ pulping of wheat and rye straw. Ind. Crops Prod. 2014, 52, 603–610. [Google Scholar] [CrossRef]
- Gao, J.Y.; Dong, M.L.; Xu, T.T.; Bian, H.Y.; Wang, S.M.; Dai, H.Q. Analysis of causes for high hydration and low drainage rate of wheat straw chemi-thermo-mechanical pulp. Ind. Crops Prod. 2023, 203, 117103. [Google Scholar] [CrossRef]
- Joelsson, T.; Pettersson, G.; Norgren, S.; Svedberg, A.; Höglund, H.; Engstrand, P. High strength paper from high yield pulps by means of hot-pressing. Nordic Pulp Paper Res. J. 2020, 35, 195–204. [Google Scholar] [CrossRef]
- Salehi, K.; Kordachia, O.; Saake, B. The Potential of Wheat Straw High Yield MEA Pulp for Enhancing Strength Properties of Recycled Paper. Bioresources 2017, 12, 8255–8271. [Google Scholar] [CrossRef]
- Kalyoncu, E.E.; Kirci, H.; Peşman, E. Evaluating suitable chelating agents for Q stage in TCF bleaching of wheat straw alkaline pulps. Cellulose Chem. Technol. 2023, 57, 831–841. [Google Scholar] [CrossRef]
- Kordsachia, T. Aufschluss von Weizenstroh und TCFF/ECF-Bleiche von Weizenstrohzellstoff. Bachelor‘s Thesis, University of Hamburg, Hamburg, Germany, September 2011. [Google Scholar]
- Caustic Soda Price Trend and Forecast. Available online: https://www.chemanalyst.com/Pricing-data/caustic-soda-3 (accessed on 13 December 2023).
- Soda Ash Price and Forecast. Available online: https://www.chemanalyst.com/Pricing-data/soda-ash-76 (accessed on 13 December 2023).
- Tofani, G.; Cornet, I.; Tavernier, S. Estimation of hydrogen peroxide effectivity during bleaching using the Kappa number. Chem. Pap. 2021, 75, 5749–5758. [Google Scholar] [CrossRef]








| Compounds | Present Study | Other Studies [17] | Spruce Wood [18] |
|---|---|---|---|
| Cellulose | 36.4 (0.2) | 32–45 | 41.6 |
| Hemicelluloses | 23.1 (0.4) | 20–45 | 25.2 |
| Lignin 1 | 20.5 (0.2) | 11–26 | 29.5 |
| Extractives | 10.5 (0.1) | ND 2 | 3.6 |
| Ash | 10.5 (0.2) | 0–2 | 0.4 |
| Compounds | Wheat Straw | Na2CO3 Pulp 1 | NaOH Pulp 2 |
|---|---|---|---|
| Ash | 10.5 (0.2) | 7.8 | 4.1 |
| Silicates | 9.7 (0.3) | 7.7 | 3.0 |
| Pulp Characteristics | A Stage | Q Stage |
|---|---|---|
| Pulp brightness, %ISO | 42.4 | 42.3 |
| Kappa number | 18.3 | 18.4 |
| Total pulp yield 1 | 58.0 | 57.4 |
| Pulp Characteristics | A–P | Q–P |
|---|---|---|
| Pulp brightness, %ISO | 67.9 | 64.5 |
| Kappa number | 10.3 | 11.6 |
| H2O2 consumption, % | 48.7 | 51.0 |
| Total pulp yield, % 1 | 55.7 | 55.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steffen, F.; Kordsachia, T.; Heizmann, T.; Eckardt, M.P.; Chen, Y.; Saake, B. Sodium Carbonate Pulping of Wheat Straw—An Alternative Fiber Source for Various Paper Applications. Agronomy 2024, 14, 162. https://doi.org/10.3390/agronomy14010162
Steffen F, Kordsachia T, Heizmann T, Eckardt MP, Chen Y, Saake B. Sodium Carbonate Pulping of Wheat Straw—An Alternative Fiber Source for Various Paper Applications. Agronomy. 2024; 14(1):162. https://doi.org/10.3390/agronomy14010162
Chicago/Turabian StyleSteffen, Friedrich, Tamas Kordsachia, Tobias Heizmann, Maximilian Paul Eckardt, Yue Chen, and Bodo Saake. 2024. "Sodium Carbonate Pulping of Wheat Straw—An Alternative Fiber Source for Various Paper Applications" Agronomy 14, no. 1: 162. https://doi.org/10.3390/agronomy14010162






