The Elimination of Levofloxacin from High-Salinity Wastewater via the Electrochlorination Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Electrochlorination Experiments
2.3. Experimental Instrument
2.4. Analytical Methods
3. Results and Discussion
3.1. Effects of Key Operational Parameters on LEV Degradation
3.1.1. Effects of Cl− Concentration
3.1.2. Effects of Current Density
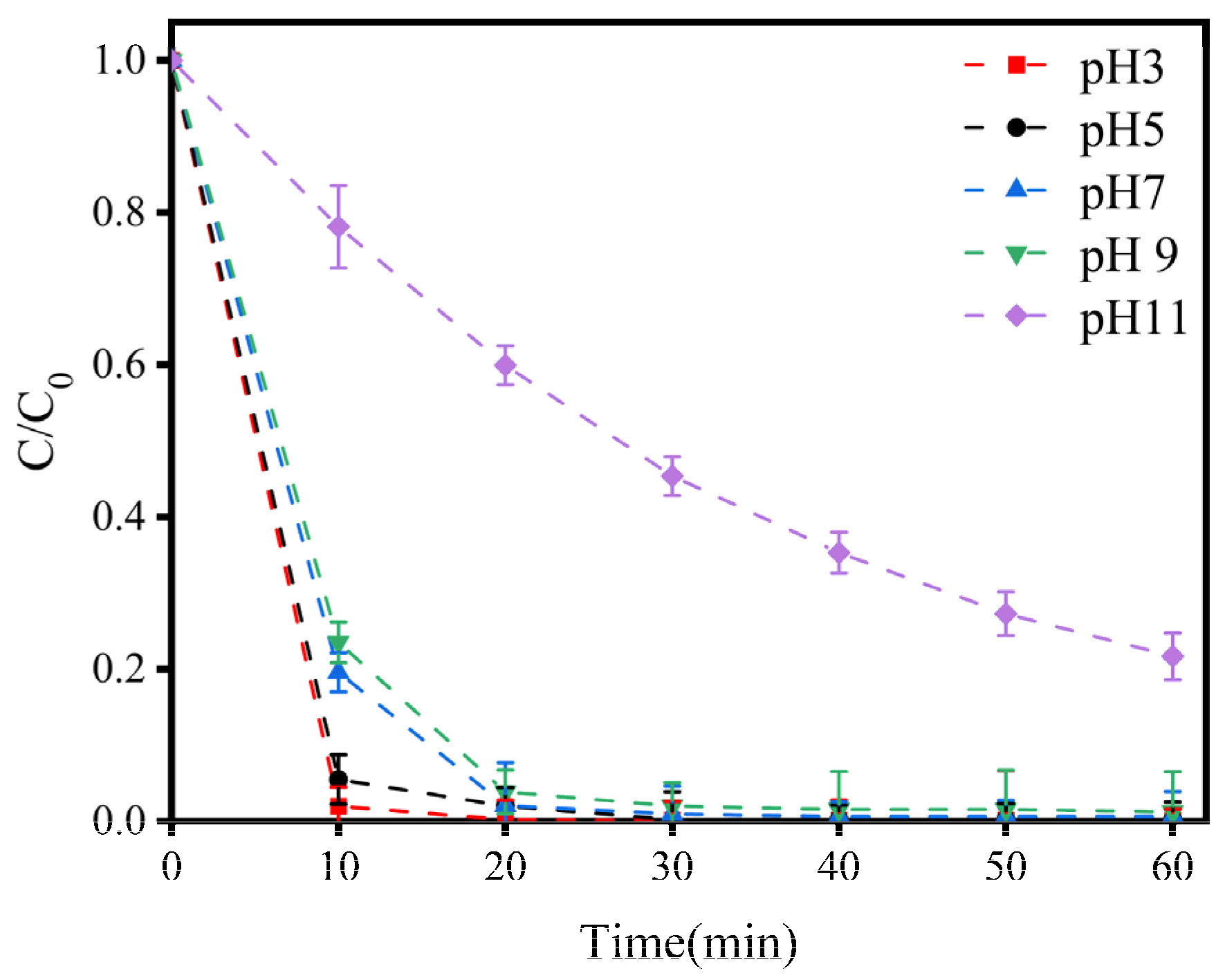
3.1.3. Effects of pH
3.1.4. Effects of Plate Spacing
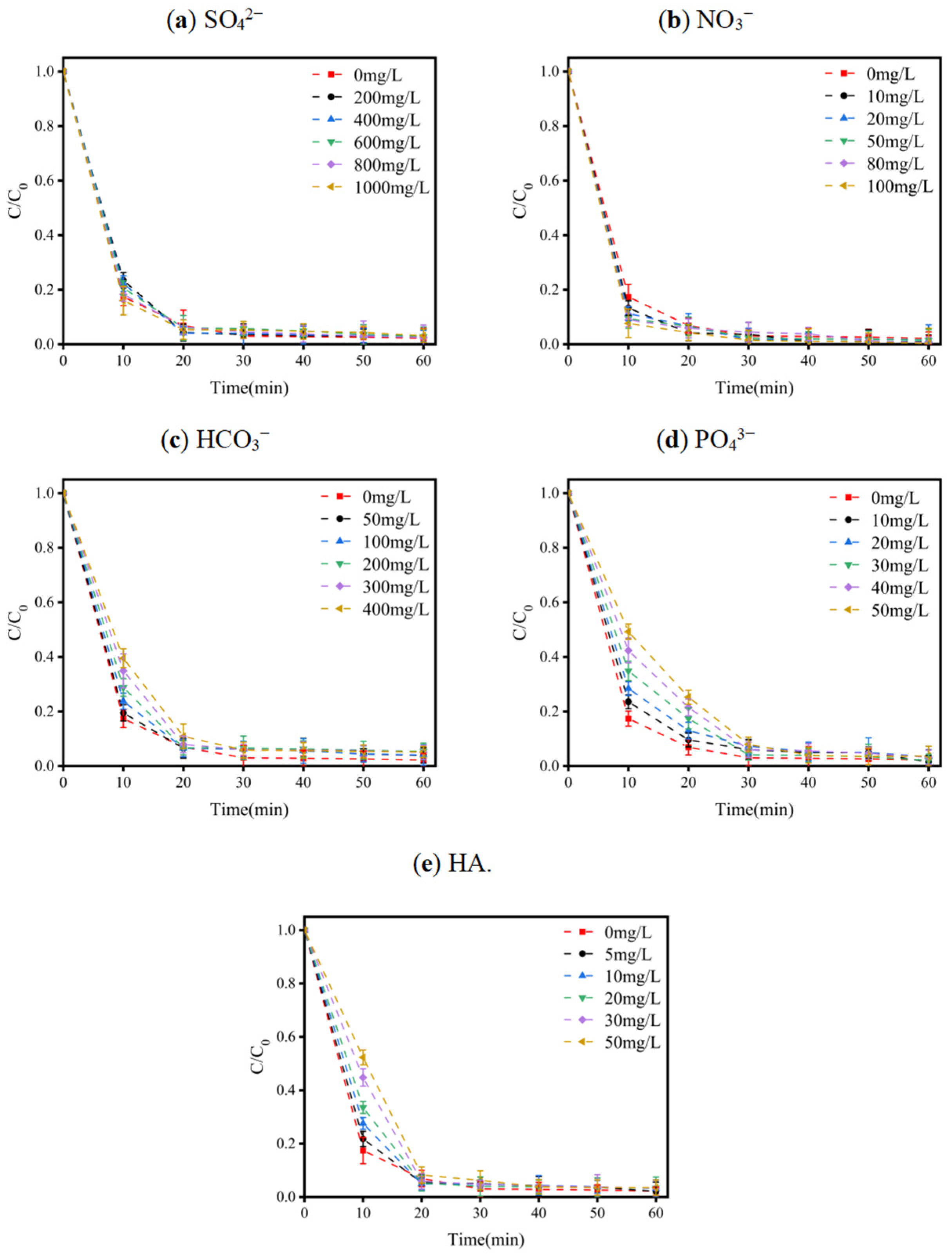
3.1.5. Effects of Coexisting Substances
3.2. Identification of Reactive Species
3.3. Possible Pathways of LEV Degradation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tawabini, B.S.; Plakas, K.V.; Fraim, M.; Safi, E.; Oyehan, T.; Karabelas, A.J. Assessing the efficiency of a pilot-scale GDE/BDD electrochemical system in removing phenol from high salinity waters. Chemosphere 2020, 239, 124714. [Google Scholar] [CrossRef]
- Cao, W.R.; Hu, C.; Zhang, P.; Qiu, T.; Wang, S.G.; Huang, G.H.; Lyu, L. Salinity-mediated water self-purification via bond network distorting of H2O molecules on DRC-surface. Proc. Natl. Acad. Sci. USA 2023, 120, e2311920120. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, X.; Ng, H.Y. Aerobic granular sludge systems for treating hypersaline pharmaceutical wastewater: Start-up, long-term performances and metabolic function. J. Hazard. Mater. 2021, 412, 125229. [Google Scholar] [CrossRef]
- Van Doorslaer, X.; Dewulf, J.; Van Langenhove, H.; Demeestere, K. Fluoroquinolone antibiotics: An emerging class of environmental micropollutants. Sci. Total Environ. 2014, 500, 250–269. [Google Scholar] [CrossRef]
- Lv, H.; Han, P.; Li, X.; Mu, Z.; Zuo, Y.; Wang, X.; Tan, Y.; He, G.; Jin, H.; Sun, C.; et al. Electrocatalytic Degradation of Levofloxacin, a Typical Antibiotic in Hospital Wastewater. Materials 2021, 14, 6814. [Google Scholar] [CrossRef]
- John, Y.; David, V.E., Jr.; Mmereki, D. A Comparative Study on Removal of Hazardous Anions from Water by Adsorption: A Review. Int. J. Chem. Eng. 2018, 2018, 3975948. [Google Scholar] [CrossRef]
- Gonzalez-Pleiter, M.; Gonzalo, S.; Rodea-Palomares, I.; Leganes, F.; Rosal, R.; Boltes, K.; Marco, E.; Fernandez-Pinas, F. Toxicity of five antibiotics and their mixtures towards photosynthetic aquatic organisms: Implications for environmental risk assessment. Water Res. 2013, 47, 2050–2064. [Google Scholar] [CrossRef]
- Hanna, N.; Sun, P.; Sun, Q.; Li, X.W.; Yang, X.W.; Ji, X.; Zou, H.Y.; Ottoson, J.; Nilsson, L.E.; Berglund, B.; et al. Presence of antibiotic residues in various environmental compartments of Shandong province in eastern China: Its potential for resistance development and ecological and human risk. Environ. Int. 2018, 114, 131–142. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.B.; Patil, D.V.; Jang, M.; Paeng, K.-J.; Jeon, B.-H. Biodegradation and metabolic fate of levofloxacin via a freshwater green alga, Scenedesmus obliquus in synthetic saline wastewater. Algal Res.-Biomass Biofuels Bioprod. 2017, 25, 54–61. [Google Scholar] [CrossRef]
- Yang, K.D.; Han, P.W.; Liu, Y.N.; Lv, H.X.; Chen, X.F.; Lei, Y.H.; Yu, L.; Ma, L.; Duan, P.Z. Boosted Electrocatalytic Degradation of Levofloxacin by Chloride Ions: Performances Evaluation and Mechanism Insight with Different Anodes. Molecules 2024, 29, 662. [Google Scholar] [CrossRef]
- Bhat, A.P.; Gogate, P.R. Degradation of nitrogen-containing hazardous compounds using advanced oxidation processes: A review on aliphatic and aromatic amines, dyes, and pesticides. J. Hazard. Mater. 2021, 403, 123657. [Google Scholar] [CrossRef]
- Fonseca Pierangeli, G.M.; Ragio, R.A.; Benassi, R.F.; Gregoracci, G.B.; Subtil, E.L. Pollutant removal, electricity generation and microbial community in an electrochemical membrane bioreactor during co-treatment of sewage and landfill leachate. J. Environ. Chem. Eng. 2021, 9, 106205. [Google Scholar] [CrossRef]
- Shenvi, S.S.; Isloor, A.M.; Ismail, A.F. A review on RO membrane technology: Developments and challenges. Desalination 2015, 368, 10–26. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, J.; Yu, S.; Chen, B.; Chen, C.; Shen, L.; Li, B.; Lin, H. The coupling of persulfate activation and membrane separation for the effective pollutant degradation and membrane fouling alleviation. Chem. Eng. J. 2023, 451, 139009. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Ma, L.; Huang, Y.; Wang, H. Characteristics and mechanisms of catalytic ozonation with Fe-shaving-based catalyst in industrial wastewater advanced treatment. J. Clean. Prod. 2019, 222, 174–181. [Google Scholar] [CrossRef]
- Yang, F.; Sheng, B.; Wang, Z.; Xue, Y.; Liu, J.; Ma, T.; Bush, R.; Kusic, H.; Zhou, Y. Performance of UV/acetylacetone process for saline dye wastewater treatment: Kinetics and mechanism. J. Hazard. Mater. 2021, 406, 124774. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Hong, M.; Peng, B.; Bao, C.; Xu, X.; Li, D.; Chen, J.; Wang, B.; Zhang, Q. Ferrocene-based resin as heterogeneous fenton-like catalyst for efficient treatment of high salinity wastewater at acidic, neutral, and basic pH. Chem. Eng. J. 2023, 464, 142450. [Google Scholar] [CrossRef]
- Radjenovic, J.; Sedlak, D.L. Challenges and Opportunities for Electrochemical Processes as Next-Generation Technologies for the Treatment of Contaminated Water. Environ. Sci. Technol. 2015, 49, 11292–11302. [Google Scholar] [CrossRef]
- Wang, A.Q.; Chen, Z.; Zheng, Z.K.; Xu, H.; Wang, H.; Hu, K.; Yan, K. Remarkably enhanced sulfate radical-based photo-Fenton-like degradation of levofloxacin using the reduced mesoporous MnO@MnOx microspheres. Chem. Eng. J. 2020, 379, 122340. [Google Scholar] [CrossRef]
- Li, M.K.; Huang, F.L.; Hu, L.; Sun, W.; Li, E.P.; Xiong, D.L.; Zhong, H.; He, Z.G. Efficient activation of peroxymonosulfate by a novel catalyst prepared directly from electrolytic manganese slag for degradation of recalcitrant organic pollutes. Chem. Eng. J. 2020, 401, 126085. [Google Scholar] [CrossRef]
- Nabgan, W.; Saeed, M.; Jalil, A.A.; Nabgan, B.; Gambo, Y.; Ali, M.W.; Ikram, M.; Fauzi, A.A.; Owgi, A.H.K.; Hussain, I.; et al. A state of the art review on electrochemical technique for the remediation of pharmaceuticals containing wastewater. Environ. Res. 2022, 210, 112975. [Google Scholar] [CrossRef]
- Nejumal, K.K.; Manoj, P.R.; Aravind, U.K.; Aravindakumar, C.T. Sonochemical degradation of a pharmaceutical waste, atenolol, in aqueous medium. Environ. Sci. Pollut. Res. 2014, 21, 4297–4308. [Google Scholar] [CrossRef]
- Hai, H.; Xing, X.; Li, S.; Xia, S.; Xia, J. Electrochemical oxidation of sulfamethoxazole in BDD anode system: Degradation kinetics, mechanisms and toxicity evaluation. Sci. Total Environ. 2020, 738, 139909. [Google Scholar] [CrossRef]
- Sheng, B.; Huang, Y.; Wang, Z.; Yang, F.; Ai, L.; Liu, J. On peroxymonosulfate-based treatment of saline wastewater: When phosphate and chloride co-exist. RSC Adv. 2018, 8, 13865–13870. [Google Scholar] [CrossRef]
- Cho, K.; Hoffmann, M.R. Urea Degradation by Electrochemically Generated Reactive Chlorine Species: Products and Reaction Pathways. Environ. Sci. Technol. 2014, 48, 11504–11511. [Google Scholar] [CrossRef]
- Kuang, W.J.; Yan, Z.; Chen, J.X.; Ling, X.T.; Zheng, W.X.; Huang, W.J.; Feng, C.H. A Bipolar Membrane-Integrated Electrochlorination Process for Highly Efficient Ammonium Removal in Mature Landfill Leachate: The Importance of ClO• Generation. Environ. Sci. Technol. 2022, 57, 18538–18549. [Google Scholar] [CrossRef]
- Huo, Z.Y.; Winter, L.R.; Wang, X.X.; Du, Y.; Wu, Y.H.; Hubner, U.; Hu, H.Y.; Elimelech, M. Synergistic Nanowire-Enhanced Electroporation and Electrochlorination for Highly Efficient Water Disinfection. Environ. Sci. Technol. 2022, 56, 10925–10934. [Google Scholar] [CrossRef]
- Khelifa, A.; Aoudj, S.; Moulay, S.; Hecini, M.; De Petris-Wery, M. Degradation of EDTA by in-situ electrogenerated active chlorine in an electroflotation cell. Desalination Water Treat. 2009, 7, 119–123. [Google Scholar] [CrossRef]
- Bonola, B.; Aguilar, D.; Fuentes-Camargo, I.; Sosa-Rodríguez, F.S.; Altamirano, R.H.; Luna-Sánchez, R.; Vazquez-Arenas, J. Implications to enhance sulfamethoxazole degradation using statistical optimization of either active chlorine concentration or ORP in an electrochemical reactor. J. Environ. Chem. Eng. 2020, 8, 104179. [Google Scholar] [CrossRef]
- Xie, J.Z.; Zhang, C.Y.; Waite, T.D. Hydroxyl radicals in anodic oxidation systems: Generation, identification and quantification. Water Res. 2022, 217, 118425. [Google Scholar] [CrossRef]
- Wojnarovits, L.; Takacs, E. Rate constants of dichloride radical anion reactions with molecules of environmental interest in aqueous solution: A review. Environ. Sci. Pollut. Res. 2021, 28, 41552–41575. [Google Scholar] [CrossRef]
- Wang, Z.; Li, K.; Guo, J.; Yang, C.; Yan, L.; Feng, W.; Zhang, Y.; Wang, J. Elimination of pesticide from high salinity wastewater by electrochlorination process: Active chlorine species and scale-up performance. Sep. Purif. Technol. 2023, 306, 122572. [Google Scholar] [CrossRef]
- Song, T.H.; Gao, Y.J.; Pang, S.Y.; Hu, R.H.; Li, H.B. Study of Fe3O4/PS System in Degrading BPA in Aqueous Solution. J. Braz. Chem. Soc. 2021, 32, 2264–2271. [Google Scholar] [CrossRef]
- Chen, H.; Lin, T.; Wang, P.F.; Wang, Y.C.; Wei, W.; Zhu, S.G. A novel solar-activated chlorine dioxide process for atrazine degradation in drinking water. Water Res. 2023, 239, 120056. [Google Scholar] [CrossRef]
- Xiang, Y.Y.; Fang, J.Y.; Shang, C. Kinetics and pathways of ibuprofen degradation by the UV/chlorine advanced oxidation process. Water Res. 2016, 90, 301–308. [Google Scholar] [CrossRef]
- Lu, X.; Wu, L.; Liang, L.; Liu, D.; Chen, Y.; Zeng, Y.; Zhong, M.; Jia, B. Levofloxacin degradation by porous Cox/CN activated peroxymonosulfate: Investigation of efficiency, mechanism, and degradation pathways. J. Water Process Eng. 2023, 56, 104427. [Google Scholar] [CrossRef]
- Wen, X.-J.; Niu, C.-G.; Guo, H.; Zhang, L.; Liang, C.; Zeng, G.-M. Photocatalytic degradation of levofloxacin by ternary Ag2CO3/CeO2/AgBr photocatalyst under visible-light irradiation: Degradation pathways, mineralization ability, and an accelerated interfacial charge transfer process study. J. Catal. 2018, 358, 211–223. [Google Scholar] [CrossRef]
- Zhang, M.X.; Xing, M.Y.; Dong, B.; Sun, X.J.; Zhang, H.X.; Wang, C.L.; Zhu, H.X. Preparation of BiVO4/CO32−-Bi2O2CO3 heterojunctions for enhanced photocatalytic activity in the degradation of levofloxacin under visible light. J. Alloys Compd. 2023, 965, 171471. [Google Scholar] [CrossRef]
- Liu, X.H.; Liu, Y.; Lu, S.Y.; Wang, Z.; Wang, Y.Q.; Zhang, G.D.; Guo, X.C.; Guo, W.; Zhang, T.T.; Xi, B.D. Degradation difference of ofloxacin and levofloxacin by UV/H2O2 and UV/PS (persulfate): Efficiency, factors and mechanism. Chem. Eng. J. 2020, 385, 123987. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.B.; Owens, G.; Chen, Z.L. Toward green nano adsorbents and catalysts: Highly active Fe/Mn nanoparticles for enhanced oxidation of oxytetracycline and levofloxacin. J. Colloid Interface Sci. 2023, 632, 299–310. [Google Scholar] [CrossRef]
- Fang, C.; Shao, C.; Wang, S.; Wu, Y.; Liu, C.; Huang, Q. Simultaneous removal of levofloxacin and sulfadiazine in water by dielectric barrier discharge (DBD) plasma: Enhanced performance and degradation mechanism. Process Saf. Environ. Prot. 2023, 171, 459–469. [Google Scholar] [CrossRef]
- Peng, J.; He, Y.Y.; Zhang, Z.Y.; Chen, X.Z.; Jiang, Y.L.; Guo, H.; Yuan, J.P.; Wang, J.H. Removal of levofloxacin by an oleaginous microalgae Chromochloris zofingiensis in the heterotrophic mode of cultivation: Removal performance and mechanism. J. Hazard. Mater. 2022, 425, 128036. [Google Scholar] [CrossRef]
- He, L.; Ma, Y.Y.; Lei, X.; Zhou, H.J.; Yuan, Y.; Du, W.T.; Liu, Z.L.; Miao, R.R.; Guan, Q.Q. Degradation of 4-chlorophenol through in situ generation of reactive chlorine species in a Zn2CoOx electrocatalytic system. J. Environ. Chem. Eng. 2024, 12, 111911. [Google Scholar] [CrossRef]
- An, T.; Yang, H.; Song, W.; Li, G.; Luo, H.; Cooper, W.J. Mechanistic Considerations for the Advanced Oxidation Treatment of Fluoroquinolone Pharmaceutical Compounds using TiO2 Heterogeneous Catalysis. J. Phys. Chem. A 2010, 114, 2569–2575. [Google Scholar] [CrossRef]
- Mahjoore, M.; Honarmand, M.; Aryafar, A. Plant-based green fabrication of CuO-CdO-bentonite S-scheme heterojunction with enhanced photocatalytic performance for the degradation of levofloxacin. Environ. Sci. Pollut. Res. 2023, 30, 44439–44456. [Google Scholar] [CrossRef]
- Kiki, C.; Rashid, A.; Zhang, Y.Q.; Li, X.; Chen, T.Y.; Adéoye, A.B.E.; Peter, P.O.; Sun, Q. Microalgal mediated antibiotic co-metabolism: Kinetics, transformation products and pathways. Chemosphere 2022, 292, 133438. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, M.; Li, J.; Jing, B.; Li, X.; Li, G. The Elimination of Levofloxacin from High-Salinity Wastewater via the Electrochlorination Process. Water 2024, 16, 1355. https://doi.org/10.3390/w16101355
Wei M, Li J, Jing B, Li X, Li G. The Elimination of Levofloxacin from High-Salinity Wastewater via the Electrochlorination Process. Water. 2024; 16(10):1355. https://doi.org/10.3390/w16101355
Chicago/Turabian StyleWei, Mingfei, Jingyu Li, Bingqing Jing, Xuankun Li, and Guanghui Li. 2024. "The Elimination of Levofloxacin from High-Salinity Wastewater via the Electrochlorination Process" Water 16, no. 10: 1355. https://doi.org/10.3390/w16101355




