Altered Expression of PDE4 Genes in Schizophrenia: Insights from a Brain and Blood Sample Meta-Analysis and iPSC-Derived Neurons
Abstract
1. Introduction
2. Methods
2.1. Identification and Selection of Eligible Gene Expression Datasets for Meta-Analysis
2.2. Statistical Analysis
2.3. iPSC-Derived Neuron Preparation and Transcription Analysis
3. Results
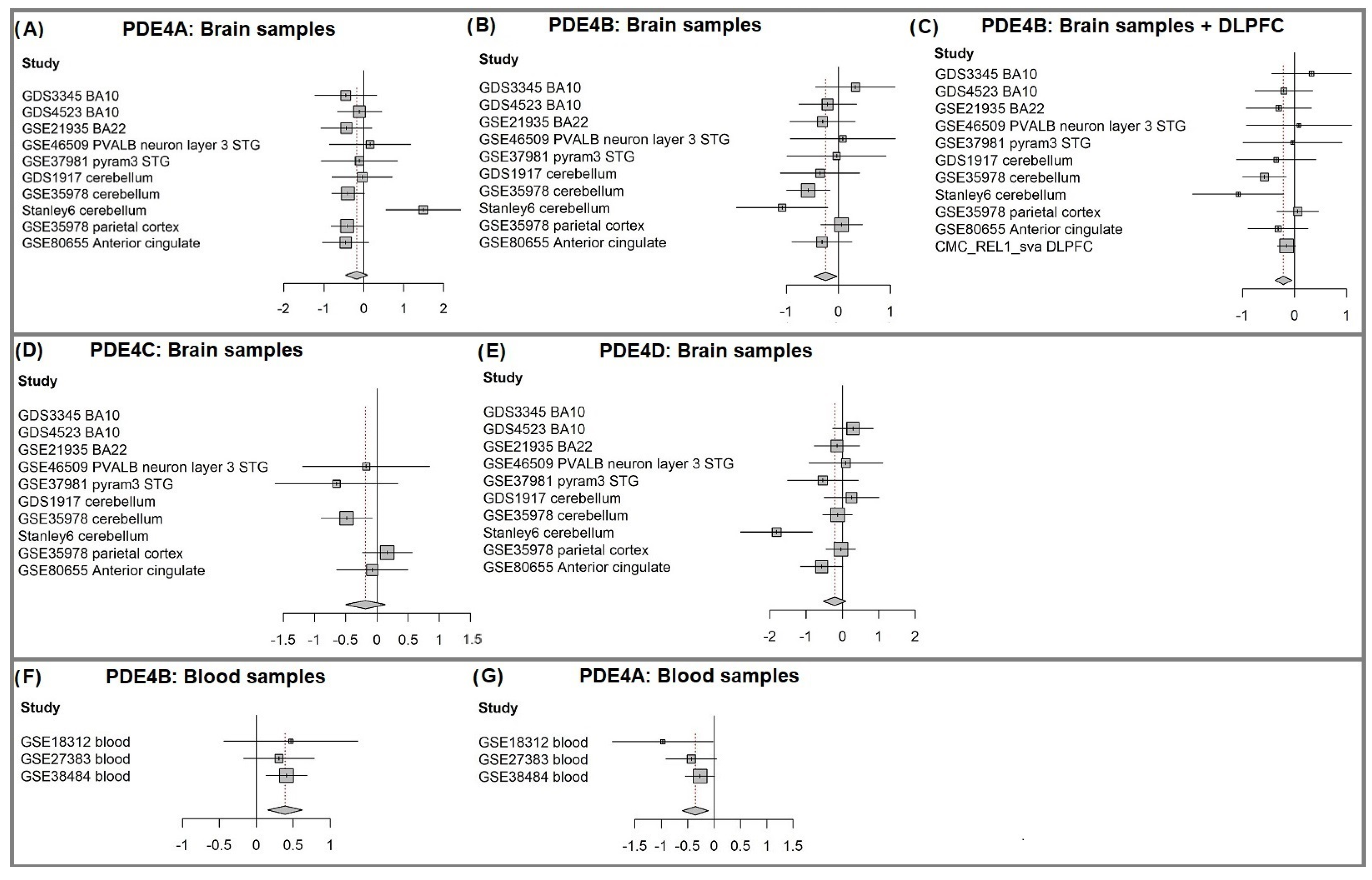
3.1. PDE4B Is Downregulated in Brain Samples of Individuals with Schizophrenia
3.2. PDE4B Downregulation Is Concentrated in a Subgroup of Patients
3.3. Blood Samples Analysis: PDE4A Is Downregulated and PDE4B Is Upregulated in Individuals with Schizophrenia
3.4. PDE4 Genes Are Upregulated in Dentate Gyrus Granule Neurons Derived from Schizophrenia Patients Compared to Controls
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGrath, J.; Saha, S.; Chant, D.; Welham, J. Schizophrenia: A Concise Overview of Incidence, Prevalence, and Mortality. Epidemiol. Rev. 2008, 30, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Cherian, J.; Gohil, K.; Atkinson, D. Schizophrenia: Overview and Treatment Options. Pharm. Ther. 2014, 39, 638. [Google Scholar]
- Lieberman, J.A.; Stroup, T.S.; McEvoy, J.P.; Swartz, M.S.; Rosenheck, R.A.; Perkins, D.O.; Keefe, R.S.E.; Davis, S.M.; Davis, C.E.; Lebowitz, B.D.; et al. Effectiveness of Antipsychotic Drugs in Patients with Chronic Schizophrenia. N. Engl. J. Med. 2009, 353, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Ripke, S.; Neale, B.M.; Corvin, A.; Walters, J.T.R.; Farh, K.H.; Holmans, P.A.; Lee, P.; Bulik-Sullivan, B.; Collier, D.A.; Huang, H.; et al. Biological Insights from 108 Schizophrenia-Associated Genetic Loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef]
- Gejman, P.V.; Sanders, A.R.; Duan, J. The Role of Genetics in the Etiology of Schizophrenia. Psychiatr. Clin. N. Am. 2010, 33, 35–66. [Google Scholar] [CrossRef]
- Trubetskoy, V.; Pardiñas, A.F.; Qi, T.; Panagiotaropoulou, G.; Awasthi, S.; Bigdeli, T.B.; Bryois, J.; Chen, C.Y.; Dennison, C.A.; Hall, L.S.; et al. Mapping Genomic Loci Implicates Genes and Synaptic Biology in Schizophrenia. Nature 2022, 604, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.R.; Göring, H.H.H.; Duan, J.; Drigalenko, E.I.; Moy, W.; Freda, J.; He, D.; Shi, J.; Gejman, P.V. Transcriptome Study of Differential Expression in Schizophrenia. Hum. Mol. Genet. 2013, 22, 5001. [Google Scholar] [CrossRef]
- Habtewold, T.D.; Rodijk, L.H.; Liemburg, E.J.; Sidorenkov, G.; Boezen, H.M.; Bruggeman, R.; Alizadeh, B.Z. A Systematic Review and Narrative Synthesis of Data-Driven Studies in Schizophrenia Symptoms and Cognitive Deficits. Transl. Psychiatry 2020, 10, 244. [Google Scholar] [CrossRef]
- Owen, M.J. Genomic Insights into Schizophrenia. R. Soc. Open Sci. 2023, 10, 230125. [Google Scholar] [CrossRef]
- Hartl, C.L.; Ramaswami, G.; Pembroke, W.G.; Muller, S.; Pintacuda, G.; Saha, A.; Parsana, P.; Battle, A.; Lage, K.; Geschwind, D.H. Coexpression Network Architecture Reveals the Brain-Wide and Multiregional Basis of Disease Susceptibility. Nat. Neurosci. 2021, 24, 1313–1323. [Google Scholar] [CrossRef]
- Van Erp, T.G.M.; Hibar, D.P.; Rasmussen, J.M.; Glahn, D.C.; Pearlson, G.D.; Andreassen, O.A.; Agartz, I.; Westlye, L.T.; Haukvik, U.K.; Dale, A.M.; et al. Subcortical Brain Volume Abnormalities in 2028 Individuals with Schizophrenia and 2540 Healthy Controls via the ENIGMA Consortium. Mol. Psychiatry 2016, 21, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Van Erp, T.G.M.; Walton, E.; Hibar, D.P.; Schmaal, L.; Jiang, W.; Glahn, D.C.; Pearlson, G.D.; Yao, N.; Fukunaga, M.; Hashimoto, R.; et al. Cortical Brain Abnormalities in 4474 Individuals with Schizophrenia and 5098 Control Subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biol. Psychiatry 2018, 84, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.; Brown, H.R.; Siemerkus, J.; Stephan, K.E. The Dysconnection Hypothesis (2016). Schizophr. Res. 2016, 176, 83. [Google Scholar] [CrossRef] [PubMed]
- Edwards, H.V.; Christian, F.; Baillie, G.S. CAMP: Novel Concepts in Compartmentalised Signalling. Semin. Cell Dev. Biol. 2012, 23, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Beavo, J.A.; Brunton, L.L. Cyclic Nucleotide Research—Still Expanding after Half a Century. Nat. Rev. Mol. Cell Biol. 2002, 3, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Beavo, J. Biochemistry and Physiology of Cyclic Nucleotide Phosphodiesterases: Essential Components in Cyclic Nucleotide Signaling. Annu. Rev. Biochem. 2007, 76, 481–511. [Google Scholar] [CrossRef]
- Baillie, G.S. Compartmentalized Signalling: Spatial Regulation of CAMP by the Action of Compartmentalized Phosphodiesterases. FEBS J. 2009, 276, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Kotera, J. Overview of PDEs and Their Regulation. Circ. Res. 2007, 100, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.; Ukkola-Vuoti, L.; Ortega-Alonso, A.; Torniainen-Holm, M.; Therman, S.; Tuulio-Henriksson, A.; Jylhä, P.; Kaprio, J.; Hovatta, I.; Isometsä, E.; et al. Variants in Regulatory Elements of PDE4D Associate with Major Mental Illness in the Finnish Population. Mol. Psychiatry 2021, 26, 816–824. [Google Scholar] [CrossRef]
- Pérez-Torres, S.; Miró, X.; Palacios, J.M.; Cortés, R.; Puigdoménech, P.; Mengod, G. Phosphodiesterase Type 4 Isozymes Expression in Human Brain Examined by in Situ Hybridization Histochemistry and [3H]Rolipram Binding Autoradiography. Comparison with Monkey and Rat Brain. J. Chem. Neuroanat. 2000, 20, 349–374. [Google Scholar] [CrossRef]
- Lynch, M.A. Long-Term Potentiation and Memory. Physiol. Rev. 2004, 84, 87–136. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Frey, S.; Frey, J.U. Regulation of the Phosphodiesterase PDE4B3-Isotype during Long-Term Potentiation in the Area Dentata in Vivo. Neuroscience 2004, 124, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Frey, J.U. Expression of the Specific Type IV Phosphodiesterase Gene PDE4B3 during Different Phases of Long-Term Potentiation in Single Hippocampal Slices of Rats in Vitro. Neuroscience 2003, 117, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Mcgirr, A.; Lipina, T.V.; Mun, H.S.; Georgiou, J.; Al-Amri, A.H.; Ng, E.; Zhai, D.; Elliott, C.; Cameron, R.T.; Mullins, J.G.; et al. Specific Inhibition of Phosphodiesterase-4B Results in Anxiolysis and Facilitates Memory Acquisition. Neuropsychopharmacology 2015, 41, 1080–1092. [Google Scholar] [CrossRef]
- Tibbo, A.J.; Baillie, G.S. Phosphodiesterase 4B: Master Regulator of Brain Signaling. Cells 2020, 9, 1254. [Google Scholar] [CrossRef] [PubMed]
- Paspalas, C.D.; Wang, M.; Arnsten, A.F.T. Constellation of HCN Channels and CAMP Regulating Proteins in Dendritic Spines of the Primate Prefrontal Cortex: Potential Substrate for Working Memory Deficits in Schizophrenia. Cereb. Cortex 2013, 23, 1643. [Google Scholar] [CrossRef] [PubMed]
- Lakics, V.; Karran, E.H.; Boess, F.G. Quantitative Comparison of Phosphodiesterase MRNA Distribution in Human Brain and Peripheral Tissues. Neuropharmacology 2010, 59, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; King, D.P.; Reutiman, T.J.; Folsom, T.D.; Laurence, J.A.; Lee, S.; Fan, Y.T.; Paciga, S.A.; Conti, M.; Menniti, F.S. PDE4B Polymorphisms and Decreased PDE4B Expression Are Associated with Schizophrenia. Schizophr. Res. 2008, 101, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Cheng, D.; Zhang, C.; Li, Y.; Zhang, Z.; Wang, J.; Shi, Y. Association of PDE4B Polymorphisms with Susceptibility to Schizophrenia: A Meta-Analysis of Case-Control Studies. PLoS ONE 2016, 11, e0147092. [Google Scholar] [CrossRef]
- Numata, S.; Ueno, S.-I.; Iga, J.-I.; Song, H.; Nakataki, M.; Tayoshi, S.; Sumitani, S.; Tomotake, M.; Itakura, M.; Sano, A.; et al. Positive Association of the PDE4B (Phosphodiesterase 4B) Gene with Schizophrenia in the Japanese Population. J. Psychiatr. Res. 2008, 43, 7–12. [Google Scholar] [CrossRef]
- Rastogi, A.; Zai, C.; Likhodi, O.; Kennedy, J.L.; Wong, A.H. Genetic Association and Post-Mortem Brain MRNA Analysis of DISC1 and Related Genes in Schizophrenia. Schizophr. Res. 2009, 114, 39–49. [Google Scholar] [CrossRef]
- Liu, D.; Meyer, D.; Fennessy, B.; Feng, C.; Cheng, E.; Johnson, J.S.; Park, Y.J.; Rieder, M.K.; Ascolillo, S.; de Pins, A.; et al. Schizophrenia Risk Conferred by Rare Protein-Truncating Variants Is Conserved across Diverse Human Populations. Nat. Genet. 2023, 55, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Poterba, T.; Curtis, D.; Akil, H.; Al Eissa, M.; Barchas, J.D.; Bass, N.; Bigdeli, T.B.; Breen, G.; Bromet, E.J.; et al. Rare Coding Variants in Ten Genes Confer Substantial Risk for Schizophrenia. Nature 2022, 604, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Takaki, H.; Wang, L.; Kuroki, T.; Nakahara, T.; Hashimoto, K.; Ninomiya, H.; Arinami, T.; Inada, T.; Ujike, H.; et al. Positive Association of Phencyclidine-Responsive Genes, PDE4A and PLAT, with Schizophrenia. Am. J. Med. Genet. Part. B Neuropsychiatr. Genet. 2011, 156, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Hossein Fatemi, S.; Reutiman, T.J.; Folsom, T.D.; Lee, S. Phosphodiesterase-4A Expression Is Reduced in Cerebella of Patients with Bipolar Disorder. Psychiatr. Genet. 2008, 18, 282–288. [Google Scholar] [CrossRef]
- Tavitian, A.; Song, W.; Schipper, H.M. Dentate Gyrus Immaturity in Schizophrenia. Neuroscientist 2019, 25, 528–547. [Google Scholar] [CrossRef]
- Santos, R.; Linker, S.B.; Stern, S.; Mendes, A.P.D.; Shokhirev, M.N.; Erikson, G.; Randolph-Moore, L.; Racha, V.; Kim, Y.; Kelsoe, J.R.; et al. Deficient LEF1 Expression Is Associated with Lithium Resistance and Hyperexcitability in Neurons Derived from Bipolar Disorder Patients. Mol. Psychiatry 2021, 26, 2440–2456. [Google Scholar] [CrossRef]
- Nakahara, S.; Turner, J.A.; Calhoun, V.D.; Lim, K.O.; Mueller, B.; Bustillo, J.R.; O’Leary, D.S.; McEwen, S.; Voyvodic, J.; Belger, A.; et al. Dentate Gyrus Volume Deficit in Schizophrenia. Psychol. Med. 2020, 50, 1267–1277. [Google Scholar] [CrossRef]
- Hoffman, G.E.; Bendl, J.; Voloudakis, G.; Montgomery, K.S.; Sloofman, L.; Wang, Y.C.; Shah, H.R.; Hauberg, M.E.; Johnson, J.S.; Girdhar, K.; et al. CommonMind Consortium Provides Transcriptomic and Epigenomic Data for Schizophrenia and Bipolar Disorder. Sci. Data 2019, 6, 180. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Wolf, A.; Yitzhaky, A.; Hertzberg, L. SMAD Genes Are Up-Regulated in Brain and Blood Samples of Individuals with Schizophrenia. J. Neurosci. Res. 2023, 101, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, K.; Kakiuchi, C.; Bundo, M.; Ikeda, K.; Kato, T. Molecular Characterization of Bipolar Disorder by Comparing Gene Expression Profiles of Postmortem Brains of Major Mental Disorders. Mol. Psychiatry 2004, 9, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Maycox, P.R.; Kelly, F.; Taylor, A.; Bates, S.; Reid, J.; Logendra, R.; Barnes, M.R.; Larminie, C.; Jones, N.; Lennon, M.; et al. Analysis of Gene Expression in Two Large Schizophrenia Cohorts Identifies Multiple Changes Associated with Nerve Terminal Function. Mol. Psychiatry 2009, 14, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.R.; Huxley-Jones, J.; Maycox, P.R.; Lennon, M.; Thornber, A.; Kelly, F.; Bates, S.; Taylor, A.; Reid, J.; Jones, N.; et al. Transcription and Pathway Analysis of the Superior Temporal Cortex and Anterior Prefrontal Cortex in Schizophrenia. J. Neurosci. Res. 2011, 89, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Pietersen, C.Y.; Mauney, S.A.; Kim, S.S.; Lim, M.P.; Rooney, R.J.; Goldstein, J.M.; Petryshen, T.L.; Seidman, L.J.; Shenton, M.E.; McCarley, R.W.; et al. Molecular Profiles of Pyramidal Neurons in the Superior Temporal Cortex in Schizophrenia. J. Neurogenet. 2014, 28, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Pietersen, C.Y.; Mauney, S.A.; Kim, S.S.; Passeri, E.; Lim, M.P.; Rooney, R.J.; Goldstein, J.M.; Petreyshen, T.L.; Seidman, L.J.; Shenton, M.E.; et al. Molecular Profiles of Parvalbumin-Immunoreactive Neurons in the Superior Temporal Cortex in Schizophrenia. J. Neurogenet. 2014, 28, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Paz, R.D.; Andreasen, N.C.; Daoud, S.Z.; Conley, R.; Roberts, R.; Bustillo, J.; Perrone-Bizzozero, N.I. Increased Expression of Activity-Dependent Genes in Cerebellar Glutamatergic Neurons of Patients with Schizophrenia. Am. J. Psychiatry 2006, 163, 1829–1831. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cheng, L.; Grennan, K.; Pibiri, F.; Zhang, C.; Badner, J.A.; Kelsoe, J.R.; Greenwood, T.A.; Nievergelt, C.M.; Barrett, T.B.; et al. Two Gene Co-Expression Modules Differentiate Psychotics and Controls. Mol. Psychiatry 2013, 18, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Ramaker, R.C.; Bowling, K.M.; Lasseigne, B.N.; Hagenauer, M.H.; Hardigan, A.A.; Davis, N.S.; Gertz, J.; Cartagena, P.M.; Walsh, D.M.; Vawter, M.P.; et al. Post-Mortem Molecular Profiling of Three Psychiatric Disorders. Genome Med. 2017, 9, 72. [Google Scholar] [CrossRef]
- Bousman, C.A.; Chana, G.; Glatt, S.J.; Chandler, S.D.; Lucero, G.R.; Tatro, E.; May, T.; Lohr, J.B.; Kremen, W.S.; Tsuang, M.T.; et al. Preliminary Evidence of Ubiquitin Proteasome System Dysregulation in Schizophrenia and Bipolar Disorder: Convergent Pathway Analysis Findings from Two Independent Samples. Am. J. Med. Genet. Part. B Neuropsychiatr. Genet. 2010, 153, 494–502. [Google Scholar] [CrossRef]
- Van Beveren, N.J.M.; Buitendijk, G.H.S.; Swagemakers, S.; Krab, L.C.; Röder, C.; de Haan, L.; van der Spek, P.; Elgersma, Y. Marked Reduction of AKT1 Expression and Deregulation of AKT1-Associated Pathways in Peripheral Blood Mononuclear Cells of Schizophrenia Patients. PLoS ONE 2012, 7, e32618. [Google Scholar] [CrossRef] [PubMed]
- De Jong, S.; Boks, M.P.M.; Fuller, T.F.; Strengman, E.; Janson, E.; de Kovel, C.G.F.; Ori, A.P.S.; Vi, N.; Mulder, F.; Blom, J.D.; et al. A Gene Co-Expression Network in Whole Blood of Schizophrenia Patients Is Independent of Antipsychotic-Use and Enriched for Brain-Expressed Genes. PLoS ONE 2012, 7, e39498. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.V. Distribution Theory for Glass’s Estimator of Effect Size and Related Estimators. J. Educ. Stat. 1981, 6, 107. [Google Scholar] [CrossRef]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Meta-Analysis with R. Use R! Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-21415-3. [Google Scholar]
- Fleiss, J.L. The Statistical Basis of Meta-Analysis. Stat. Methods Med. Res. 1993, 2, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.; Zhang, L.; Wang, M.; Wright, R.; Rosh, I.; Hussein, Y.; Stern, T.; Choudhary, A.; Tripathi, U.; Reed, P.; et al. Monozygotic Twins Discordant for Schizophrenia Differ in Maturation and Synaptic Transmission. Mol. Psychiatry 2024, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bohlken, M.M.; Brouwer, R.M.; Mandl, R.C.W.; van den Heuvel, M.P.; Hedman, A.M.; de Hert, M.; Cahn, W.; Kahn, R.S.; Hulshoff Pol, H.E. Structural Brain Connectivity as a Genetic Marker for Schizophrenia. JAMA Psychiatry 2016, 73, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.; Sarkar, A.; Galor, D.; Stern, T.; Mei, A.; Stern, Y.; Mendes, A.P.D.; Randolph-Moore, L.; Rouleau, G.; Bang, A.G.; et al. A Physiological Instability Displayed in Hippocampal Neurons Derived from Lithium-Nonresponsive Bipolar Disorder Patients. Biol. Psychiatry 2020, 88, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.; Sarkar, A.; Stern, T.; Mei, A.; Mendes, A.P.D.; Stern, Y.; Goldberg, G.; Galor, D.; Nguyen, T.; Randolph-Moore, L.; et al. Mechanisms Underlying the Hyperexcitability of CA3 and Dentate Gyrus Hippocampal Neurons Derived from Patients with Bipolar Disorder. Biol. Psychiatry 2020, 88, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010. [Google Scholar]
- Schulmann, A.; Marenco, S.; Vawter, M.P.; Akula, N.; Limon, A.; Mandal, A.; Auluck, P.K.; Patel, Y.; Lipska, B.K.; McMahon, F.J. Antipsychotic Drug Use Complicates Assessment of Gene Expression Changes Associated with Schizophrenia. Transl. Psychiatry 2023, 13, 93. [Google Scholar] [CrossRef]
- Delhaye, S.; Bardoni, B. Role of Phosphodiesterases in the Pathophysiology of Neurodevelopmental Disorders. Mol. Psychiatry 2021, 26, 4570–4582. [Google Scholar] [CrossRef]
- Giorgi, M.; Modica, A.; Pompili, A.; Pacitti, C.; Gasbarri, A. The Induction of Cyclic Nucleotide Phosphodiesterase 4 Gene (PDE4D) Impairs Memory in a Water Maze Task. Behav. Brain Res. 2004, 154, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Richter, W.; Menniti, F.S.; Zhang, H.T.; Conti, M. PDE4 as a Target for Cognition Enhancement. Expert Opin. Ther. Targets 2013, 17, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Bryois, J.; Calini, D.; Macnair, W.; Foo, L.; Urich, E.; Ortmann, W.; Iglesias, V.A.; Selvaraj, S.; Nutma, E.; Marzin, M.; et al. Cell-Type-Specific Cis-EQTLs in Eight Human Brain Cell Types Identify Novel Risk Genes for Psychiatric and Neurological Disorders. Nat. Neurosci. 2022, 25, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Giralt, A.; Saavedra, A.; Carretón, O.; Xifró, X.; Alberch, J.; Pérez-navarro, E. Increased PKA Signaling Disrupts Recognition Memory and Spatial Memory: Role in Huntington’s Disease. Hum. Mol. Genet. 2011, 20, 4232–4247. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, S.B.; Mogulkoc, R.; Baltaci, A.K. Molecular Mechanisms of Early and Late LTP. Neurochem. Res. 2019, 44, 281–296. [Google Scholar] [CrossRef]
- Rutten, K.; Wallace, T.L.; Works, M.; Prickaerts, J.; Blokland, A.; Novak, T.J.; Santarelli, L.; Misner, D.L. Enhanced Long-Term Depression and Impaired Reversal Learning in Phosphodiesterase 4B-Knockout (PDE4B−/−) Mice. Neuropharmacology 2011, 61, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Bowen, E.F.W.; Burgess, J.L.; Granger, R.; Kleinman, J.E.; Rhodes, C.H. DLPFC Transcriptome Defines Two Molecular Subtypes of Schizophrenia. Transl. Psychiatry 2019, 9, 147. [Google Scholar] [CrossRef]
- Dolmetsch, R.; Geschwind, D.H. The Human Brain in a Dish: The Promise of IPSC-Derived Neurons. Cell 2011, 145, 831. [Google Scholar] [CrossRef]
- Kang, S.; Chen, X.; Gong, S.; Yu, P.; Yau, S.; Su, Z.; Zhou, L.; Yu, J.; Pan, G.; Shi, L. Characteristic Analyses of a Neural Differentiation Model from IPSC-Derived Neuron According to Morphology, Physiology, and Global Gene Expression Pattern. Sci. Rep. 2017, 7, 12233. [Google Scholar] [CrossRef]
- Bell, S.; Hettige, N.C.; Silveira, H.; Peng, H.; Wu, H.; Jefri, M.; Antonyan, L.; Zhang, Y.; Zhang, X.; Ernst, C. Differentiation of Human Induced Pluripotent Stem Cells (IPSCs) into an Effective Model of Forebrain Neural Progenitor Cells and Mature Neurons. Bio-Protocol 2019, 9, e3188. [Google Scholar] [CrossRef]
- Hong, Y.; Yang, Q.; Song, H.; Ming, G. li Opportunities and Limitations for Studying Neuropsychiatric Disorders Using Patient-Derived Induced Pluripotent Stem Cells. Mol. Psychiatry 2023, 28, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Ohline, S.M.; Liu, X.; Ibrahim, M.F.; Mockett, B.M.; Empson, R.M.; Abraham, W.C.; Iremonger, K.J.; Jones, P.P. Altered Membrane Properties but Unchanged Intrinsic Excitability and Spontaneous Postsynaptic Currents in an Aged APPswe/PS1dE9 Model of Alzheimer’s Disease. Front. Cell Neurosci. 2022, 16, 958876. [Google Scholar] [CrossRef] [PubMed]
- Brant, B.; Stern, T.; Shekhidem, H.A.; Mizrahi, L.; Rosh, I.; Stern, Y.; Ofer, P.; Asleh, A.; Umanah, G.K.E.; Jada, R.; et al. IQSEC2 Mutation Associated with Epilepsy, Intellectual Disability, and Autism Results in Hyperexcitability of Patient-Derived Neurons and Deficient Synaptic Transmission. Mol. Psychiatry 2021, 26, 7498–7508. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.M.; Simkin, D.; Disterhoft, J.F. Intrinsic Hippocampal Excitability Changes of Opposite Signs and Different Origins in CA1 and CA3 Pyramidal Neurons Underlie Aging-Related Cognitive Deficits. Front. Syst. Neurosci. 2016, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.; Beccano-Kelly, D.A.; Talbot, K.; Llana, O.C.; Wade-Martins, R. Hyperexcitability in Young IPSC-Derived C9ORF72 Mutant Motor Neurons Is Associated with Increased Intracellular Calcium Release. Sci. Rep. 2022, 12, 7378. [Google Scholar] [CrossRef] [PubMed]
- Von Bartheld, C.S.; Bahney, J.; Herculano-Houzel, S. The Search for True Numbers of Neurons and Glial Cells in the Human Brain: A Review of 150 Years of Cell Counting. J. Comp. Neurol. 2016, 524, 3865. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, S.; Yitzhaky, A.; Hertzberg, L. Gene Expression Meta-Analysis Reveals the up-Regulation of CREB1 and CREBBP in Brodmann Area 10 of Patients with Schizophrenia. Psychiatry Res. 2020, 292, 113311. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, G.E.; Hartley, B.J.; Flaherty, E.; Ladran, I.; Gochman, P.; Ruderfer, D.M.; Stahl, E.A.; Rapoport, J.; Sklar, P.; Brennand, K.J. Transcriptional Signatures of Schizophrenia in HiPSC-Derived NPCs and Neurons Are Concordant with Post-Mortem Adult Brains. Nat. Commun. 2017, 8, 2225. [Google Scholar] [CrossRef] [PubMed]
- Burke, E.E.; Chenoweth, J.G.; Shin, J.H.; Collado-Torres, L.; Kim, S.K.; Micali, N.; Wang, Y.; Colantuoni, C.; Straub, R.E.; Hoeppner, D.J.; et al. Dissecting Transcriptomic Signatures of Neuronal Differentiation and Maturation Using IPSCs. Nat. Commun. 2020, 11, 462. [Google Scholar] [CrossRef]
- Verheijen, M.C.T.; Krauskopf, J.; Caiment, F.; Nazaruk, M.; Wen, Q.F.; van Herwijnen, M.H.M.; Hauser, D.A.; Gajjar, M.; Verfaillie, C.; Vermeiren, Y.; et al. IPSC-Derived Cortical Neurons to Study Sporadic Alzheimer Disease: A Transcriptome Comparison with Post-Mortem Brain Samples. Toxicol. Lett. 2022, 356, 89–99. [Google Scholar] [CrossRef]
- Nascimento, J.M.; Saia-Cereda, V.M.; Zuccoli, G.S.; Reis-de-Oliveira, G.; Carregari, V.C.; Smith, B.J.; Rehen, S.K.; Martins-de-Souza, D. Proteomic Signatures of Schizophrenia-Sourced IPSC-Derived Neural Cells and Brain Organoids Are Similar to Patients’ Postmortem Brains. Cell Biosci. 2022, 12, 189. [Google Scholar] [CrossRef]
- Bilecki, W.; Maćkowiak, M. Gene Expression and Epigenetic Regulation in the Prefrontal Cortex of Schizophrenia. Genes 2023, 14, 243. [Google Scholar] [CrossRef] [PubMed]
- Wainger, B.J.; Degennaro, M.; Santoro, B.; Siegelbaum, S.A.; Tibbs, G.R. Molecular Mechanism of CAMP Modulation of HCN Pacemaker Channels. Nature 2001, 411, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Kase, D.; Imoto, K. The Role of HCN Channels on Membrane Excitability in the Nervous System. J. Signal Transduct. 2012, 2012, 619747. [Google Scholar] [CrossRef] [PubMed]
- Nucifora, L.G.; MacDonald, M.L.; Lee, B.J.; Peters, M.E.; Norris, A.L.; Orsburn, B.C.; Yang, K.; Gleason, K.; Margolis, R.L.; Pevsner, J.; et al. Increased Protein Insolubility in Brains from a Subset of Patients with Schizophrenia. Am. J. Psychiatry 2019, 176, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.J.; Mirnics, K. Neurodevelopment, GABA System Dysfunction, and Schizophrenia. Neuropsychopharmacology 2015, 40, 190–206. [Google Scholar] [CrossRef]
- Gogtay, N.; Vyas, N.S.; Testa, R.; Wood, S.J.; Pantelis, C. Age of Onset of Schizophrenia: Perspectives from Structural Neuroimaging Studies. Schizophr. Bull. 2011, 37, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Oldham, M.C.; Konopka, G.; Iwamoto, K.; Langfelder, P.; Kato, T.; Horvath, S.; Geschwind, D.H. Functional Organization of the Transcriptome in Human Brain. Nat. Neurosci. 2008, 11, 1271–1282. [Google Scholar] [CrossRef]
- Andrews, D.F. A Robust Method for Multiple Linear Regression. Technometrics 1974, 16, 523–531. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]




| Brain Samples Gene Expression Studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Accession | Publication | Brain Region; Brain Bank | # SZ | # CNT | Platform | Mean Age (Standard Dev.) | Mean PMI (Standard Dev.) | Mean pH (Standard Dev.) |
| GDS3345 | Iwamoto 2004 [42] | BA10; SMRI | 12 7M:5F | 15 9M:6F | HG U95 Av.2 | SZ: 45 (14) CNT: 48 (11) p = 0.47 | SZ: 33 (16) CNT: 24 (10) p = 0.07 | SZ: 6.2 (0.2) CNT: 6.3 (0.2) p = 0.37 |
| GDS4523 | Maycox 2009 [43] | BA10; CCHPC | 27 19M:8F | 23 12M:11F | HG U133 Plus 2.0 | SZ: 73 (15) CNT: 69 (22) p = 0.45 | SZ: 8.2 (7) CNT: 10 (4) p = 0.3 | SZ:6.1 (0.2) CNT: 6.5 (0.3) p = 8 × 10−6 |
| GSE21935 | Barnes 2011 [44] | BA22; CCHPC | 23 13M:10F | 17 9M:8F | U133 Plus 2.0 Array | SZ: 72 (17) CNT: 65 (22) p = 0.25 | SZ: 7 (6) CNT: 9 (4) p = 0.36 | SZ: 6.2 (0.2) CNT: 6.5 (0.3) p = 2.3 × 10−6 |
| GSE37981 | Pietersen 2014 pyramidal [45] | STG; HBTRC | 9 4M:5F | 8 4M:4F | U133 X3P Array | SZ: 67 (20) CNT: 67 (21) p = 0.99 | SZ: 17 (5) CNT: 18 (3) p = 0.71 | Not provided |
| GSE46509 | Pietersen 2014 parvalbumin [46] | STG; HBTRC | 7 3M:4F | 8 4M:4F | U133 X3P Array | SZ: 69 (22) CNT: 67 (21) p = 0.87 | SZ: 15.8 (6) CNT: 18 (3) p = 0.38 | Not provided |
| GDS1917 | Paz 2006 [47] | CRBLM; Maryland | 13 13M:0F | 14 14M:0F | U133 Plus 2.0 Array | SZ: 46 (12) CNT: 43 (10) p = 0.5 | SZ: 12.8 (5) CNT: 15.6 (6) p = 0.18 | Not provided |
| GSE35978 | Chen 2013 [48] | CRBLM; SMRI | 44 32M:12F | 50 31M:19F | Gene 1.0 ST Array | SZ: 43 (9); CNT: 46 (9) p = 0.18 | SZ: 33 (15) CNT: 28 (11) p = 0.042 | SZ: 6.4 (0.2) CNT: 6.5 (0.3) p = 0.44 |
| Stanley#6 | CRBLM; SMRI | 10 7M:3F | 14 9M:5F | U95 Av2 Array | SZ: 46 (14); CNT: 47 (9) p = 0.83 | SZ: 34 (14) CNT: 24 (10) p = 0.071 | SZ: 6.2 (0.2) CNT: 6.3 (0.2) p = 0.82 | |
| GSE35978 | Chen 2013 [48] | Parietal cortex; SMRI | 51 37M:14F | 45 31M:14F | Gene 1.0 ST Array | SZ: 43 (10); CNT: 46 (9) p = 0.14 | SZ: 31 (16) CNT: 27 (12) p = 0.17 | SZ: 6.4 (0.3) CNT: 6.5 (0.3) p = 0.015 |
| GSE80655 | Ramaker 2017 [49] | ACC; Pritzker | 23 20M:3F | 24 21M:3F | Illumina HiSeq 2000 | SZ: 43 (9); CNT: 50 (13) p = 0.043 | SZ: 21 (9) CNT: 22 (7) p = 0.62 | SZ: 6.8 (0.2) CNT: 6.9 (0.1) p = 0.044 |
| Overall: 219 155M:64F | Overall: 218 144M:74F | SZ: 52.1 (18) CNT: 51.8 (16) p = 0.85 | SZ: 23.5 (16) CNT: 21.6 (11) p = 0.15 | SZ: 6.4 (0.3) CNT: 6.5 (0.3) p = 5 × 10−7 | ||||
| CMC Release 1 | Hoffman 2019 [39] | DLPFC; MSSM Penn Pitt HBCC | 244 154M:90F | 271 157M:114F | Illumina HiSeq 2500 | SZ: 69 (17) CNT: 65 (19) p = 0.012 | SZ: 20 (13) CNT: 14 (8) p = −1012 | SZ: 6.5 (0.3) CNT: 6.6 (0.3) p = 0.003 |
| Blood Sample Gene Expression Studies | ||||||||
| Accession | Publication | Blood Samples Type | # SZ | # CNT | Platform | Mean Age (Standard Dev.) | ||
| GSE18312 | Bousman 2010 [50] | Whole blood | 12 9M:3F | 8 5M:3F | HuEx-1_0-st | SZ: 43 (9); CNT: 45 (7) p = 0.57 | ||
| GSE27383 | Van Beveren 2012 [51] | Peripheral Blood Mononuclear Cells (PBMCs) | 41 41M:0F | 29 29M:0F | HG-U133_Plus_2 | Not provided | ||
| GSE38484 | De Jong 2012 [52] | Whole blood | 104 75M:29F | 96 42M:54F | Illumina HumanHT-12 V3.0 | SZ: 40 (11); CNT: 39 (14) p = 0.85 | ||
| Overall: 167 | Overall: 133 | |||||||
| Twin Type | Type | Sex | Diagnosis DSM IV | Age | Age Onset | Sex |
|---|---|---|---|---|---|---|
| MZ | Patient | M | Paranoid schizophrenia | 46 | 22 | M |
| MZ | Co-twin | M | Depressive Disorder NOS | 46 | 24 | M |
| MZ | Patient | F | Paranoid schizophrenia | 50 | 35 | F |
| MZ | Co-twin | F | Major Depressive Disorder, Recurrent, In Partial Remission | 50 | 25 | F |
| MZ | Control | M | No diagnosis or condition on Axis I or Axis 2 | 35 | - | M |
| MZ | Control | M | 35 | - | M | |
| MZ | Control | F | 46 | - | F | |
| MZ | Control | F | 46 | - | F | |
| MZ | Control | F | 32 | - | F | |
| MZ | Control | F | 32 | - | F |
| Gene Symbol | Random Effects Hedges | Lower | Upper |
|---|---|---|---|
| PDE4A | −0.18 | −0.46 | 0.1 |
| PDE4B | −0.25 | −0.47 | −0.03 |
| PDE4C | −0.18 | −0.5 | 0.13 |
| PDE4D | −0.21 | −0.53 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burrack, N.; Yitzhaky, A.; Mizrahi, L.; Wang, M.; Stern, S.; Hertzberg, L. Altered Expression of PDE4 Genes in Schizophrenia: Insights from a Brain and Blood Sample Meta-Analysis and iPSC-Derived Neurons. Genes 2024, 15, 609. https://doi.org/10.3390/genes15050609
Burrack N, Yitzhaky A, Mizrahi L, Wang M, Stern S, Hertzberg L. Altered Expression of PDE4 Genes in Schizophrenia: Insights from a Brain and Blood Sample Meta-Analysis and iPSC-Derived Neurons. Genes. 2024; 15(5):609. https://doi.org/10.3390/genes15050609
Chicago/Turabian StyleBurrack, Nitzan, Assif Yitzhaky, Liron Mizrahi, Meiyan Wang, Shani Stern, and Libi Hertzberg. 2024. "Altered Expression of PDE4 Genes in Schizophrenia: Insights from a Brain and Blood Sample Meta-Analysis and iPSC-Derived Neurons" Genes 15, no. 5: 609. https://doi.org/10.3390/genes15050609
APA StyleBurrack, N., Yitzhaky, A., Mizrahi, L., Wang, M., Stern, S., & Hertzberg, L. (2024). Altered Expression of PDE4 Genes in Schizophrenia: Insights from a Brain and Blood Sample Meta-Analysis and iPSC-Derived Neurons. Genes, 15(5), 609. https://doi.org/10.3390/genes15050609






