Mineral Assemblages and Ore-Forming Physicochemical Conditions of the La’erma and Qiongmo Au–Se Deposits in the Western Qinling Orogen, Central China
Abstract
:1. Introduction
2. Geological Setting
2.1. Regional Geology
2.2. Deposit Geology
2.2.1. Stratigraphy
2.2.2. Structural Geology
2.2.3. Orebody Geology

3. Sample and Analytical Methods
4. Results
4.1. Native Gold
4.2. Selenides
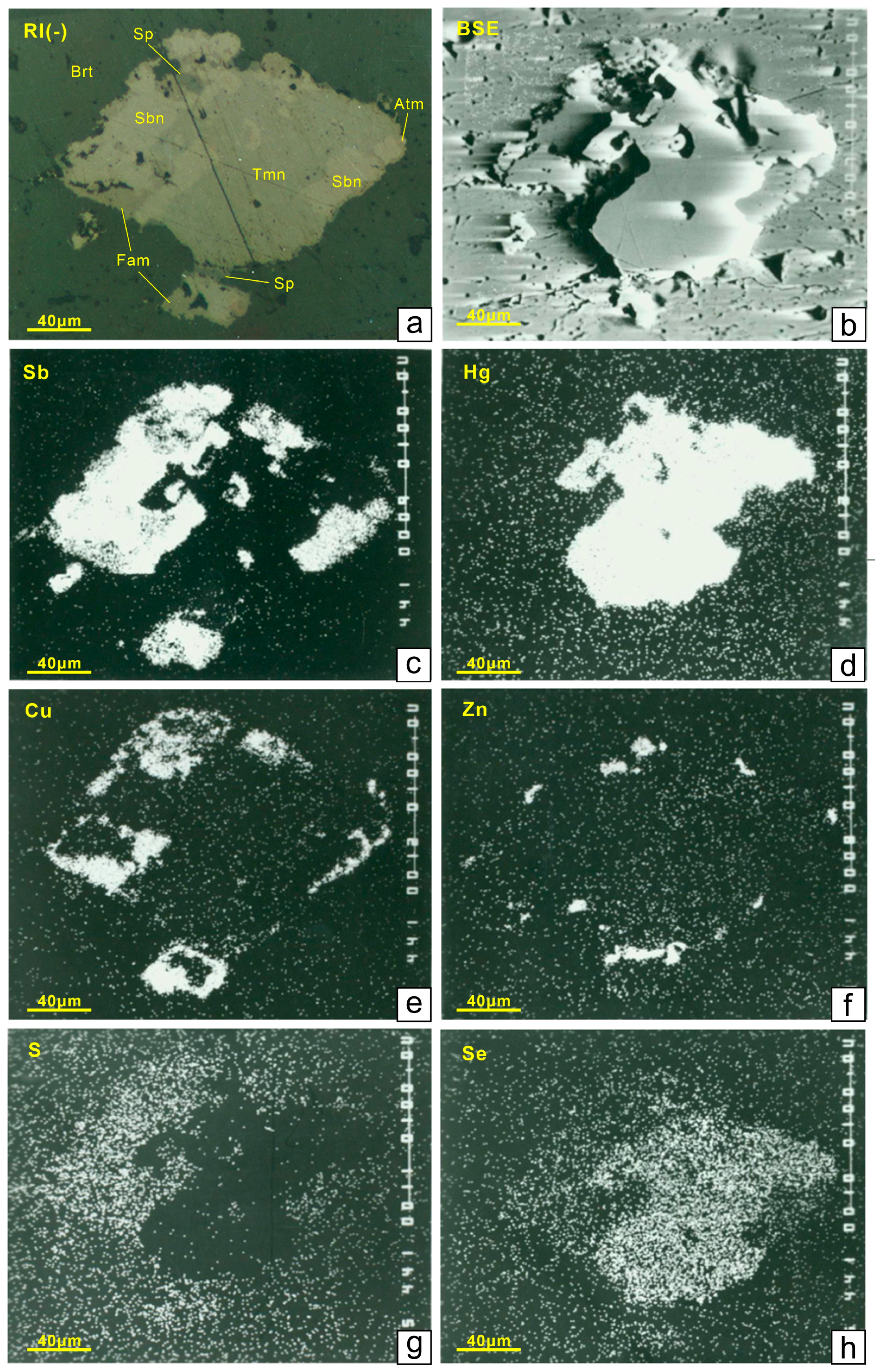

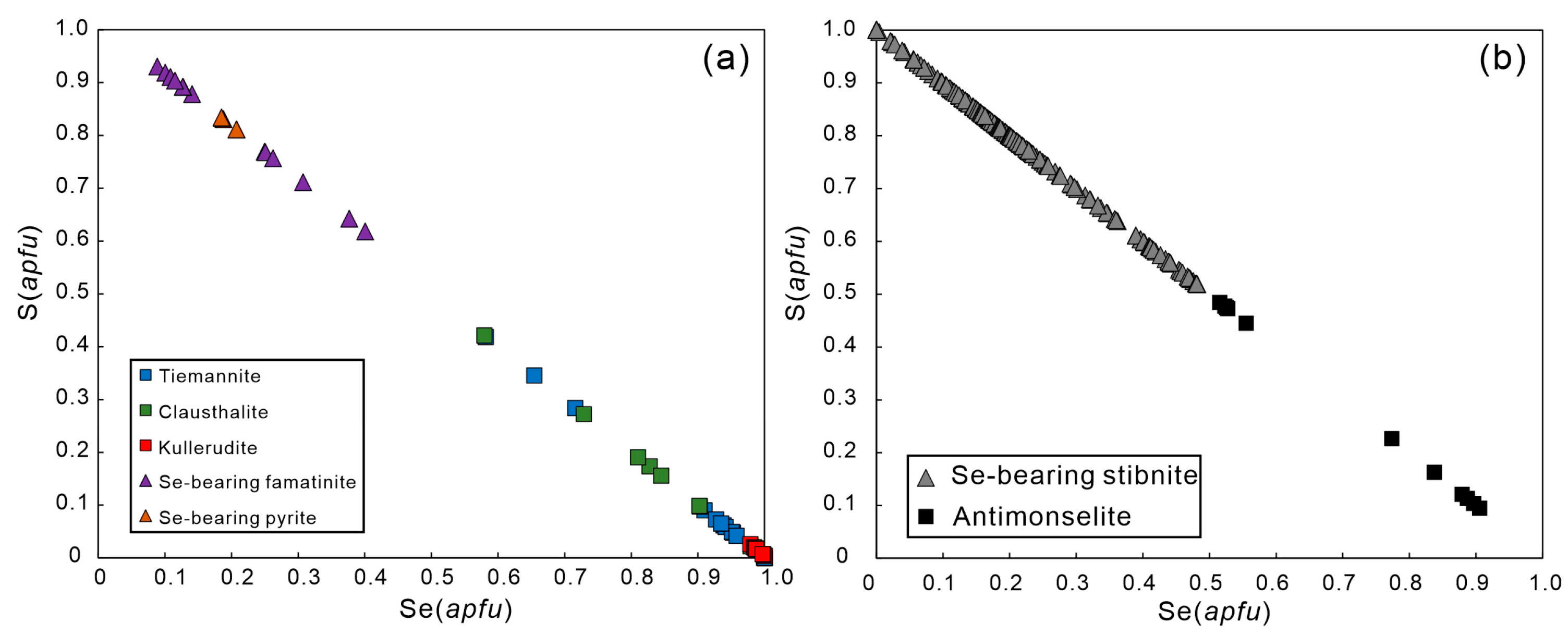
4.3. Se-Bearing Sulfides
5. Discussion
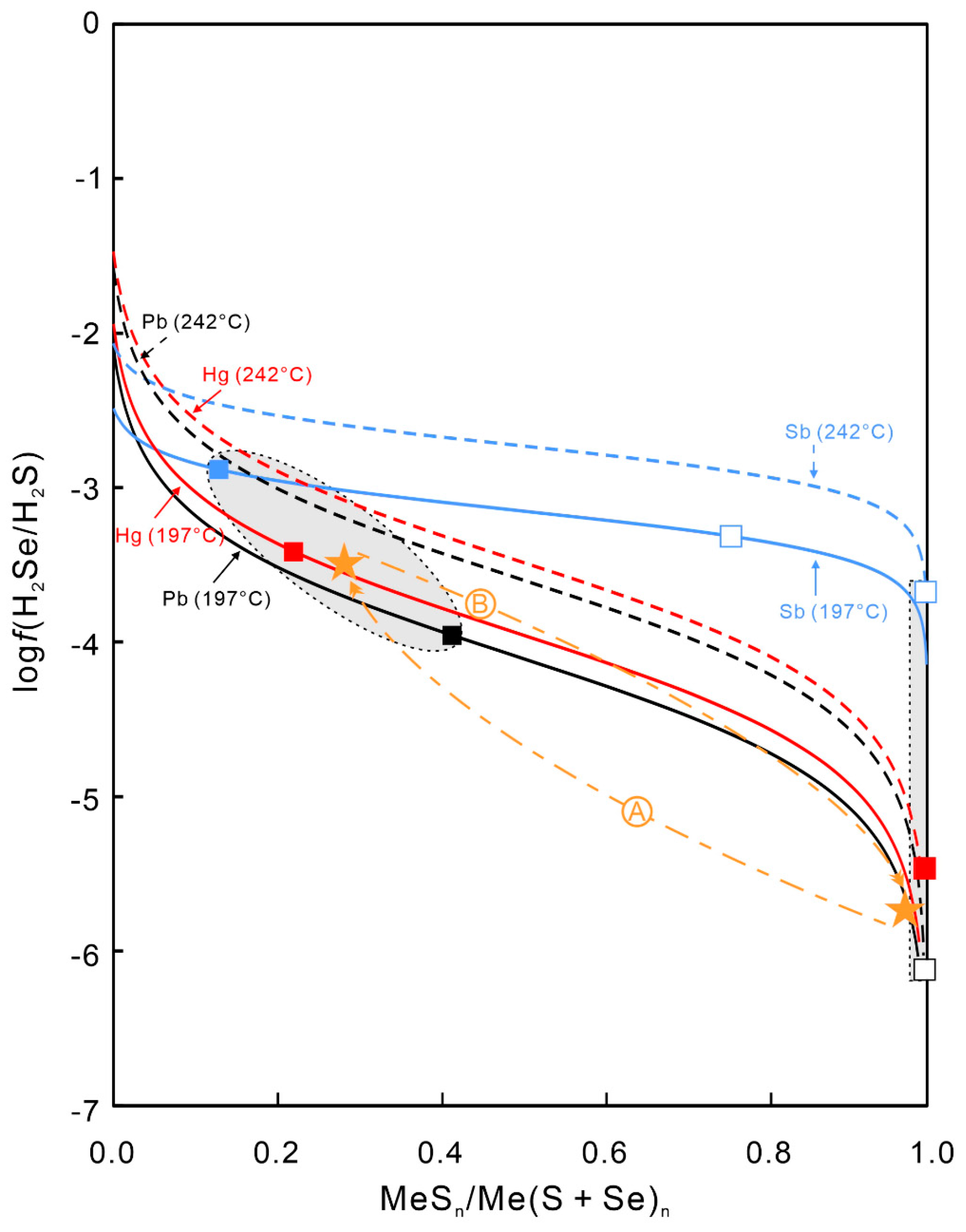
5.1. Physicochemical Conditions of Mineral Deposition
5.2. Reasons for Se-Enrichment

6. Conclusions
- The La’erma and Qiongmo Au–Se deposits developed a variety of selenides, native gold, and stibnite that coexist with baryte. Based on the mineral assemblage and metallogenic temperature, the logfS2 values of the sulfide assemblage at stage II probably ranged between −10.44 and −14.60 with logfSe2 being less than −10.70. Comparably, during stage III, which is characterized by many selenides, the logfS2 and logfSe2 ranged from −7.13 to −12.20 and −13.98 to −8.82, respectively.
- The redox conditions were consistently oxidizing, as confirmed by the persistent presence of baryte. However, the oxidizing condition was only a fundamental prerequisite for selenides’ deposition. The ultimate controlling factor for the precipitation of selenides was a high ∑Se/S ratio of the fluid. Simulations of phase diagrams revealed that an increase in the ∑Se/S ratio of the fluid through water–rock reactions promoted the precipitation of selenides rather than sulfides.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rudnick, R.L.; Gao, S. Composition of the continental crust. Treatise Geochem. 2003, 3, 659. [Google Scholar]
- Hu, Z.C.; Gao, S. Upper crustal abundances of trace elements: A revision and update. Chem. Geol. 2008, 253, 205–221. [Google Scholar] [CrossRef]
- Simon, G.; Kesler, S.E.; Essene, E.J. Phase relations among selenides, sulfides, tellurides and oxides: II. applications to selenide-bearing ore deposits. Econ. Geol. 1997, 92, 465–484. [Google Scholar] [CrossRef]
- Zhai, D.G.; Williams-Jones, A.E.; Liu, J.J.; Tombros, S.F.; Cook, N.J. Mineralogical, fluid inclusion, and multiple isotope (H-O-S-Pb) constraints on the genesis of the Sandaowanzi epithermal Au-Ag-Te Deposit, NE China. Econ. Geol. 2018, 113, 1359–1382. [Google Scholar] [CrossRef]
- Wang, D.Z.; Zhen, S.M.; Liu, J.J.; Carranza, E.J.M.; Wang, J.; Zha, Z.J.; Li, Y.S.; Bai, H.J. Mineral paragenesis and hydrothermal evolution of the Dabaiyang tellurium-gold deposit, Hebei Province, China: Constraints from fluid inclusions, H-O-He-Ar isotopes, and physicochemical conditions. Ore Geol. Rev. 2021, 130, 103904. [Google Scholar] [CrossRef]
- Zhu, J.; Zuo, W.; Liang, X.; Li, S.; Zheng, B. Occurrence of native selenium in Yutangba and its environmental implications. Appl. Geochem. 2004, 19, 461–467. [Google Scholar] [CrossRef]
- So, C.S.; Dunchenko, V.Y.; Yun, S.T.; Park, M.E.; Choi, S.G.; Shelton, K.L. Te- and Se-bearing epithermal Au-Ag mineralization, Prasolovskoye, Kunashir Island, Kuril island arc. Econ. Geol. 1995, 90, 105–117. [Google Scholar] [CrossRef]
- Finkelman, R.B.; Mrose, M.E. Downeyite, the first verified natural occurrence of SeO2. Am. Mineral. 1977, 62, 316–320. [Google Scholar]
- Luo, K.D.; Wei, J.; Zhang, J.Y.; Gu, Q.F. Chalcomenite—A new selenous acid oxysalts mineral. Chin. Sci. Bull. 1980, 2, 85–89. (In Chinese) [Google Scholar]
- Weng, G.M.; Liu, J.J.; Carranza, E.J.M.; Zhai, D.G.; Zhang, F.F.; Wang, Y.H.; Yu, C.; Zhang, B.; Liu, X.H.; Sun, B.; et al. Mineralogy and geochemistry of tellurides, selenides and sulfides from the Zhaishang gold deposit, western Qinling, China: Implications for metallogenic processes. J. Asian Earth Sci. 2023, 244, 105536. [Google Scholar] [CrossRef]
- Liu, J.J.; Zheng, M.H. First discovery of a compound in the antimony selenium-sulfur series. Chin. Sci. Bull. 1992, 9, 864. (In Chinese) [Google Scholar]
- Wang, X.; Liu, J.; Carranza, E.J.M.; Zhai, D.G.; Zhao, Q.Q.; Weng, G.M.; Zhang, B. Characteristics and formation conditions of Se-Bearing metacinnabar in the Wanshan mercury ore field, eastern Guizhou. Minerals 2023, 13, 173. [Google Scholar] [CrossRef]
- Shao, Y.J.; Wang, W.S.; Liu, Q.Q.; Zhang, Y. Trace element analysis of pyrite from the Zhengchong gold deposit, northeast Hunan province, China: Implications for the ore-forming process. Minerals 2018, 8, 262. [Google Scholar] [CrossRef]
- Beliaeva, T.V.; Palyanova, G.A. Silver Sulfides and Selenides in Ores from Au–Ag Epithermal Deposits of the Okhotsk—Chukotka Volcanic Belt. Geol. Ore Depos. 2023, 65, 72–105. [Google Scholar] [CrossRef]
- Liu, J.J.; Zheng, M.H.; Liu, J.M.; Su, W.C. Geochemistry of the La’erma and Qiongmo Au–Se deposits in the western Qinling Mountains, China. Ore Geol. Rev. 2000, 17, 91–111. [Google Scholar] [CrossRef]
- Sidorov, E.G.; Borovikov, A.A.; Tolstykh, N.D.; Bukhanova, D.S.; Palyanova, G.A.; Chubarov, V.M. Gold mineralization at the maletoyvayam deposit (Koryak Highland, Russia) and physicochemical conditions of its formation. Minerals 2020, 10, 1093. [Google Scholar] [CrossRef]
- Kovalenker, V.A.; Plotinskaya, O.Y.; Prokof’ev, V.Y.; Pomortsev, V.V.; Koneev, R.I. Mineralogy, geochemistry, and genesis of gold-sulfide-selenide-telluride ores from the Kairagach deposit (Uzbekistan). Geol. Ore Depos. 2003, 45, 171–200. [Google Scholar]
- Redwood, S.D. Famous mineral localities: The Pacajake selenium mine, Potosi, Bolivia. Mineral. Rec. 2003, 34, 339. [Google Scholar]
- Hans-Jürgen, F.; Luca, B.; Chris, J.S. Grundmannite, CuBiSe2, the Se-analogue of emplectite, a new mineral from the El Dragon mine, Potosi, Bolivia. Eur. J. Mineral. 2016, 28, 467–477. [Google Scholar] [CrossRef]
- Song, C.Z. A brief description of the Yutangba sedimentary type selenium mineralized area in southwestern Hubei. Miner. Depos. 1989, 3, 83–89, (In Chinese with English Abstract). [Google Scholar]
- Dai, S.F.; Yang, J.Y.; Ward, C.R.; Hower, J.C.; Liu, H.D.; Garrison, T.M.; French, D.; O’Keefe, J.M.K. Geochemical and mineralogical evidence for a coal-hosted uranium deposit in the Yili Basin, Xinjiang, northwestern China. Ore Geol. Rev. 2015, 70, 1–30. [Google Scholar] [CrossRef]
- Dill, H.G. The “chessboard” classification scheme of mineral deposits: Mineralogy and geology from aluminum to zirconium. Earth-Sci. Rev. 2010, 100, 1–420. [Google Scholar] [CrossRef]
- Saunders, J.A.; Brueseke, M.E. Volatility of Se and Te during subduction-related distillation and the geochemistry of epithermal ores of the western United states. Econ. Geol. 2012, 107, 165–172. [Google Scholar] [CrossRef]
- Shuai, D.Q.; Hu, X.Q. Mineral component features and gold migration patterns of La’erma gold deposit in Gansu-Sichuan. Geol. Explor. 1995, 31, 28–35, (In Chinese with English Abstract). [Google Scholar]
- Dong, Y.P.; Santosh, M. Tectonic architecture and multiple orogeny of the Qinling Orogenic Belt, Central China. Gondwana Res. 2016, 29, 1–40. [Google Scholar] [CrossRef]
- Dong, Y.P.; Sun, S.S.; Santosh, M.; Zhao, J.; Sun, J.P.; He, D.F.; Shi, X.H.; Hui, B.; Cheng, C.; Zhang, G.W. Central China Orogenic Belt and amalgamation of East Asian continents. Gondwana Res. 2021, 100, 131–194. [Google Scholar] [CrossRef]
- Zhang, G.W.; Guo, A.L.; Dong, Y.P.; Yao, A.P. Rethinking of the Qinling Orogen. J. Geomech. 2019, 25, 746–768, (In Chinese with English Abstract). [Google Scholar]
- Dong, Y.P.; Zhang, G.W.; Neubauer, F.; Liu, X.M.; Genser, J.; Hauzenberger, C. Tectonic evolution of the Qinling orogen, China: Review and synthesis. J. Asian Earth Sci. 2011, 41, 213–237. [Google Scholar] [CrossRef]
- Qiu, K.F.; Deng, J.; He, D.Y.; Rosenbaum, G.; Zheng, X.; Williams-Jones, A.E.; Yu, H.C.; Balen, D. Evidence of vertical slab tearing in the late triassic Qinling Orogen (central China) from multiproxy geochemical and isotopic imaging. JCR Solid Earth 2023, 128, e2022JB025514. [Google Scholar] [CrossRef]
- Liu, J.J.; Dai, H.Z.; Zhai, D.G.; Wang, J.P.; Wang, Y.H.; Yang, L.B.; Mao, G.J.; Liu, X.H.; Liao, Y.F.; Yu, C.; et al. Geological and geochemical characteristics and formation mechanisms of the Zhaishang Carlin-like type gold deposit, western Qinling Mountains, China. Ore Geol. Rev. 2015, 64, 273–298. [Google Scholar] [CrossRef]
- Zhang, G.W.; Zhang, B.R.; Yuan, X.C.; Xiao, Q.H. Qinling Orogenic Belt and Continental Dynamics; Science Press: Beijing, China, 2001; 855p. (In Chinese) [Google Scholar]
- Meng, Q.R.; Zhang, G.W. Geologic framework and tectonic evolution of the Qinling orogen, central China. Tectonophysics 2000, 323, 183–196. [Google Scholar] [CrossRef]
- Hu, F.Y.; Liu, S.W.; Ducea, M.N.; Chapman, J.B.; Wu, F.Y.; Kusky, T. Early Mesozoic magmatism and tectonic evolution of the Qinling Orogen: Implications for oblique continental collision. Gondwana Res. 2020, 88, 296–332. [Google Scholar] [CrossRef]
- Liu, J.J.; Liu, J.M.; Zheng, M.H.; Liu, X.F. Au–Se paragenesis in Cambrian stratabound gold deposits, western Qinling Mountains, China. Int. Geol. Rev. 2000, 42, 1037–1045. [Google Scholar] [CrossRef]
- Liu, J.J.; Zheng, M.H.; Liu, J.M. Selenium enrichement in Cambrian stratabound gold deposits in western Qinling Mountains: Characteristics, origin and prospects. Acta Geol. Sin. 1997, 71, 266–273, (In Chinese with English Abstract). [Google Scholar]
- Liu, J.J.; Zheng, M.H. The gold occurence of La’erma stratabound gold deposit. Gold 1994, 15, 7–12, (In Chinese with English Abstract). [Google Scholar]
- Liu, J.J.; Zheng, M.H.; Liu, J.M.; Zhou, Y.F.; Gu, X.X.; Zhang, B. The geological and geochemical characteristics of Cambrian chert and their sedimentary environmental implications in western Qinling. Acta Petrol. Sin. 1999, 15, 145–154, (In Chinese with English Abstract). [Google Scholar]
- Xu, L. Geological, Geochemical Characteristics and Genesis of the La’erma Gold Deposit in Gansu Province. Ms.D. Thesis, China University of Geosciences, Beijing, China, 2020. [Google Scholar]
- He, W.M.; Li, W.J.; Ning, J.W.; Li, D.Z.; Wang, H.K. The geological characteristics and ore-controlling factors of La’erma gold deposit in Luqu county, Gansu province. Miner. Resour. Geol. 2017, 31, 99–105, (In Chinese with English Abstract). [Google Scholar]
- Mao, J.W.; Qiu, Y.M.; Goldfarb, R.J.; Zhang, Z.C.; Garwin, S.; Fengshou, R. Geology, distribution, and classification of gold deposits in the western Qinling belt, central China. Mineral. Depos. 2002, 37, 352–377. [Google Scholar] [CrossRef]
- Chen, Y.Y. Ore prospecting indicator and exploration model of gold deposits in South Gansu: Comparative analysis of Dashui, Zaozigou and La’erma gold deposits. Miner. Resour. Geol. 2020, 34, 7–18, (In Chinese with English Abstract). [Google Scholar]
- Liu, J.J.; Zheng, M.H.; Liu, J.M.; Zhou, D.A. Sulfur istopic composition and its geological significance of the Cambrian gold deposits in western Qinling, China. J. Chang. Univ. Sci. Technol. 2000, 30, 150–156, (In Chinese with English Abstract). [Google Scholar]
- Liu, J.J.; Zheng, M.H.; Liu, J.M.; Lu, W.Q. A description of possible new Ni-As-S-Se mineral phase. Acta Mineral. Sin. 1995, 15, 425–427, (In Chinese with English Abstract). [Google Scholar]
- Wen, H.J.; Qiu, Y.Z. Study on the organic/inorganic combined states of elements and the occurrence state of selenium in the La’erma Au–Se deposit. Sci. China (Ser. D) 1999, 29, 426–432. (In Chinese) [Google Scholar]
- Liu, J.J.; Liu, C.H.; Wang, J.P.; Zhu, L.M. Classification and mineralization of the gold deposits in the western Qinling region, China. Earth Sci. Front. 2019, 26, 1–16, (In Chinese with English Abstract). [Google Scholar]
- Liu, J.J.; Zhai, D.G.; Wang, D.Z.; Gao, S. Classification and mineralization of the Au-(Ag)-Te-Se deposits. Earth Sci. Front. 2020, 27, 79–98, (In Chinese with English Abstract). [Google Scholar]
- Liu, J.J.; Zheng, M.H. The first discovery of selenio-sulfantimonide mineral series. Chin. Sci. Bull. 1992, 37, 1495–1496. [Google Scholar]
- Liu, J.J.; Liu, J.M.; Liu, C.Q.; Lu, W.Q.; Liu, S.R.; Su, W.C. Mineralogy of the stibnite-antimonselite series. Int. Geol. Rev. 1999, 41, 1042–1050. [Google Scholar]
- Wood, M.J. Viral infections in neutropenia-Current problems and chemotherapeutic control. J. Antimicrob. Chemoth. 1998, 41, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.; Essene, E.J. Phase relations among selenides, sulfides, tellurides, and oxides; I, Thermodynamic properties and calculated equilibria. Econ. Geol. 1996, 91, 1183–1208. [Google Scholar] [CrossRef]
- Shock, E.L.; Helgeson, H.C.; Sverjernsky, D.A. Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: Standard partial molal properties of inorganic neutral species. Geochim. Cosmochim. Acta 1989, 53, 2157–2183. [Google Scholar] [CrossRef]
- Keith, M.; Smith, D.J.; Jenkin, G.R.T.; Holwell, D.A.; Dye, M.D. A review of Te and Se systematics in hydrothermal pyrite from precious metal deposits: Insights into ore-forming processes. Ore Geol. Rev. 2018, 96, 269–282. [Google Scholar] [CrossRef]
- Škácha, P.; Sejkora, J.; Plášil, J. Selenide mineralization in the Příbram Uranium and base-metal district (Czech Republic). Minerals 2017, 7, 91. [Google Scholar] [CrossRef]
- Ohomoto, H.; Rye, R.O. Isotopes of sulfur and carbon. In Geochemistry Hydrothermal Ore Depos; Barnes, H.L., Ed.; Wiley and Sons: New York, NY, USA, 1979; pp. 509–567. [Google Scholar]
- Ohmoto, H. Systematics of sulfur and carbon isotopes in hydrothermal ore deposit. Econ. Geol. 1972, 67, 551–578. [Google Scholar] [CrossRef]
- Rajabpour, S.; Behzadi, M.; Jiang, S.Y.; Rasa, I.; Lehmann, B.; Ma, Y. Sulfide chemistry and sulfur isotope characteristics of the Cenozoic volcanic-hosted Kuh-Pang copper deposit, Saveh county, northwestern central Iran. Ore Geol. Rev. 2017, 86, 563–583. [Google Scholar] [CrossRef]
- Liu, J.J.; Zheng, M.H.; Zhou, D.A.; Liu, J.M.; Zhou, Y.F.; Gu, X.X.; Zhang, B.; Lin, L. The components and evolution of the hydrogen and oxygen isotopes of ore-forming fluids from La’erma gold ore belt. J. Chang. Univ. Sci. Technol. 1998, 28, 43–49, (In Chinese with English Abstract). [Google Scholar]
- Williams-Jones, A.E.; Bowell, R.J.; Migdisov, A.A. Gold in Solution. Elements 2009, 5, 281–287. [Google Scholar] [CrossRef]
- Tolstykh, N.; Vymazalová, A.; Tuhý, M.; Shapovalova, M. Conditions of formation of Au–Se–Te mineralization in the Gaching ore occurrence (Maletoyvayam ore field), Kamchatka, Russia. Mineral. Mag. 2018, 82, 649–674. [Google Scholar] [CrossRef]
- Huston, D.L.; Sie, S.H.; Suter, G.F.; Cooke, D.R.; Both, R.A. Trace elements in sulfide minerals from eastern Australian volcanic-hosted massive sulfide deposits; Part I, Proton microprobe analyses of pyrite, chalcopyrite, and sphalerite, and Part II, Selenium levels in pyrite; comparison with delta 34S values and implications for the source of sulfur in volcanogenic hydrothermal systems. Econ. Geol. 1995, 90, 1167–1196. [Google Scholar]
- Liu, J.J.; Zheng, M.H. Antimonselite—No longer just synthetic minerals. Chin. J. 1992, 37, 1438. (In Chinese) [Google Scholar]
- Kolova, E.E.; Savva, N.E.; Zhuravkova, T.V.; Glukhov, A.N.; Palyanova, G.A. Au-Ag-S-Se-Cl-Br mineralization at the Corrida deposit (Russia) and physicochemical conditions of ore formation. Minerals 2021, 11, 144. [Google Scholar] [CrossRef]







| Minerals | Native Gold | Tiemannite | Antimonselite * | Clausthalite | Kullerudite | Ni3As3S3Se | Se-Bearing Stibnite * | Se-Bearing Famatinite | Se-Bearing Pyrite |
|---|---|---|---|---|---|---|---|---|---|
| Au | 90.15~99.82 | ||||||||
| Ag | 0.00~7.51 | 0.03 | 0.00~0.33 | ||||||
| Hg | 0.00~7.02 | 67.04~77.20 | 0.00~3.69 | 0.23~4.97 | 0.32~2.16 | 5.36 | 0.00~2.08 | 0.00~1.49 | |
| Sb | 0.00~0.18 | 48.94~59.13 | 58.47~72.99 | 5.40~8.07 | |||||
| Pb | 71.79~77.83 | ||||||||
| Ni | 24.80~27.71 | 29.38 | 0.00~0.11 | 3.97~4.59 | |||||
| As | 33.90 | 0.00~2.01 | |||||||
| Fe | 0.35~3.17 | 0.23 | 0.00~0.05 | 32.42~33.50 | |||||
| Cu | 0.46 | 36.60~39.94 | |||||||
| Zn | 0.00~0.44 | ||||||||
| Se | 0.00~4.56 | 17.73~33.01 | 29.20~46.86 | 16.79~25.19 | 67.67~73.06 | 12.67 | 0.00~29.12 | 6.11~23.89 | 19.71~22.61 |
| S | 0.44~5.18 | 1.99~11.43 | 0.23~4.97 | 0.09~0.72 | 15.56 | 11.86~28.76 | 14.49~25.74 | 32.86~35.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Weng, G.; Carranza, E.J.M.; Zhai, D.; Wang, Y.; Zhang, F.; Gao, S.; Xu, L. Mineral Assemblages and Ore-Forming Physicochemical Conditions of the La’erma and Qiongmo Au–Se Deposits in the Western Qinling Orogen, Central China. Minerals 2024, 14, 507. https://doi.org/10.3390/min14050507
Liu J, Weng G, Carranza EJM, Zhai D, Wang Y, Zhang F, Gao S, Xu L. Mineral Assemblages and Ore-Forming Physicochemical Conditions of the La’erma and Qiongmo Au–Se Deposits in the Western Qinling Orogen, Central China. Minerals. 2024; 14(5):507. https://doi.org/10.3390/min14050507
Chicago/Turabian StyleLiu, Jiajun, Guoming Weng, Emmanuel John M. Carranza, Degao Zhai, Yinhong Wang, Fangfang Zhang, Shen Gao, and Lei Xu. 2024. "Mineral Assemblages and Ore-Forming Physicochemical Conditions of the La’erma and Qiongmo Au–Se Deposits in the Western Qinling Orogen, Central China" Minerals 14, no. 5: 507. https://doi.org/10.3390/min14050507





